Performance of Toluene Removal in a Nonthermal Plasma Catalysis System over Flake-Like HZSM-5 Zeolite with Tunable Pore Size and Evaluation of Its Byproducts
Abstract
1. Introduction
2. Experimental
2.1. Preparation
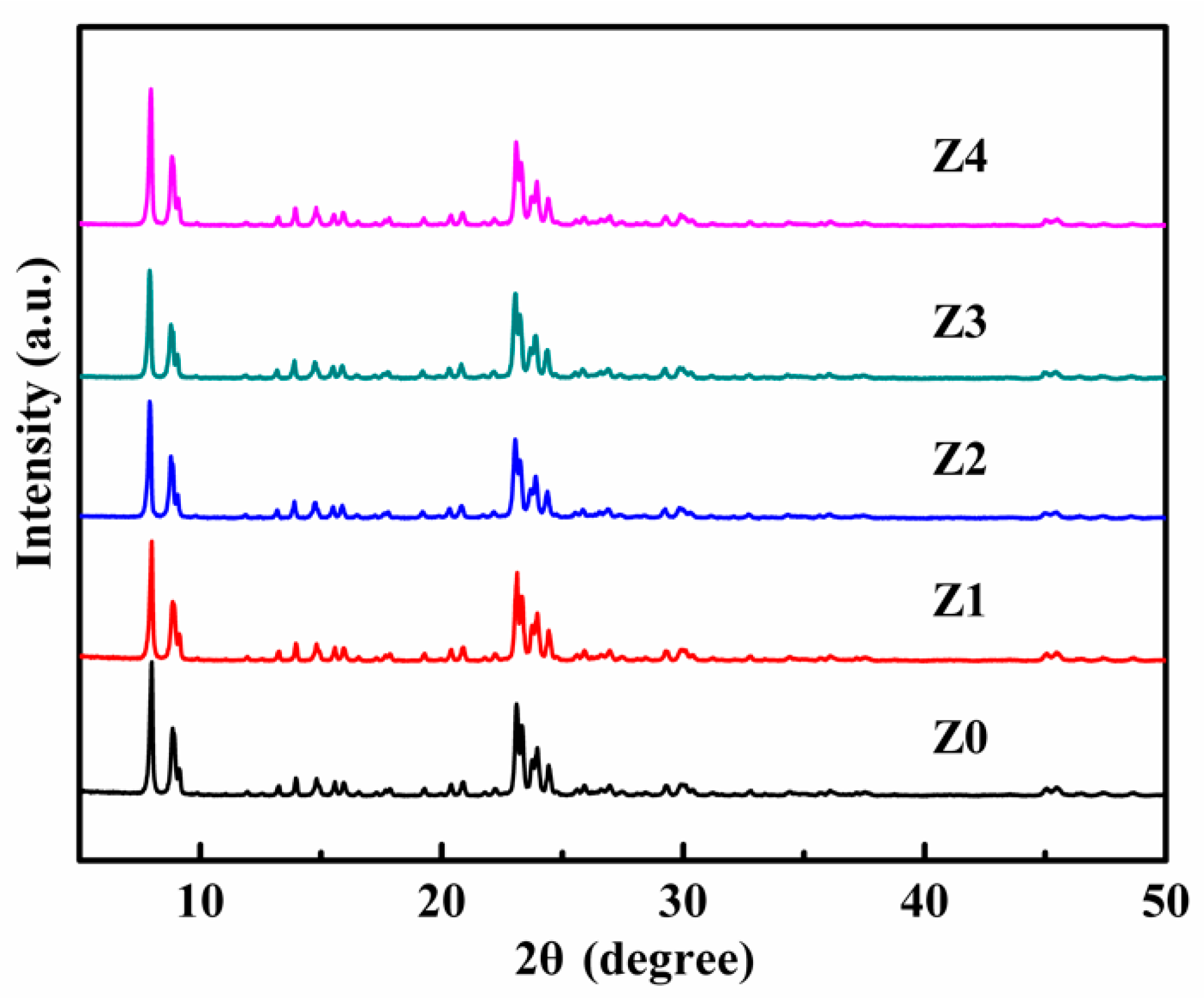
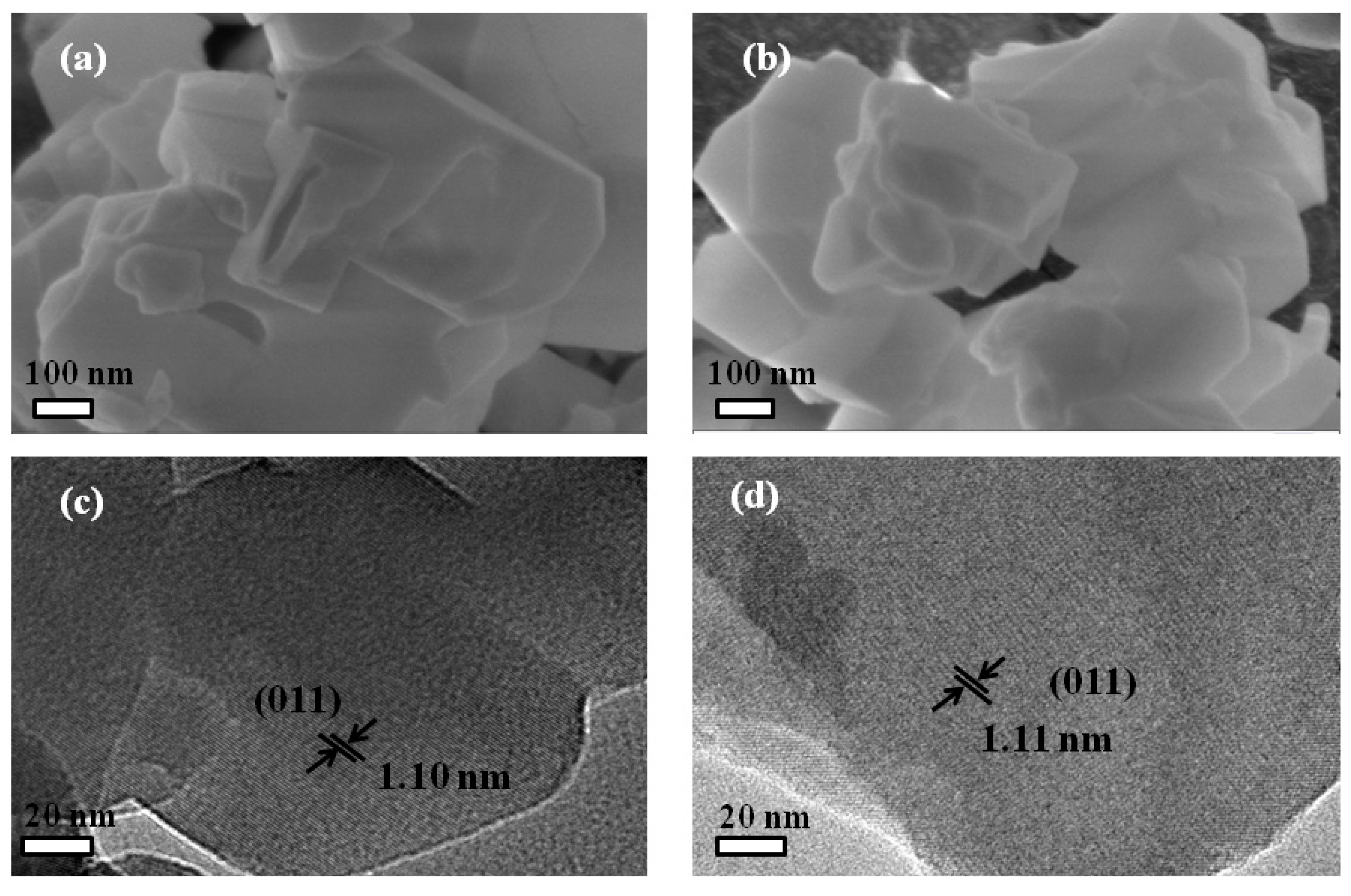
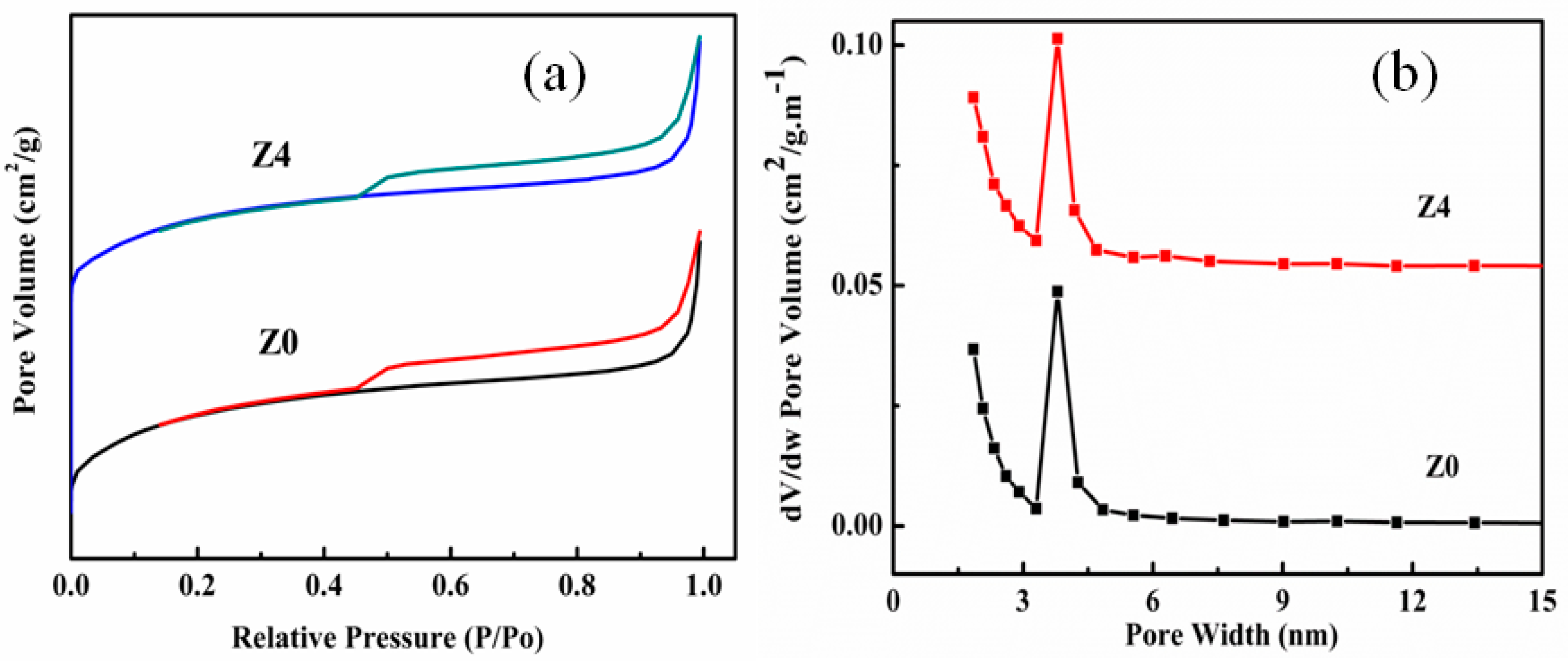
2.2. Characterization
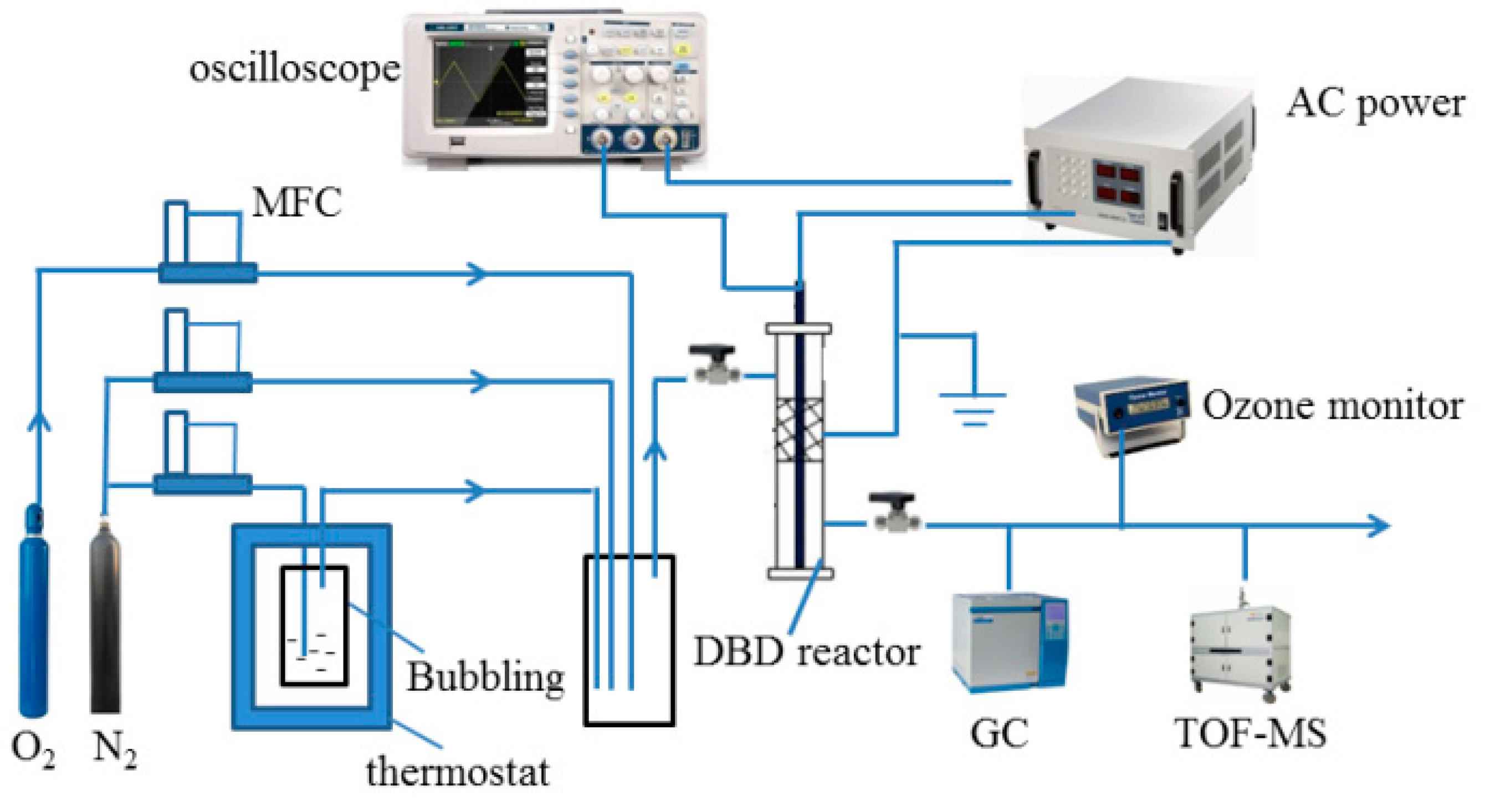
2.3. Experimental Setup
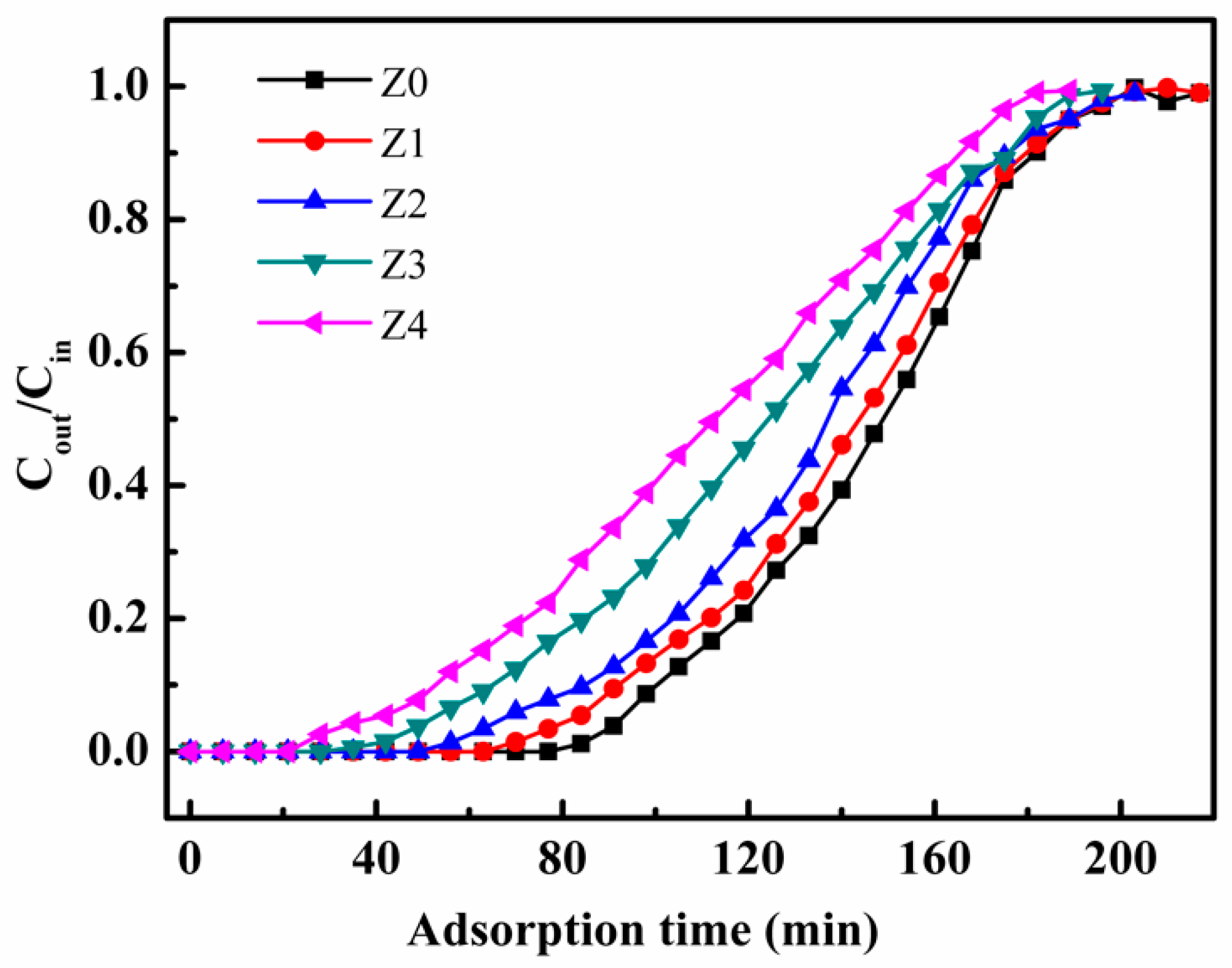
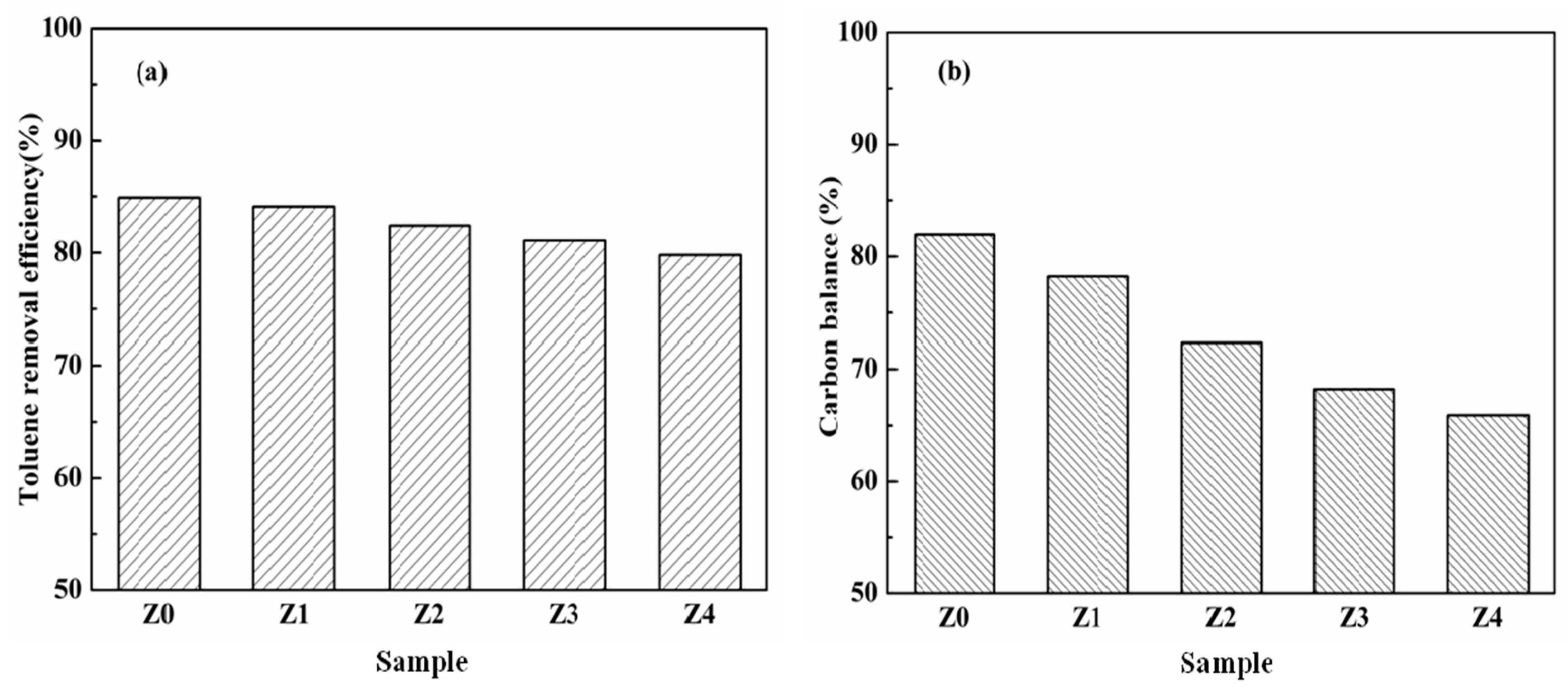
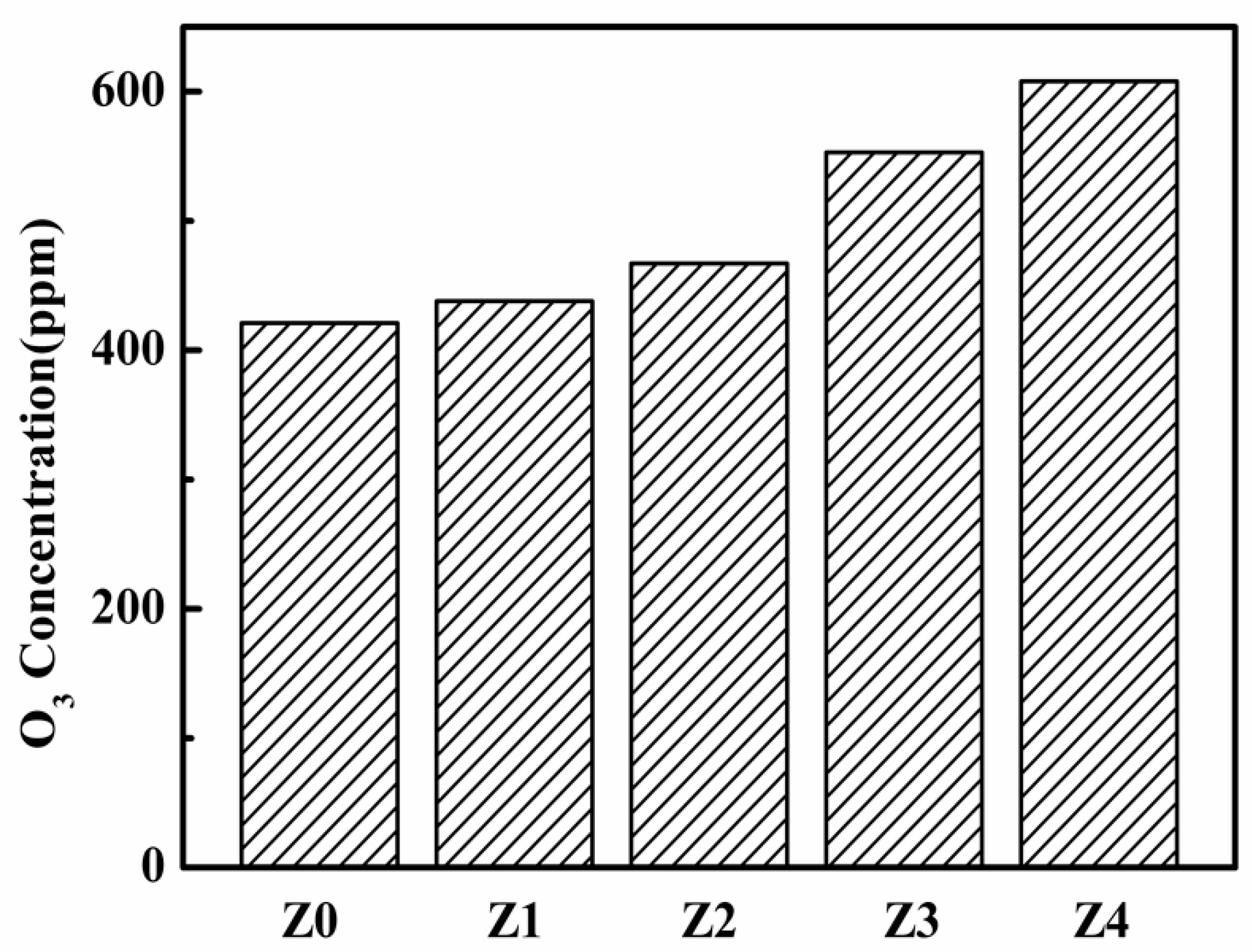
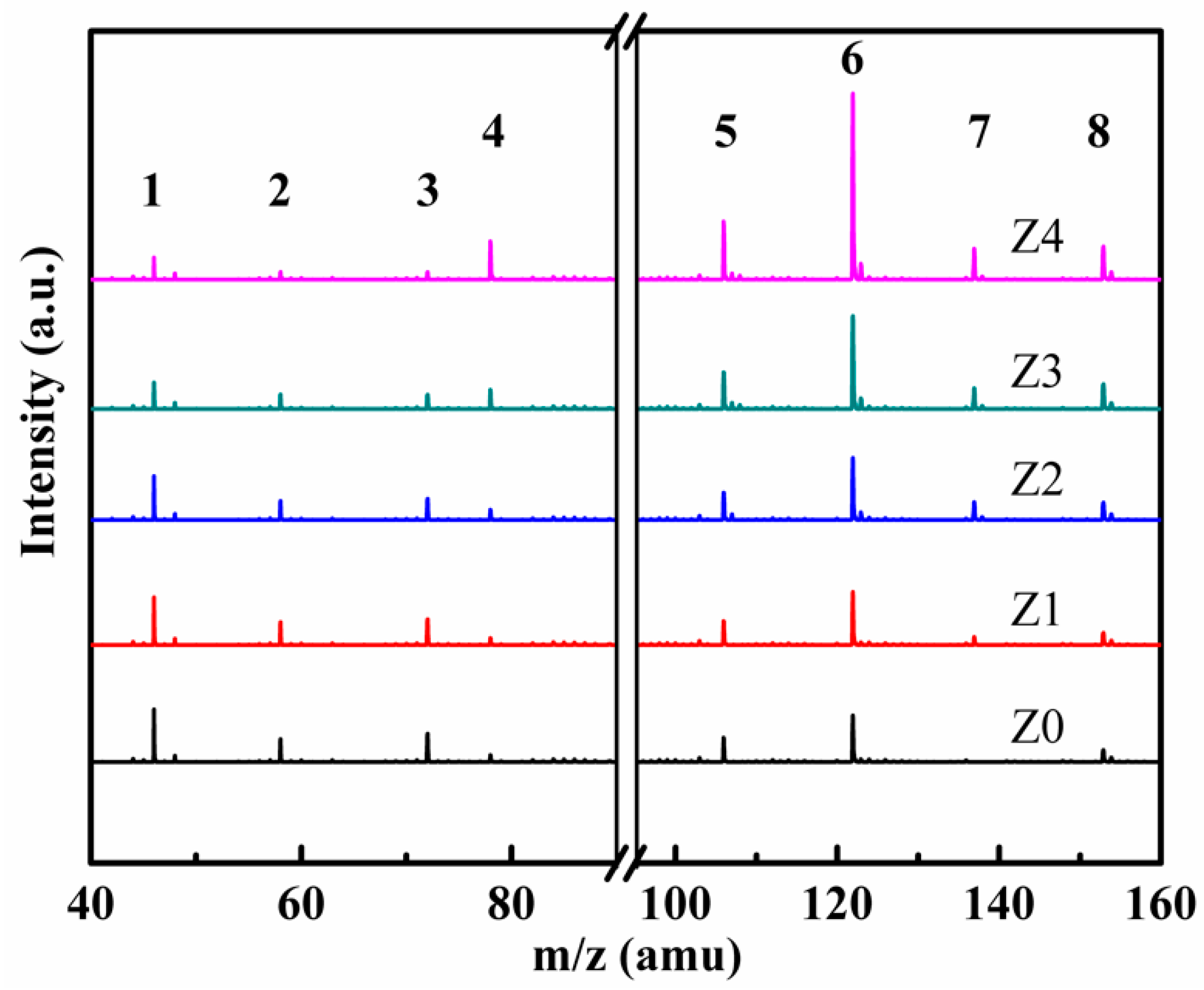
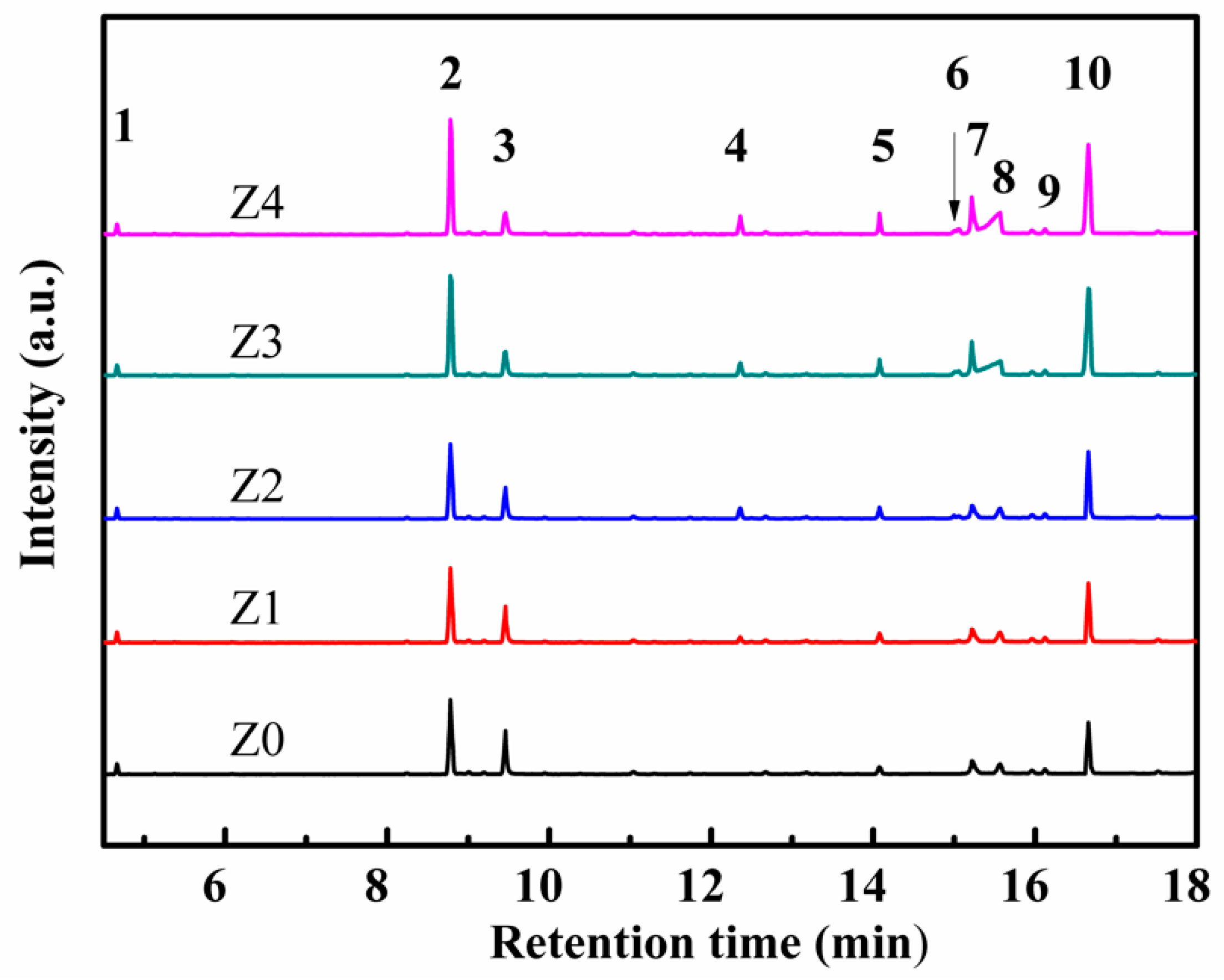
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abraham, M.H.; Gola, J.M.; Cometto-Muniz, J.E. An assessment of air quality reflecting the chemosensory irritation impact of mixtures of volatile organic compounds. Environ. Int. 2016, 86, 84–91. [Google Scholar] [CrossRef]
- Xu, N.; Fu, W.; He, C.; Cao, L.; Liu, X.; Zhao, J.; Pan, H. Benzene removal using nonthermal plasma with CuO/AC catalyst: reaction condition optimization and decomposition mechanism. Plasma Chem. Plasma Process. 2014, 34, 1387–1402. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Aunan, K.; Martin Seip, H.; Hao, J. Air pollution and lung cancer risks in China—A meta-analysis. Sci. Total Environ. 2006, 366, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lee, S.C.; Chan, L.Y.; Li, W.M. Risk assessment of exposure to volatile organic compounds in different indoor environments. Environ. Res. 2004, 94, 57–66. [Google Scholar] [CrossRef]
