Silver-Nanocellulose Composite Used as SERS Substrate for Detecting Carbendazim
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents and Aparatus
2.2. Preparation of NCF-Ag
2.3. Sample Preparation
2.4. Measurement
2.5. Data Analysis
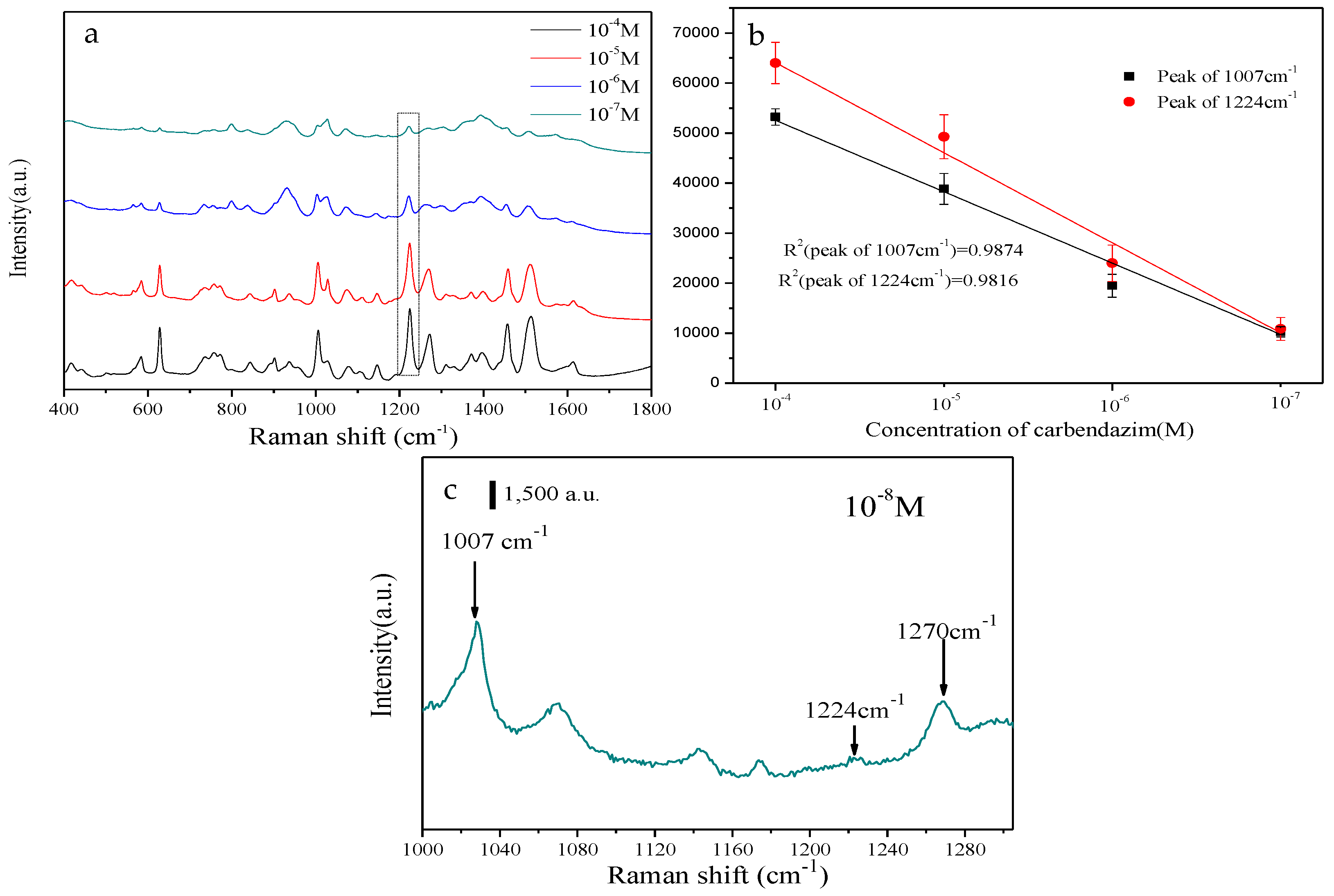
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veneziano, A. Determination of carbendazim, thiabendazole and thiophanate-methyl in banana (Musa acuminata) samples imported to Italy. Food Chem. 2004, 87, 383–386. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Li, B.; Tang, L. Carbendazim residues in vegetables in China between 2014 and 2016 and a chronic carbendazim exposure risk assessment. Food Control 2018, 91, 20–25. [Google Scholar] [CrossRef]
- Daundkar, P.S.; Rampal, S. Evaluation of ameliorative potential of selenium on carbendazim induced oxidative stress in male goats. Environ. Toxicol. Pharmacol. 2014, 38, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Han, Z.; Liu, H.; He, S.; Yang, L.; Liu, J. Three-dimensional hotspots in evaporating nanoparticle sols for ultrahigh Raman scattering: Solid-liquid interface effects. Nanoscale 2015, 7, 6619–6626. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liu, Y.; Xie, L.; Wang, X. Involvement of Sertoli cells in spermatogenic failure induced by carbendazim. Environ. Toxicol. Pharmacol. 2009, 27, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Ebrahim, H.; ElMazoudy, R.; Kadous, E. Developmental Toxicity of Fungicide Carbendazim in Female Mice. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Huan, Z.; Luo, J.; Xu, Z.; Xie, D. Acute Toxicity and Genotoxicity of Carbendazim, Main Impurities and Metabolite to Earthworms (Eisenia foetida). Bull. Environ. Contam. Toxicol. 2016, 96, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Kumaraguru, A.K.; Ramakritinan, C.M.; Anand, M. Toxicity, biochemical and clastogenic response of chlorpyrifos and carbendazim in milkfish Chanos chanos. Int. J. Environ. Sci. Technol. 2013, 11, 765–774. [Google Scholar] [CrossRef]
- Andrade, T.S.; Henriques, J.F.; Almeida, A.R.; Machado, A.L.; Koba, O.; Giang, P.T.; Soares, A.; Domingues, I. Carbendazim exposure induces developmental, biochemical and behavioural disturbance in zebrafish embryos. Aquat. Toxicol. 2016, 170, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wu, S.; Wang, Y.; An, X.; Cai, L.; Zhao, X.; Wu, C. Carbendazim has the potential to induce oxidative stress, apoptosis, immunotoxicity and endocrine disruption during zebrafish larvae development. Toxicol. In Vitro 2015, 29, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, K.; Yang, Y.; Ye, X.; Liu, J.; Li, F. Deleterious effects of benomyl and carbendazim on human placental trophoblast cells. Reprod. Toxicol. 