Micro- and Nanostructured Polyaniline for Instant Identification of Metal Ions in Solution
Abstract
1. Introduction
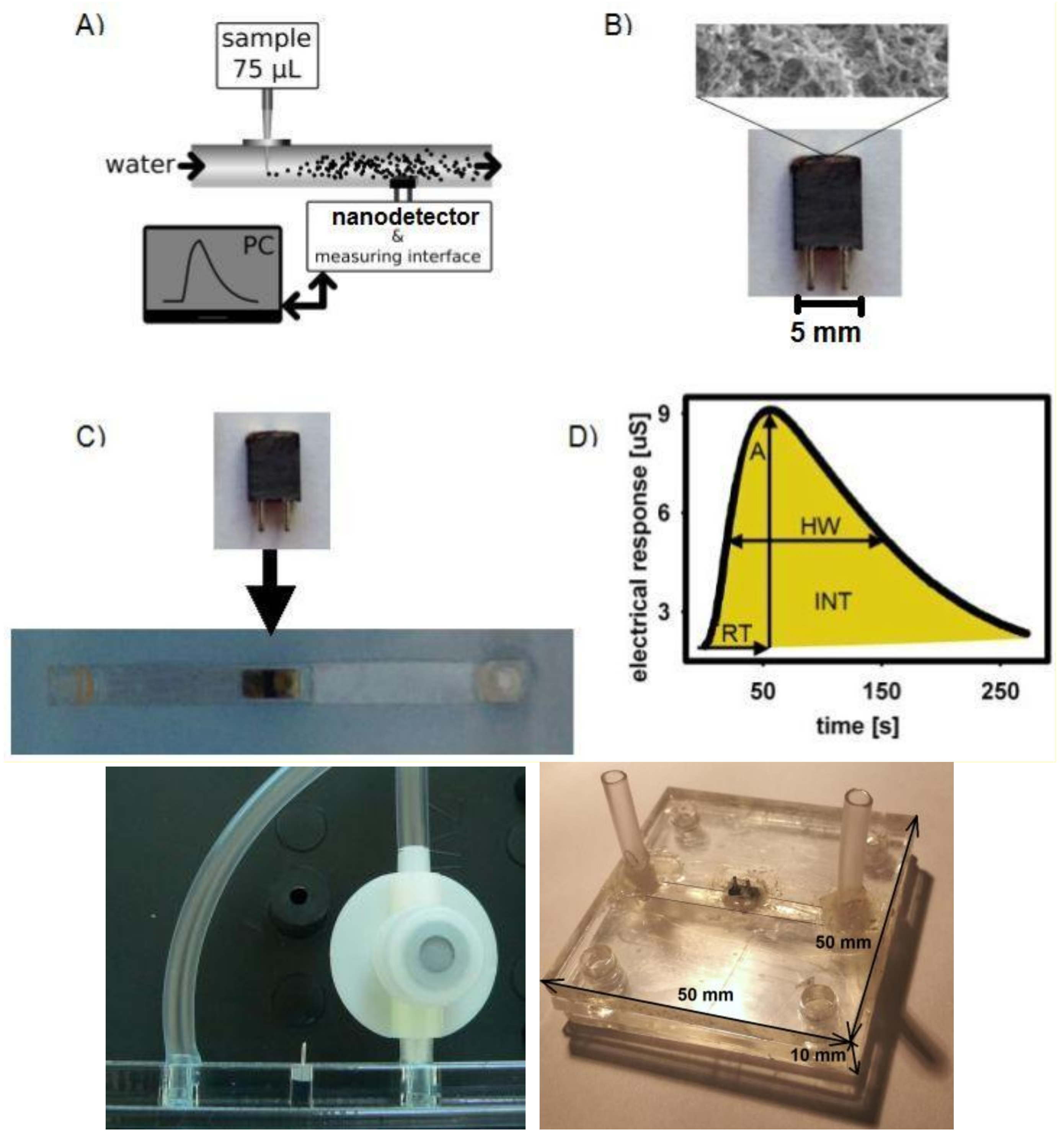
2. Materials and Methods