- Wang, Y.X.; Tao, H.; Yu, D.G.; Chang, C.T. Performance assessment of ordered porous electrospun honeycomb fibers for the removal of atmospheric polar volatile organic compounds. Nanomaterials 2018, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Zhou, H.L.; Zong, C.X.; Li, Y.X.; Jin, W.Q. Study on membrane performance in vapor permeation of VOC/N2 mixtures via modified constant volume/variable pressure method. Sep. Purif. Technol. 2018, 200, 273–283. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Huang, H.B.; Huang, H.L.; Feng, Q.Y.; Liu, G.Y.; Zhan, Y.J.; Wu, M.Y.; Lu, H.X.; Shu, Y.J. Catalytic oxidation of benzene over Mn modified TiO2/ZSM-5 under vacuum UV irradiation. Appl. Catal. B Environ. 2017, 203, 870–878. [Google Scholar] [CrossRef]
- Ren, Q.M.; Mo, S.P.; Peng, R.S.; Feng, Z.T.; Zhang, M.Y.; Cheng, L.M.; Fu, M.L.; Wu, J.L.; Ye, D.Q. Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A 2018, 6, 498–509. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Leys, C.; Van Langenhove, H. Combining non-thermal plasma with heterogeneous catalysis in waste gas treatment: A review. Appl. Catal. B Environ. 2008, 78, 324–333. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Soutrel, I.; Petit, P.; Medimagh, K.; Wolbert, D. A study of pollution removal in exhaust gases from animal quartering centers by combining photocatalysis with surface discharge plasma: From pilot to industrial scale. Chem. Eng. Process. 2017, 111, 1–6. [Google Scholar] [CrossRef]