2015, 51, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.M.; Rohit, J.V.; Singhal, R.K.; Kailasa, S.K. Recognition of carbendazim fungicide in environmental samples by using 4-aminobenzenethiol functionalized silver nanoparticles as a colorimetric sensor. Sens. Actuators B Chem. 2015, 206, 684–691. [Google Scholar] [CrossRef]
- Phansawan, B.; Prapamontol, T.; Thavornyutikarn, P.; Chantara, S.; Mangklabruks, A.; Santasup, C. A Sensitive Method for Determination of Carbendazim Residue in Vegetable Samples Using HPLC-UV and Its Application in Health Risk Assessment. Chiang Mai J. Sci. 2015, 42, 681–690. [Google Scholar]
- Razzino, C.A.; Sgobbi, L.F.; Canevari, T.C.; Cancino, J.; Machado, S.A. Sensitive determination of carbendazim in orange juice by electrode modified with hybrid material. Food Chem. 2015, 170, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ilktac, R.; Aksuner, N.; Henden, E. Selective and sensitive fluorimetric determination of carbendazim in apple and orange after preconcentration with magnetite-molecularly imprinted polymer. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 174, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Furini, L.N.; Constantino, C.J.L.; Sanchez-Cortes, S.; Otero, J.C.; Lopez-Tocon, I. Adsorption of carbendazim pesticide on plasmonic nanoparticles studied by surface-enhanced Raman scattering. J. Colloid Interface Sci. 2016, 465, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Krishnan, V. Fabrication of highly sensitive biomimetic SERS substrates for detection of herbicides in trace concentration. Sens. Actuators B Chem. 2018, 262, 710–719. [Google Scholar] [CrossRef]