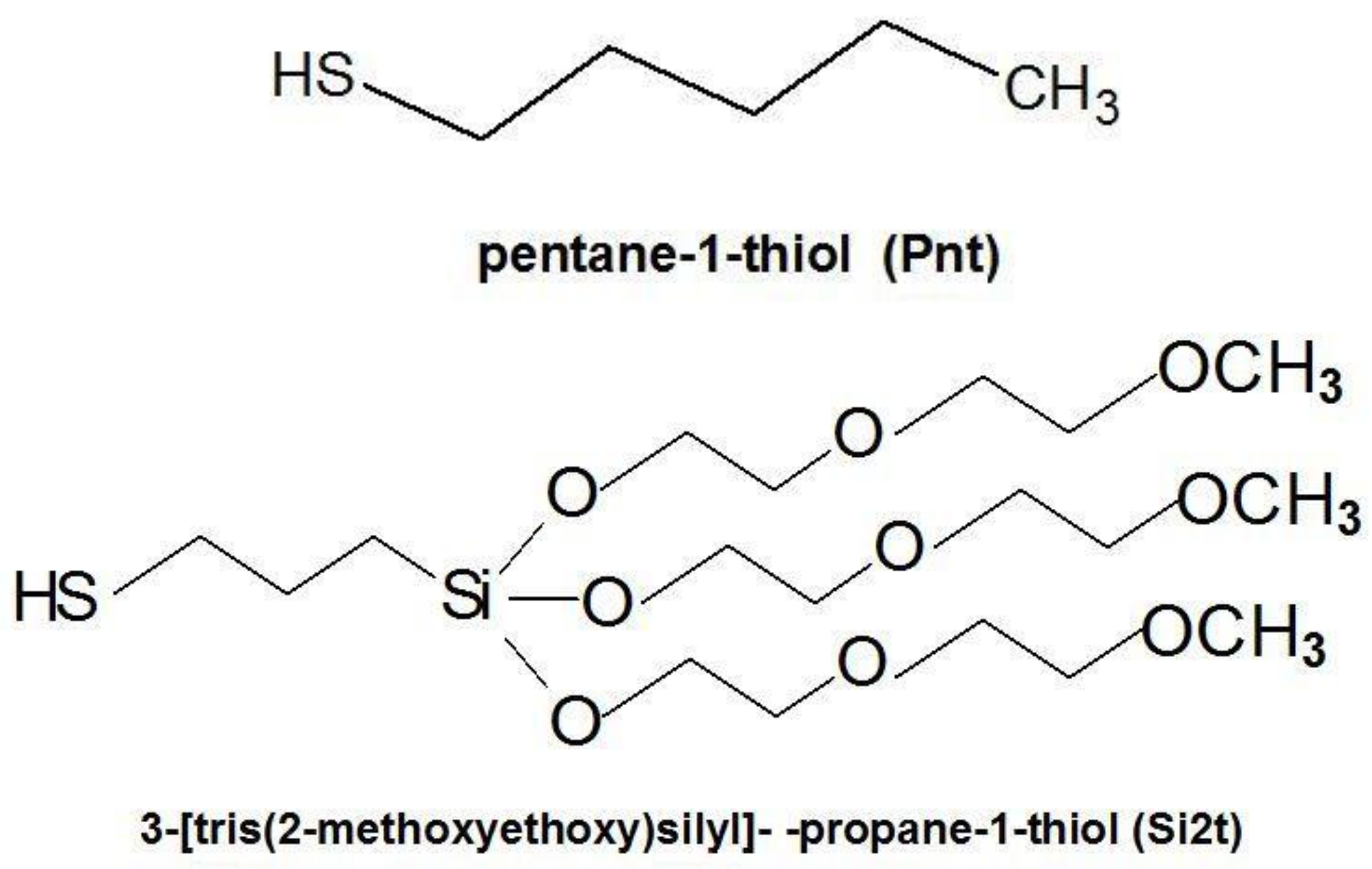
2.1. Materials
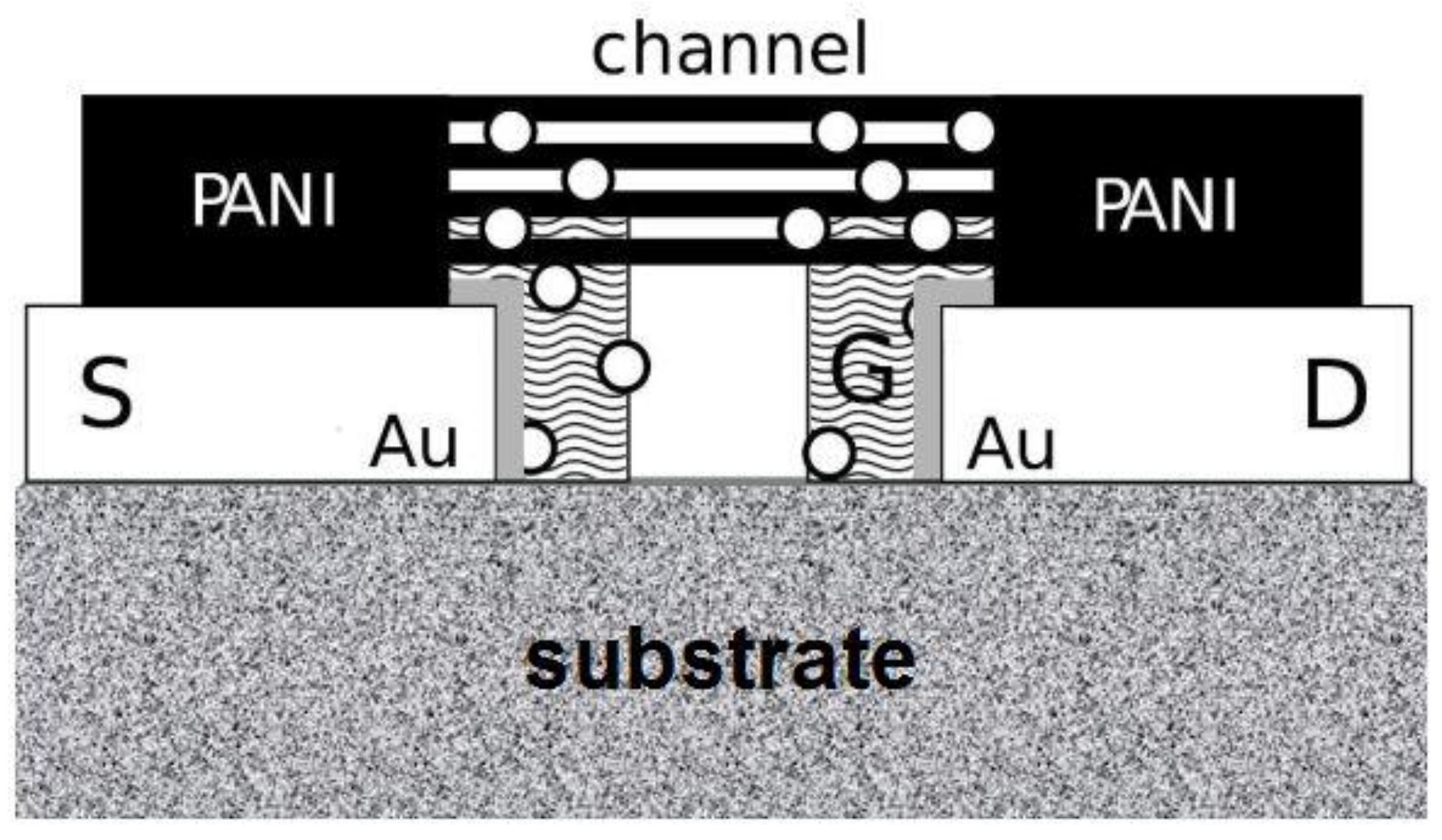