- Liang, W.J.; Ma, L.; Liu, H.; Li, J. Toluene degradation by non-thermal plasma combined with a ferroelectric catalyst. Chemosphere 2013, 92, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Vandenbroucke, A.M.; Leys, C.; De Geyter, N.; Morent, R. Abatement of VOCs with alternate adsorption and plasma-assisted regeneration: A review. Catalysts 2015, 5, 718–746. [Google Scholar] [CrossRef]
- Fan, X.; Zhu, T.; Wang, M.; Li, X. Removal of low-concentration BTX in air using a combined plasma catalysis system. Chemosphere 2009, 75, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.C.; Xu, X.X.; Wu, J.L.; Fu, M.L.; Ye, D.Q. Removal of toluene in adsorption–discharge plasma systems over a nickel modified SBA-15 catalyst. RSC Adv. 2016, 6, 104104–104111. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.H.; Song, Y.H.; Choi, W.C.; Park, Y.K.; Kim, D.H. Synergistic effect of non-thermal plasma–catalysis hybrid system on methane complete oxidation over Pd-based catalysts. Chem. Eng. J. 2018, 347, 447–454. [Google Scholar]
- Zhu, X.B.; Gao, X.; Qin, R.; Zeng, Y.X.; Qu, R.Y.; Zheng, C.H.; Tu, X. Plasma-catalytic removal of formaldehyde over Cu–Ce catalysts in a dielectric barrier discharge reactor. Appl. Catal. B Environ. 2015, 170–171, 293–300. [Google Scholar] [CrossRef]
- Li, Y.Z.; Fan, Z.Y.; Shi, J.W.; Liu, Z.Y.; Zhou, J.W.; Shangguan, W.F. Modified manganese oxide octahedral molecular sieves M-OMS-2 (M = Co,Ce,Cu) as catalysts in post plasma-catalysis for acetaldehyde degradation. Catal. Today 2015, 256, 178–185. [Google Scholar] [CrossRef]