- Lin, J.; Cai, Y.; Wang, X.; Ding, B.; Yu, J.; Wang, M. Fabrication of biomimetic superhydrophobic surfaces inspired by lotus leaf and silver ragwort leaf. Nanoscale 2011, 3, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Bell, S.E.J.; McCourt, M.R. SERS enhancement by aggregated Au colloids: Effect of particle size. Phys. Chem. Chem. Phys. 2009, 11, 7455–7462. [Google Scholar] [CrossRef] [PubMed]
- Pucetaite, M.; Velicka, M.; Pilipavicius, J.; Beganskiene, A.; Ceponkus, J.; Sablinskas, V. Uric acid detection by means of SERS spectroscopy on dried Ag colloidal drops. J. Raman Spectrosc. 2016, 47, 681–686. [Google Scholar] [CrossRef]
- Wang, R.J.; Yao, Y.F.; Shen, M.; Wang, X.S. Green synthesis of Au@Ag nanostructures through a seed-mediated method and their application in SERS. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 492, 263–272. [Google Scholar] [CrossRef]
- Tang, X.L.; Jiang, P.; Ge, G.L.; Tsuji, M.; Xie, S.S.; Guo, Y.J. Poly(N-vinyl-2-pyrrolidone) (PVP)-capped dendritic gold nanoparticles by a one-step hydrothermal route and their high SERS effect. Langmuir 2008, 24, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Severyukhina, A.N.; Parakhonskiy, B.V.; Prikhozhdenko, E.S.; Gorin, D.A.; Sukhorukov, G.B.; Mohwald, H.; Yashchenok, A.M. Nanoplasmonic Chitosan Nanofibers as Effective SERS Substrate for Detection of Small Molecules. ACS Appl. Mater. Interfaces 2015, 7, 15466–15473. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.D.; Feng, S.Y.; Liu, X.Y.; Chen, L.H. Surface-Enhanced Raman Scattering Study of Silver Nanoparticles Prepared by Using MC as a Template. J. Nanomater. 2013, 8, 170. [Google Scholar] [CrossRef]
- Xiong, Z.Y.; Chen, X.W.; Liou, P.; Lin, M.S. Development of nanofibrillated cellulose coated with gold nanoparticles for measurement of melamine by SERS. Cellulose 2017, 24, 2801–2811. [Google Scholar] [CrossRef]
- Xiong, Z.Y.; Lin, M.S.; Lin, H.T.; Huang, M.Z. Facile synthesis of cellulose nanofiber nanocomposite as a SERS substrate for detection of thiram in juice. Carbohydr. Polym. 2018, 189, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.; Balakrishnan, A.; Sanosh, K.P.; Marsano, E. Hydroxy propyl cellulose capped silver nanoparticles produced by simple dialysis process. Mater. Res. Bull. 2010, 45, 989–992. [Google Scholar] [CrossRef]
- Lu, Y.D.; Wu, C.J.; Wu, Y.; You, R.Y.; Lin, G.; Chen, Y.Q.; Feng, S.Y. Ag-Coated Cellulose Fibers as Surface-Enhanced Raman Scattering Substrates for Adsorptive Detection of Malachite Green. Materials 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jiang, Q.; Liu, K.K.; Luan, J.; Naik, R.R.; Singamaneni, S. Bacterial Nanocellulose-Based Flexible Surface Enhanced Raman Scattering Substrate. Adv. Mater. Interfaces 2016, 3, 1600214. [Google Scholar] [CrossRef]
