Investigation of the Surface of Ga–Sn–Zn Eutectic Alloy by the Characterisation of Oxide Nanofilms Obtained by the Touch-Printing Method
Abstract
:1. Introduction
2. Experimental
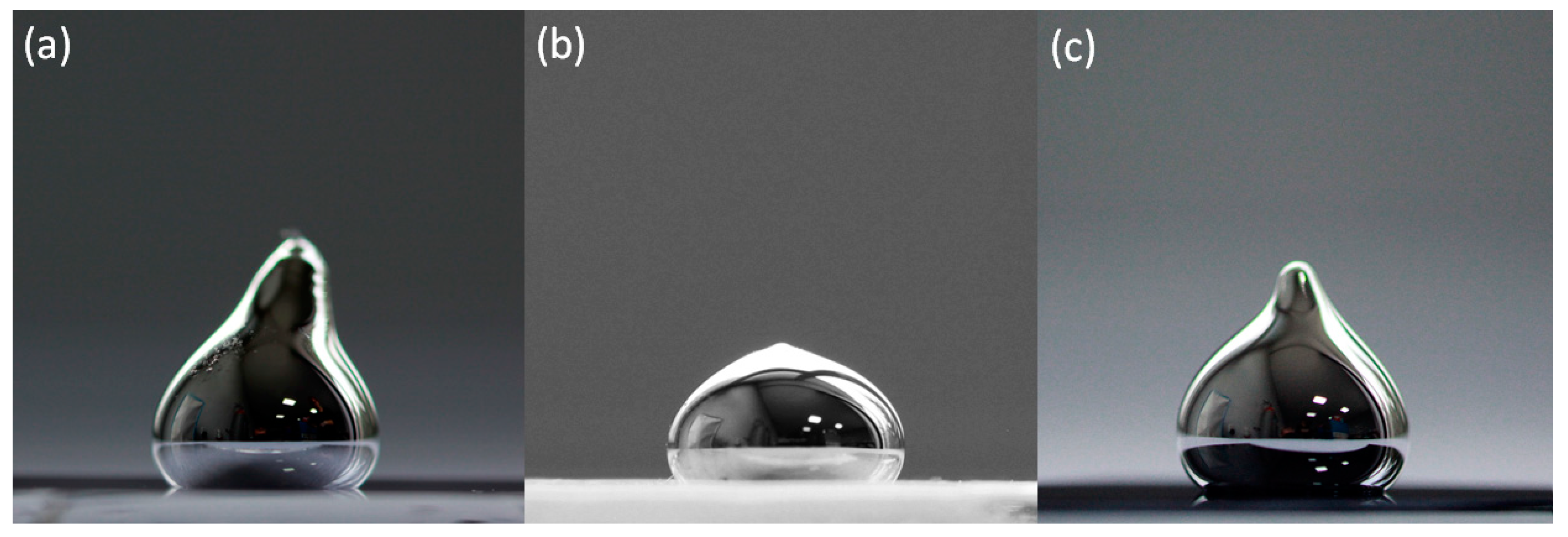
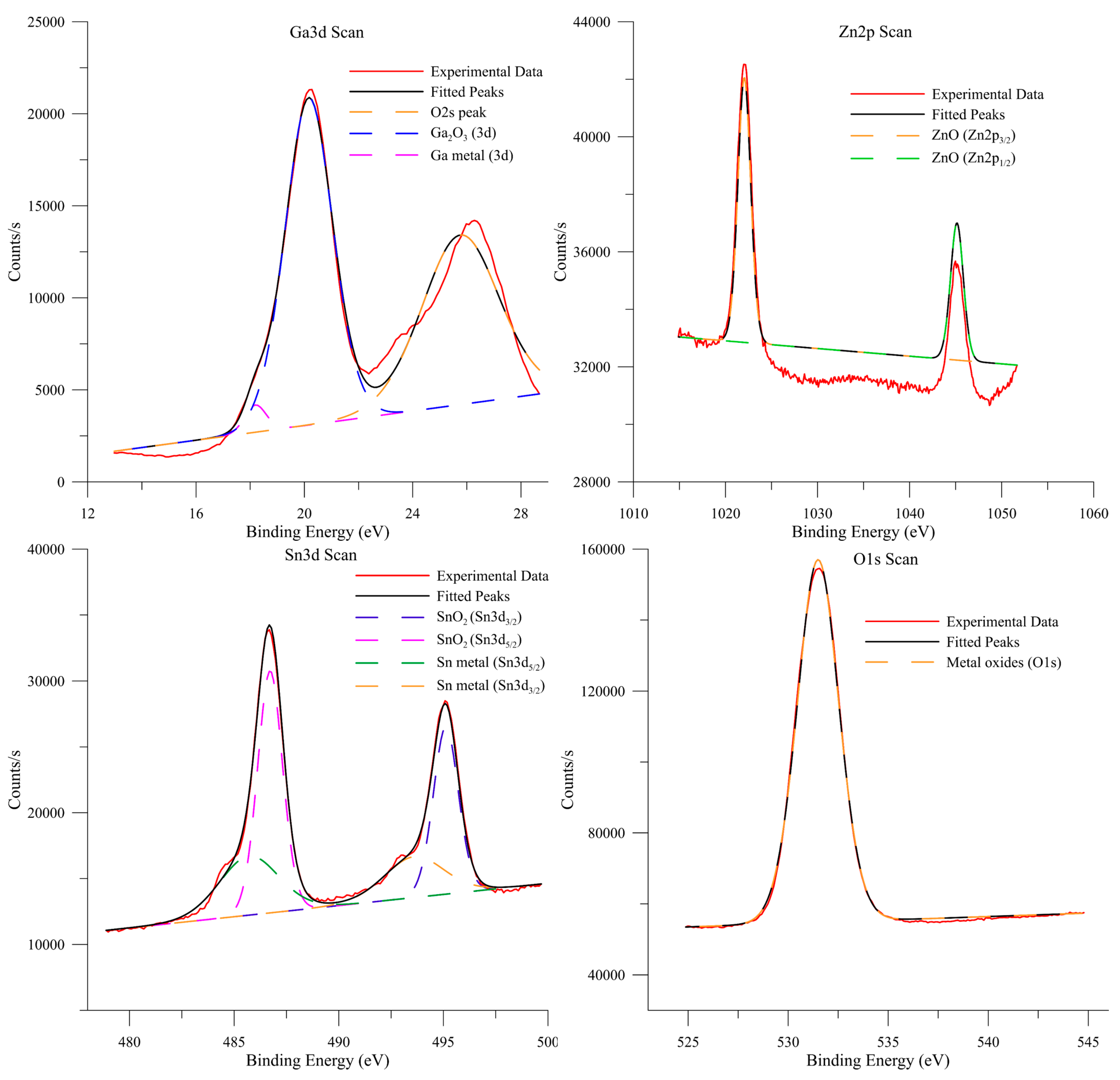
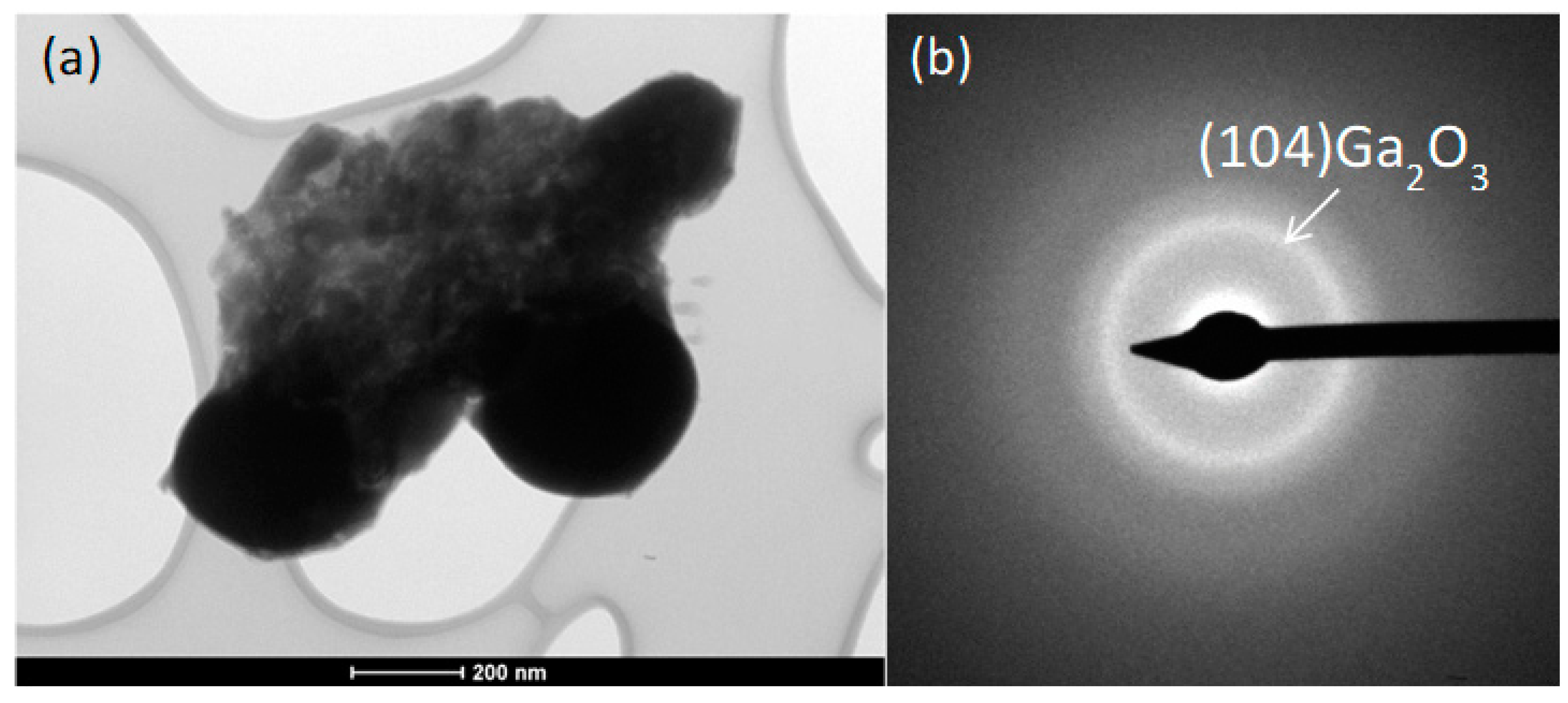
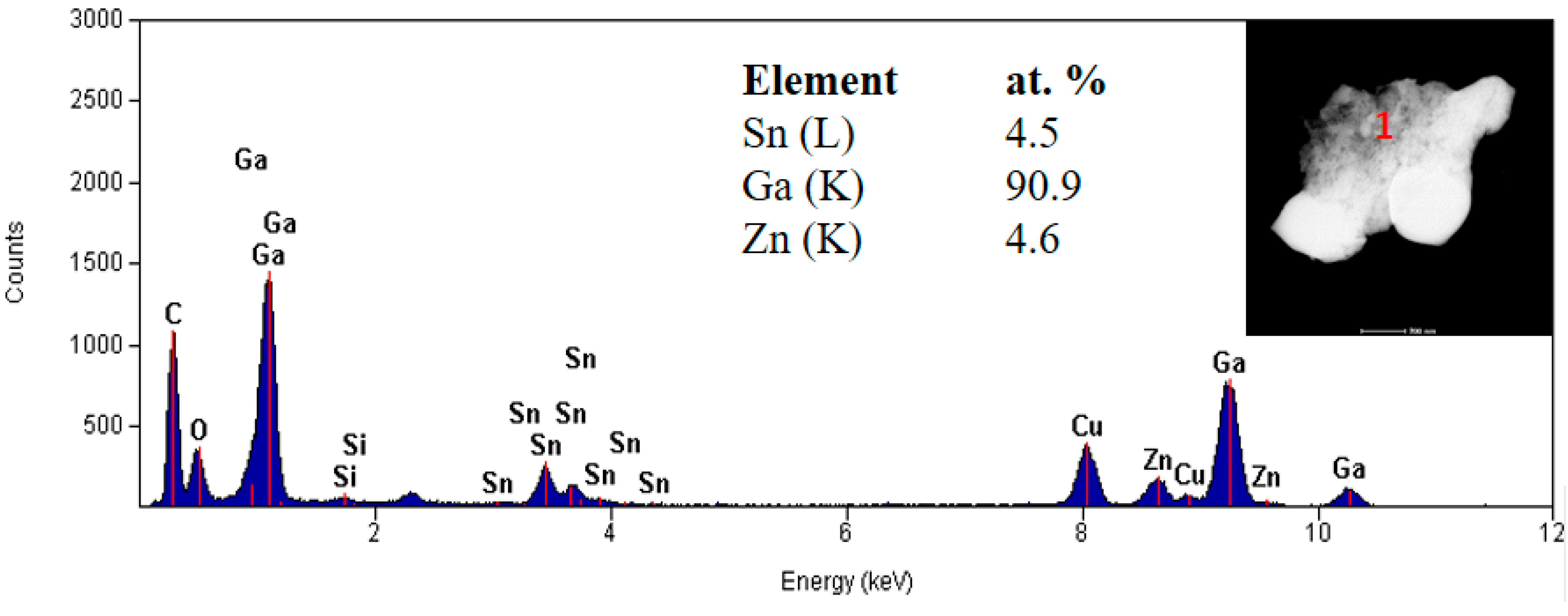
3. Results and discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lorenzin, N.; Abanades, A. A review on the application of liquid metals as heat transfer fluid in Concentrated Solar Power technologies. Int. J. Hydrog. Energy 2016, 41, 6990–6995. [Google Scholar] [CrossRef]
- Yue, L.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar]
- Kyle, A.M.; Leahy-Glass, N.; Combs, W.; March, K.L. Potential of gallium-based leads for cardiac rhythm management devices. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 341–344. [Google Scholar]
- Irshad, W.; Peroulis, D. A silicon-based galinstan magnetohydrodynamic pump. In Proceedings of the 9th International Workshop on Micro and Nanotechnology for Power Generation and Energy Conversion Applications, Washington DC, USA, 1–4 December 2009; pp. 127–129. [Google Scholar]