- NguyenDinh, M.T.; Giraudon, J.M.; Lamonier, J.F.; Vandenbroucke, A.; De Geyter, N.; Leys, C.; Morent, R. Plasma-catalysis of low TCE concentration in air using LaMnO3+δ as catalyst. Appl. Catal. B Environ. 2014, 147, 904–911. [Google Scholar]
- Xu, W.C.; Wang, N.; Chen, Y.D.; Chen, J.D.; Xu, X.X.; Yu, L.; Chen, L.M.; Wu, J.L.; Fu, M.L.; Zhu, A.M.; Ye, D.Q. In situ FT-IR study and evaluation of toluene abatement in different plasma catalytic systems over metal oxides loaded γ-Al2O3. Catal. Commun. 2016, 84, 61–66. [Google Scholar] [CrossRef]
- Qin, C.H.; Huang, X.M.; Zhao, J.J.; Huang, J.Y.; Kang, Z.L.; Dang, X.Q.J. Removal of toluene by sequential adsorption-plasma oxidation: Mixed support and catalyst deactivation. Hazard. Mater. 2017, 334, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hu, F.Y.; Peng, Y.; Li, K.Z.; Bai, S.P.; Li, J.H. Non-thermal plasma catalysis for chlorobenzene removal over CoMn/TiO2 and CeMn/TiO2: Synergistic effect of chemical catalysis and dielectric constant. Chem. Eng. J. 2018, 347, 447–454. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Sysmans, W.; Leys, C.; Van Langenhove, H. Abatement and degradation pathways of toluene in indoor air by positive corona discharge. Chemosphere 2007, 68, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Z.; Li, X.S.; Shi, C.; Fan, H.Y.; Zhu, A.M. Low-concentration formaldehyde removal from air using a cycled storage–discharge (CSD) plasma catalytic process. Chem. Eng. Sci. 2011, 66, 3922–3929. [Google Scholar] [CrossRef]