- Pang, X.; He, Y.; Jung, J.; Lin, Z. 1D nanocrystals with precisely controlled dimensions, compositions, and architectures. Science 2016, 353, 1268. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef]
- Zhao, J.; Lui, H.; McLean, D.I.; Zeng, H. Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Appl. Spectrosc. 2007, 61, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Tao, B.; Lei, Z.; Jean-Rene, E.M.; Sijun, L.; Nowinski, A.K.; Shaoyi, J.; Qiuming, Y. Sensitive and fast detection of fructose in complex media via symmetry breaking and signal amplification using surface-enhanced Raman spectroscopy. Anal. Chem. 2014, 86, 2387–2394. [Google Scholar]
- Furini, L.N.; Sanchez-Cortes, S.; Lopez-Tocon, I.; Otero, J.C.; Aroca, R.F.; Constantino, C.J.L. Detection and quantitative analysis of carbendazim herbicide on Ag nanoparticles via surface-enhanced Raman scattering. J. Raman Spectrosc. 2015, 46, 1095–1101. [Google Scholar] [CrossRef]
- Strickland, A.D.; Batt, C.A. Detection of Carbendazim by Surface-Enhanced Raman Scattering Using Cyclodextrin Inclusion Complexes on Gold Nanorods. Anal. Chem. 2009, 81, 2895–2903. [Google Scholar] [CrossRef] [PubMed]

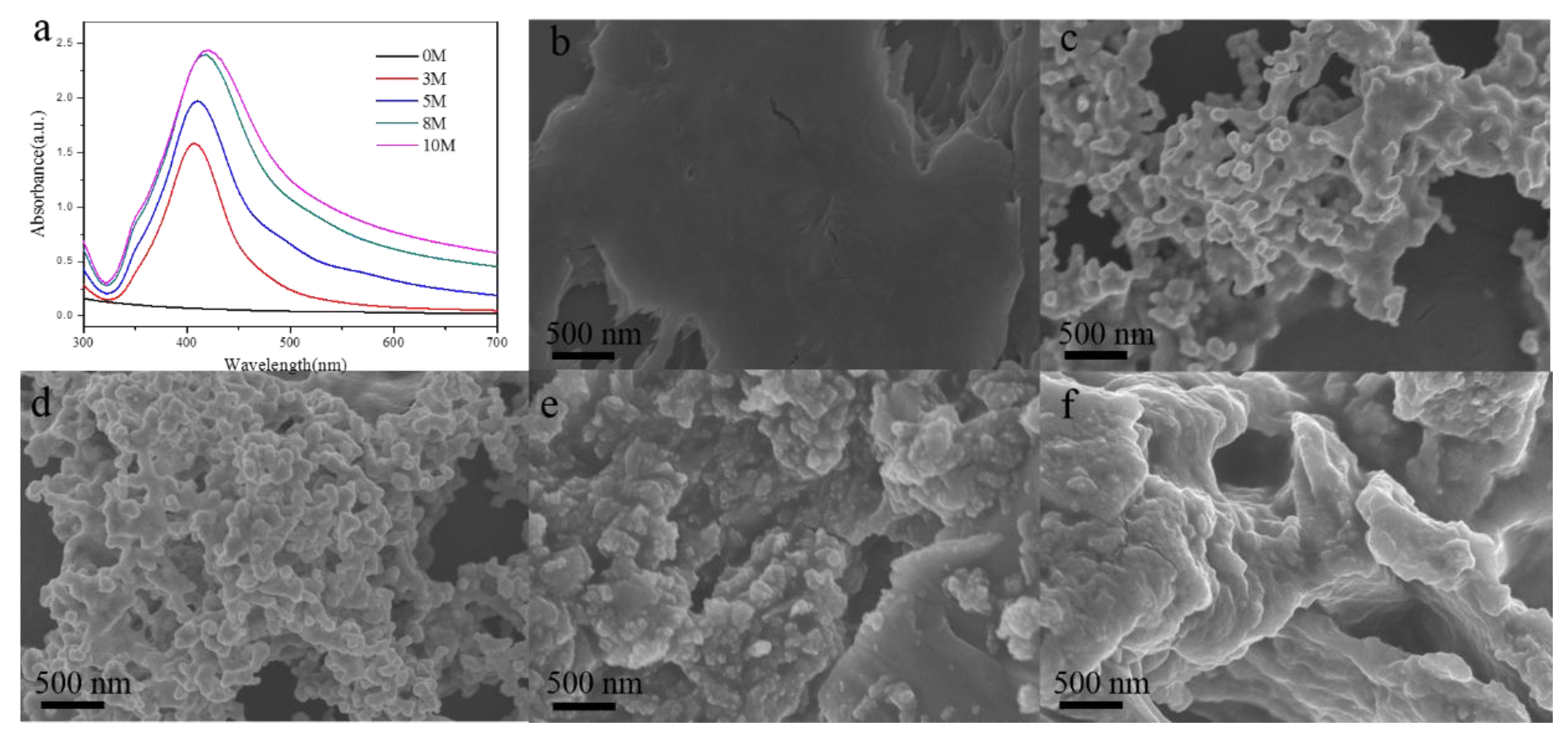
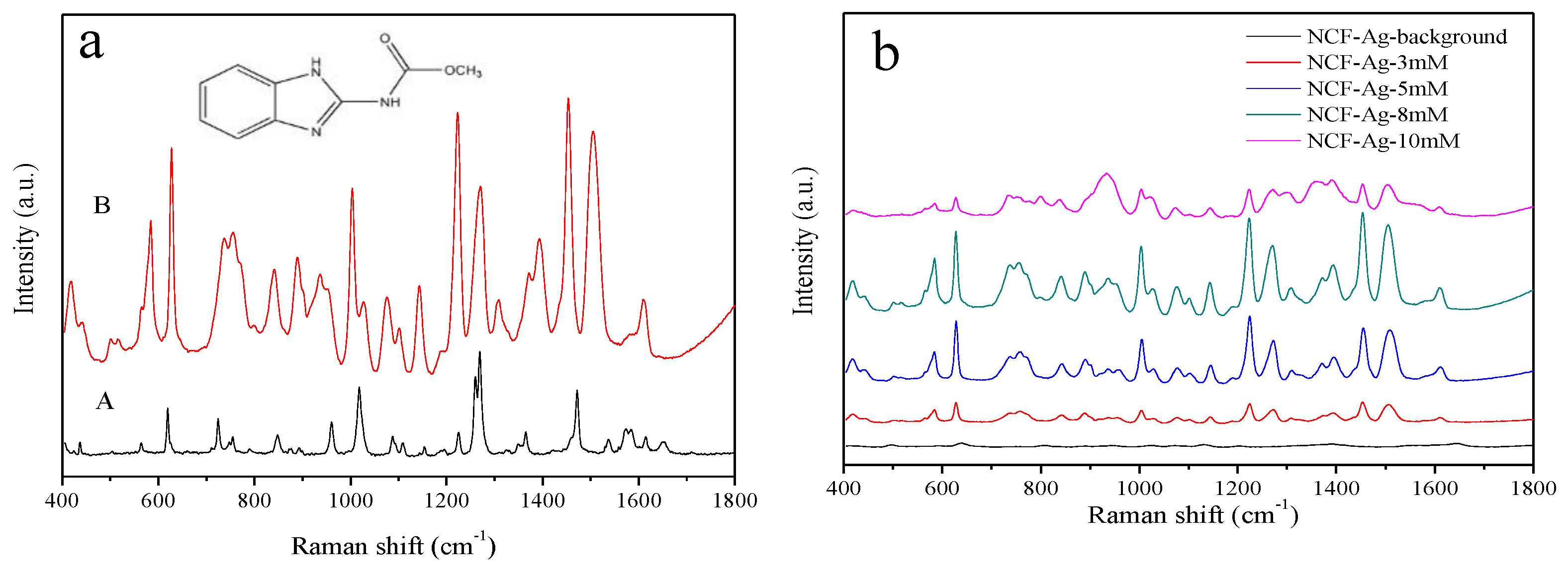
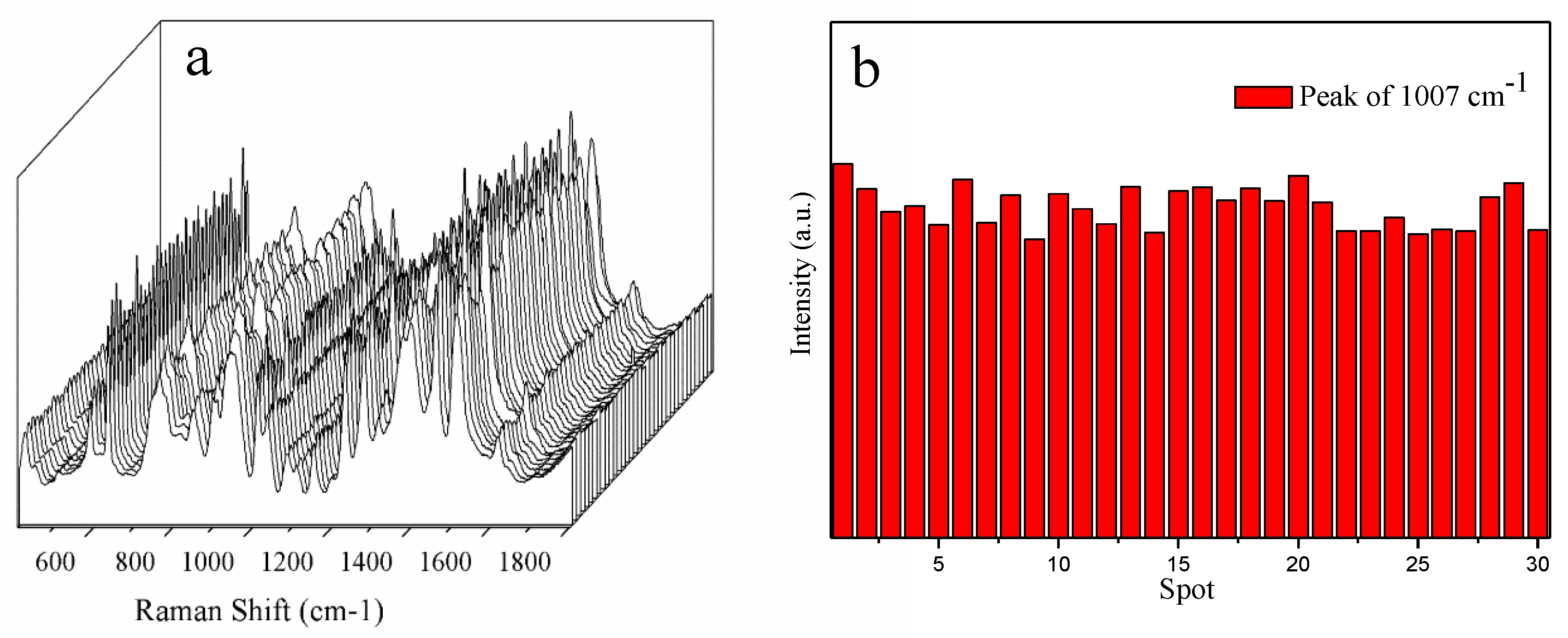
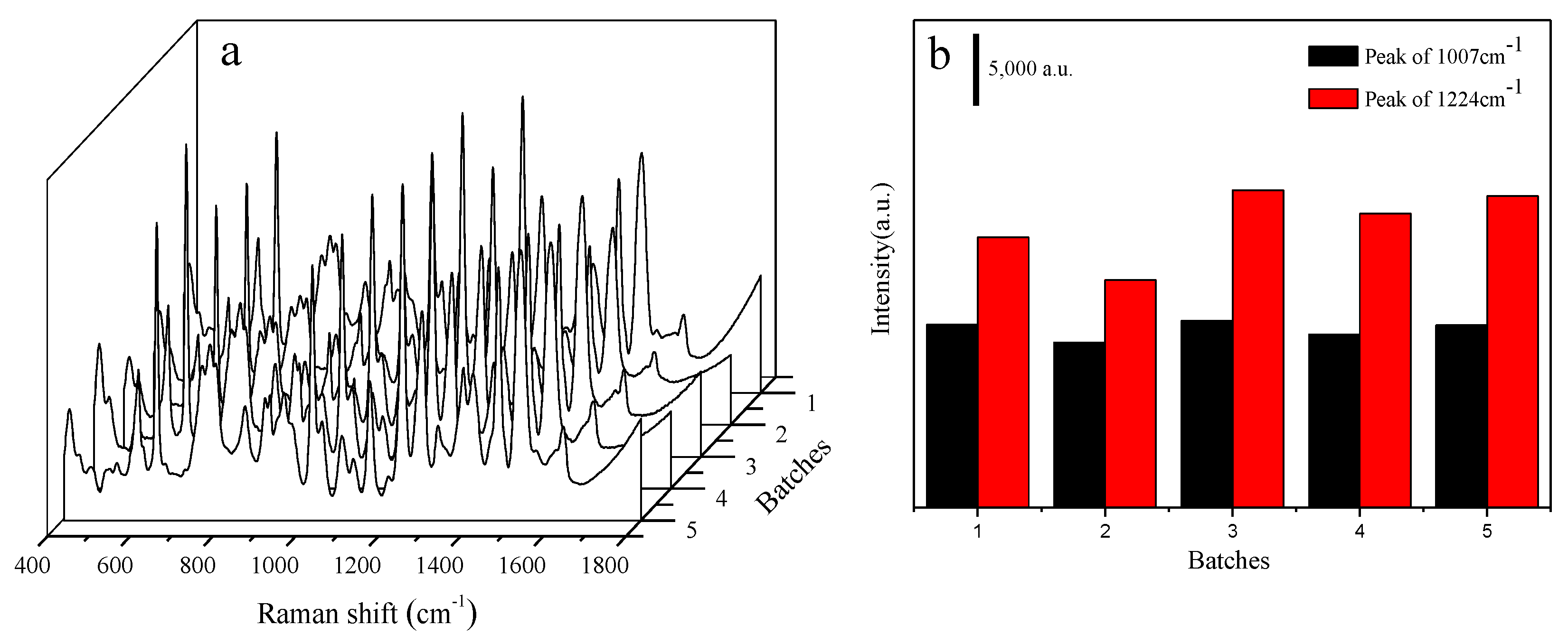
| Solid Raman | SERS | Vibrational Description |
|---|---|---|