- Hodes, M.; Zhang, R.; Lam, L.S.; Wilcoxon, R.; Lower, N. On the potential of galinstan-based minichannel and minigap cooling. IEEE Trans. Compon. Packag. Manuf. Technol. 2014, 4, 46–56. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Tang, S.Y.; Khoshmanesh, K.; Ghorbani, K. An integrated liquid cooling system based on galinstan liquid metal droplets. ACS Appl. Mater. Interfaces 2016, 8, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Gancarz, T. Thermophysical properties of the liquid Ga–Sn–Zn eutectic alloy. Fluid Phase Equilib. 2016, 465, 1–9. [Google Scholar] [CrossRef]
- Fowler, B.A.; Sexton, M.J. Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 787–795. [Google Scholar]
- Ladd, C.; So, J.H.; Muth, J.; Dickey, M.D. 3D printing of free-standing liquid metal microstructures. Adv. Mater. 2013, 25, 5081–5085. [Google Scholar] [CrossRef] [PubMed]
- Boley, J.W.; White, E.L.; Chiu, G.T.C.; Kramer, R.K. Direct writing of gallium-indium alloy for stretchable electronics. Adv. Funct. Mater. 2014, 24, 3501–3507. [Google Scholar] [CrossRef]
- Dickey, M.D. Emerging applications of liquid metals featuring surface oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.J.; Tostmann, H.; Pershan, P.S.; Magnussen, O.M.; DiMasi, E.; Ocko, B.M.; Deutsch, M. X-ray study of the oxidation of liquid-gallium surfaces. Phys. Rev. B 1997, 55, 10786–10790. [Google Scholar] [CrossRef]