- Xu, X.X.; Wu, J.L.; Xu, W.C.; Ye, D.Q. High-efficiency non-thermal plasma-catalysis of cobalt incorporated mesoporous MCM-41 for toluene removal. Catal. Today 2017, 281, 527–533. [Google Scholar] [CrossRef]
- Huang, R.; Lu, M.J.; Wang, P.T.; Chen, Y.D.; Wu, J.L.; Fu, M.L.; Chen, L.M.; Ye, D.Q. Enhancement of the non-thermal plasma-catalytic system with different zeolites for toluene removal. RSC Adv. 2015, 5, 72113–72120. [Google Scholar] [CrossRef]
- Zuhairi, A.; Zailani, M.; Bhatia, S. React. Kinet. Comparative study of the deactivation of Cr-BEA, Cr-MOR and Cr-ZSM-5 in catalytic decomposition of VOC. Catal. Lett. 2003, 79, 143–148. [Google Scholar] [CrossRef]
- Xu, X.X.; Wang, P.T.; Xu, W.C.; Ye, D.Q. Plasma-catalysis of metal loaded SBA-15 for toluene removal: comparison of continuously introduced and adsorption-discharge plasma system. Chem. Eng. J. 2016, 283, 276–284. [Google Scholar] [CrossRef]
- Narayanan, S.; JudithVijaya, J.; Sivasanker, S.; John Kennedy, L.; Jesudoss, S.K. Structural, morphological and catalytic investigations on hierarchical ZSM-5 zeolite hexagonal cubes by surfactant assisted hydrothermal method. Powder Technol. 2015, 274, 338–348. [Google Scholar] [CrossRef]
- Zang, Y.H.; Dong, X.F.; Ping, D.; Geng, J.M.; Dang, H.F. Green routes for the synthesis of hierarchical HZSM-5 zeolites with low SiO2/Al2O3 ratios for enhanced catalytic performance. Catal. Commun. 2018, 113, 51–54. [Google Scholar] [CrossRef]