| 617 cm−1 | 628 cm−1 | ring stretching and C–C bending |
| 723 cm−1 | 737 cm−1 | C–C bending and C–O–CH3 bending |
| 960 cm−1 | – | C–H bending |
| 1018 cm−1 | 1007 cm−1 | C–N bending and C– bending and C–O–CH3 stretching |
| 1260 cm−1 | 1224 cm−1 | C–C bending and N–H bending |
| 1270 cm−1 | 1270 cm−1 | C–H bending and N–H bending |
| 1473 cm−1 | 1460 cm−1 | C–H bending and N–H bending |
| – | 1510 cm−1 | N–H bending and C–N stretch |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Wu, C.; Xie, L.; Yuan, X.; Wei, X.; Huang, Q.; Chen, Y.; Lu, Y. Silver-Nanocellulose Composite Used as SERS Substrate for Detecting Carbendazim. Nanomaterials 2019, 9, 355. https://doi.org/10.3390/nano9030355
Huang L, Wu C, Xie L, Yuan X, Wei X, Huang Q, Chen Y, Lu Y. Silver-Nanocellulose Composite Used as SERS Substrate for Detecting Carbendazim. Nanomaterials. 2019; 9(3):355. https://doi.org/10.3390/nano9030355
Chicago/Turabian StyleHuang, Luqiang, Changji Wu, Lijuan Xie, Xue Yuan, Xinyu Wei, Qun Huang, Youqiang Chen, and Yudong Lu. 2019. "Silver-Nanocellulose Composite Used as SERS Substrate for Detecting Carbendazim" Nanomaterials 9, no. 3: 355. https://doi.org/10.3390/nano9030355
APA StyleHuang, L., Wu, C., Xie, L., Yuan, X., Wei, X., Huang, Q., Chen, Y., & Lu, Y. (2019). Silver-Nanocellulose Composite Used as SERS Substrate for Detecting Carbendazim. Nanomaterials, 9(3), 355. https://doi.org/10.3390/nano9030355