- Chabala, J.M. Oxide-growth kinetics and fractal-like patterning across liquid gallium surfaces. Phys. Rev. B 1992, 46, 11346–11357. [Google Scholar] [CrossRef]
- Anderson, T.J.; Ansara, I. The Ga-Sn (gallium-tin) system. J. Fluid Phase Equilib. 1992, 13, 181–189. [Google Scholar] [CrossRef]
- Dutkiewicz, J.; Moser, Z.; Zabdyr, L.; Gohil, D.D.; Chart, T.G.; Ansara, I.; Girard, C. The Ga-Zn (gallium-zinc) system. Bull. Alloy Phase Diagr. 1990, 11, 77–82. [Google Scholar] [CrossRef]
- Moser, Z.; Dutkiewicz, J.; Gąsior, W.; Salawa, J. The Sn− Zn (Tin-Zinc) system. Bull. Alloy Phase Diagr. 1985, 6, 330–334. [Google Scholar] [CrossRef]
- Dobosz, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Gancarz, T. The application of liquid metals in cooling systems: A study of the thermophysical properties of eutectic Ga-Sn-Zn with Al additions. Int. J. Heat Mass Transf. 2018, 126, 414–420. [Google Scholar] [CrossRef]
- Dobosz, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Tkach, O.; Gancarz, T. The thermophysical properties of eutectic Ga-Sn-Zn with In additions. J. Mol. Liq. 2018, 271, 942–948. [Google Scholar] [CrossRef]