- Rasamimanana, S.; Mignard, S.; Gener, I.B. Hierarchical zeolites as adsorbents for mesosulfuron-methyl removal in aqueous phase. Mesopor. Mat. 2016, 226, 153–161. [Google Scholar] [CrossRef]
- Bosnick, K.; Ban, S.; Hiebert, W.; Shi, Z.; Huang, C.; Lister, R.; Mleczko, M. Organic vapor adsorption on in situ grown carbon nanotube films. Carbon 2011, 49, 3639–3644. [Google Scholar] [CrossRef]
- Haag, S.; Hanebuth, M.; Mabande, G.T.P.; Avhale, A.; Schwieger, W.; Dittmeyer, R. On the use of a catalytic H-ZSM-5 membrane for xylene isomerization. Micropor. Mesopor. Mat. 2006, 96, 168–176. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Uguina, M.A.; Valverde, J.L.; Serrano, D.P. Kinetics of Toluene Alkylation with Methanol over Mg-Modified ZSM-5. Ind. Eng. Chem. Res. 1993, 32, 2548–2554. [Google Scholar] [CrossRef]
- Oh, S.M.; Kim, H.H.; Ogata, A.; Einaga, H.; Futamura, S.; Park, D.W. Effect of zeolite in surface discharge plasma on the decomposition of toluene. Catal. Lett. 2005, 99, 101–104. [Google Scholar] [CrossRef]
- Li, J.T.; Lou, L.L.; Xu, C.L.; Liu, S.X. Synthesis, characterization of Al-rich ZSM-12 zeolite and their catalytic performance in liquid-phase tert-butylation of phenol. Catal. Commun. 2014, 50, 97–100. [Google Scholar] [CrossRef]
- Saoud, W.A.; Assadi, A.A.; Guiza, M.; Bouzaza, A.; Aboussaoud, W.; Ouederni, A.; Soutrel, I.; Wolbert, D.; Rtimi, S. Study of synergetic effect, catalytic poisoning and regeneration using dielectric barrier discharge and photocatalysis in a continuous reactor: Abatement of pollutants in air mixture system. Appl. Catal. B Environ. 