- Zavabeti, A.; Ou, J.Z.; Carey, B.J.; Syed, N.; Orrell-Trigg, R.; Mayes, E.L.; Xu, C.; Kavehei, O.; O’mullane, A.P.; Kaner, R.B.; Kalantar-Zadeh, K. A liquid metal reaction environment for the room-temperature synthesis of atomically thin metal oxides. Science 2017, 358, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Sberveglieri, G. Recent developments in semiconducting thin-film gas sensors. Sens. Actuators B Chem. 1995, 23, 103–109. [Google Scholar] [CrossRef]
- Davis, E.; Ndao, S. On the Wetting States of Low Melting Point Metal Galinstan® on Silicon Microstructured Surfaces. Adv. Eng. Mater. 2018, 20, 1700829. [Google Scholar] [CrossRef]
- Liu, T.; Sen, P.; Kim, C.J. Characterization of nontoxic liquid-metal alloy galinstan for applications in microdevices. J. Microelectromech. Syst. 2012, 21, 443–450. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s handbook of chemistry, 15th ed.; McGraw-Hill, Inc.: New York, NY, USA, 1999; pp. 6.1–6.158. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobosz, A.; Daeneke, T.; Zavabeti, A.; Zhang, B.Y.; Orrell-Trigg, R.; Kalantar-Zadeh, K.; Wójcik, A.; Maziarz, W.; Gancarz, T. Investigation of the Surface of Ga–Sn–Zn Eutectic Alloy by the Characterisation of Oxide Nanofilms Obtained by the Touch-Printing Method. Nanomaterials 2019, 9, 235. https://doi.org/10.3390/nano9020235
Dobosz A, Daeneke T, Zavabeti A, Zhang BY, Orrell-Trigg R, Kalantar-Zadeh K, Wójcik A, Maziarz W, Gancarz T. Investigation of the Surface of Ga–Sn–Zn Eutectic Alloy by the Characterisation of Oxide Nanofilms Obtained by the Touch-Printing Method. Nanomaterials. 2019; 9(2):235. https://doi.org/10.3390/nano9020235
Chicago/Turabian StyleDobosz, Alexandra, Torben Daeneke, Ali Zavabeti, Bao Yue Zhang, Rebecca Orrell-Trigg, Kourosh Kalantar-Zadeh, Anna Wójcik, Wojciech Maziarz, and Tomasz Gancarz. 2019. "Investigation of the Surface of Ga–Sn–Zn Eutectic Alloy by the Characterisation of Oxide Nanofilms Obtained by the Touch-Printing Method" Nanomaterials 9, no. 2: 235. https://doi.org/10.3390/nano9020235
APA StyleDobosz, A., Daeneke, T., Zavabeti, A., Zhang, B. Y., Orrell-Trigg, R., Kalantar-Zadeh, K., Wójcik, A., Maziarz, W., & Gancarz, T. (2019). Investigation of the Surface of Ga–Sn–Zn Eutectic Alloy by the Characterisation of Oxide Nanofilms Obtained by the Touch-Printing Method. Nanomaterials, 9(2), 235. https://doi.org/10.3390/nano9020235