2017, 213, 53–61. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Wolbert, D. Study of synergetic effect by surface discharge plasma/TiO2 combination for indoor air treatment: Sequential and continuous configurations at pilot scale. J. Photoch. Photobio. A 2015, 310, 148–154. [Google Scholar] [CrossRef]
- Li, J.; Na, H.B.; Zeng, X.L.; Zhu, T.L.; Liu, Z.M.I. In situ DRIFTS investigation for the oxidation of toluene by ozone over Mn/HZSM-5, Ag/HZSM-5 and Mn-Ag/HZSM-5 catalysts. Appl. Surf. Sci. 2014, 311, 690–696. [Google Scholar] [CrossRef]
- Zhang, Y.; Morton, J.J.L.; Sambrook, M.R.; Porfyrakis, K.; Ardavan, A.; Briggs, G.A.D. The effects of a pyrrolidine functional group on the magnetic properties of N@C60. Chem. Phys. Lett. 2006, 432, 523–527. [Google Scholar] [CrossRef]
- Huang, H.B.; Ye, D.Q.; Leung, D.Y.C.; Feng, F.; Guan, X. Byproducts and pathways of toluene destruction via plasma-catalysis. J. Mol. Catal. A Chem. 2011, 336, 87–93. [Google Scholar] [CrossRef]
| Sample | HZSM-5 | Cyclohexane | TEOS |
|---|---|---|---|
| Z0 | pure HZSM-5 | 0 | 0 |
| Z1 | 1g | 10 mL | 0.2 mL |
| Z2 | 1g | 10 mL | 0.8 mL |
| Z3 | 1g | 10 mL | 1.6 mL |
| Z4 | 1g | 10 mL | 2.4 mL |
| Sample | SBET (m2/g) | Smic (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Dmic (nm) |
|---|---|---|---|---|---|
| Z0 | 366 | 330 | 0.180 | 0.145 | 0.533 |
| Z1 | 360 | 328 | 0.179 | 0.145 | 0.531 |
| Z2 | 354 | 325 | 0.178 | 0.146 | 0.526 |
| Z3 | 349 | 318 | 0.174 | 0.141 | 0.523 |
| Z4 | 341 | 313 | 0.175 | 0.140 | 0.522 |
| Sample | Equilibrium Adsorption Capacity (mg/g) | ||
|---|---|---|---|
| Toluene | p-xylene | m-xylene | |
| Z0 | 39.70 | 34.23 | 10.58 |
| Z1 | 38.92 | 34.15 | 9.17 |
| Z2 | 36.53 | 34.02 | 6.96 |
| Z3 | 33.31 | 31.42 | 3.84 |
| Z4 | 30.32 | 30.17 | 1.10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Lin, K.; Ye, D.; Jiang, X.; Liu, J.; Chen, Y. Performance of Toluene Removal in a Nonthermal Plasma Catalysis System over Flake-Like HZSM-5 Zeolite with Tunable Pore Size and Evaluation of Its Byproducts. Nanomaterials 2019, 9, 290. https://doi.org/10.3390/nano9020290
Xu W, Lin K, Ye D, Jiang X, Liu J, Chen Y. Performance of Toluene Removal in a Nonthermal Plasma Catalysis System over Flake-Like HZSM-5 Zeolite with Tunable Pore Size and Evaluation of Its Byproducts. Nanomaterials. 2019; 9(2):290. https://doi.org/10.3390/nano9020290
Chicago/Turabian StyleXu, Weicheng, Kaichun Lin, Daiqi Ye, Xueding Jiang, Junxing Liu, and Yangda Chen. 2019. "Performance of Toluene Removal in a Nonthermal Plasma Catalysis System over Flake-Like HZSM-5 Zeolite with Tunable Pore Size and Evaluation of Its Byproducts" Nanomaterials 9, no. 2: 290. https://doi.org/10.3390/nano9020290
APA StyleXu, W., Lin, K., Ye, D., Jiang, X., Liu, J., & Chen, Y. (2019). Performance of Toluene Removal in a Nonthermal Plasma Catalysis System over Flake-Like HZSM-5 Zeolite with Tunable Pore Size and Evaluation of Its Byproducts. Nanomaterials, 9(2), 290. https://doi.org/10.3390/nano9020290