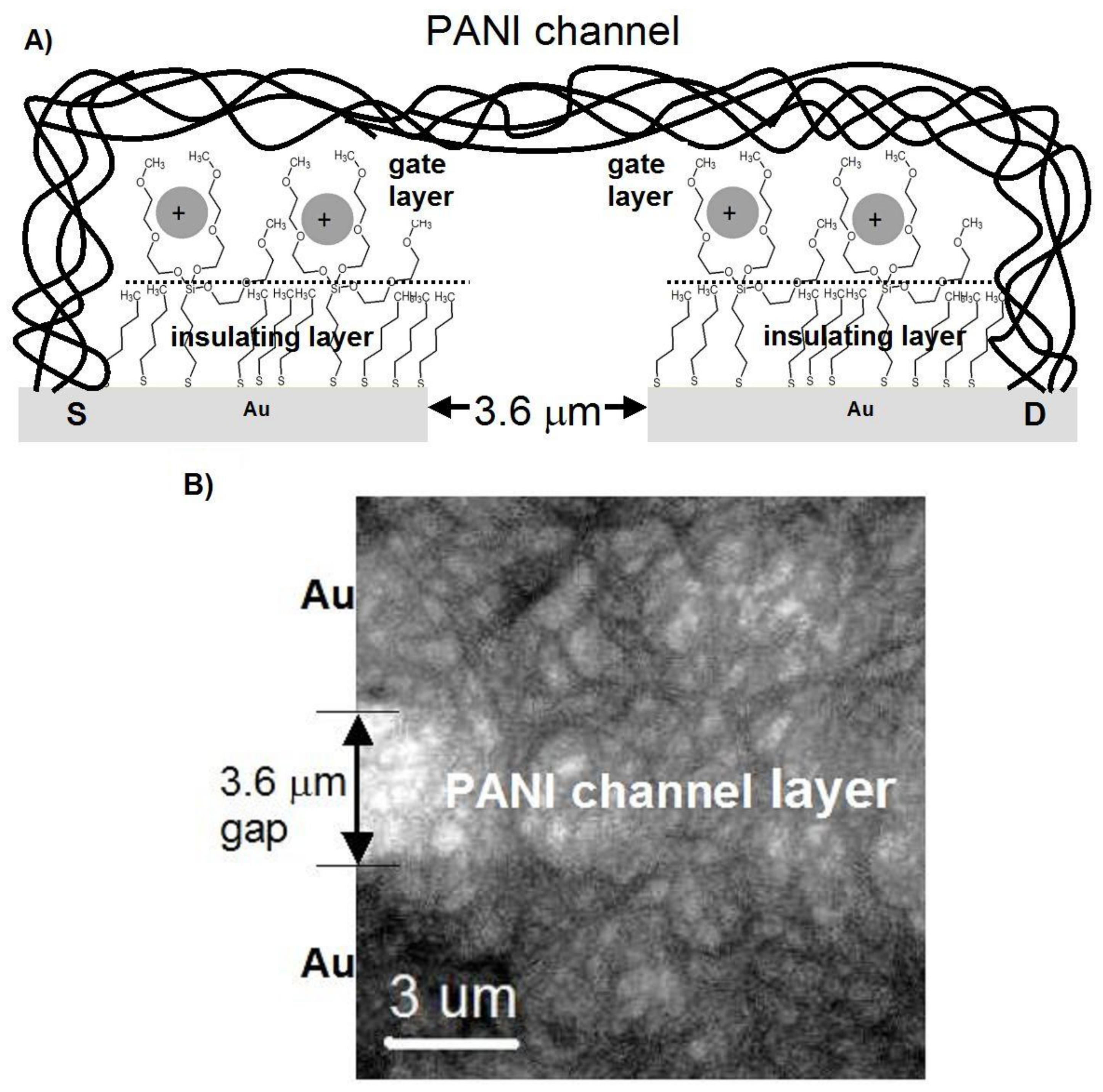
2.2. Preparation
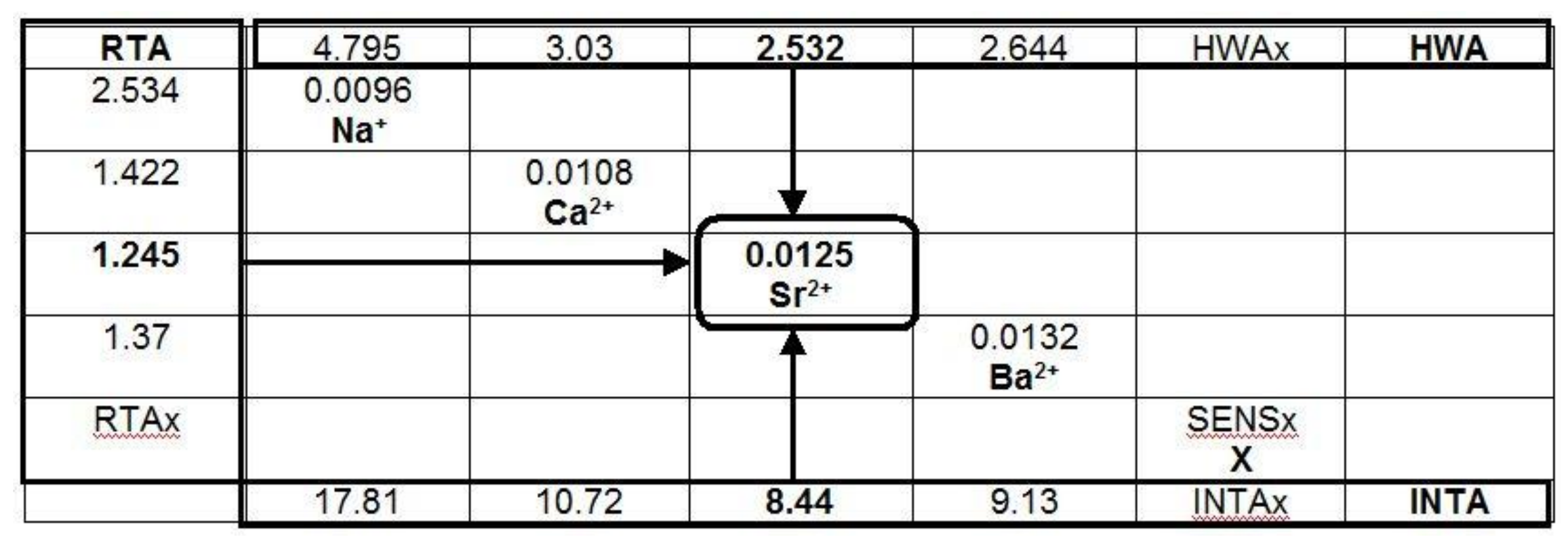
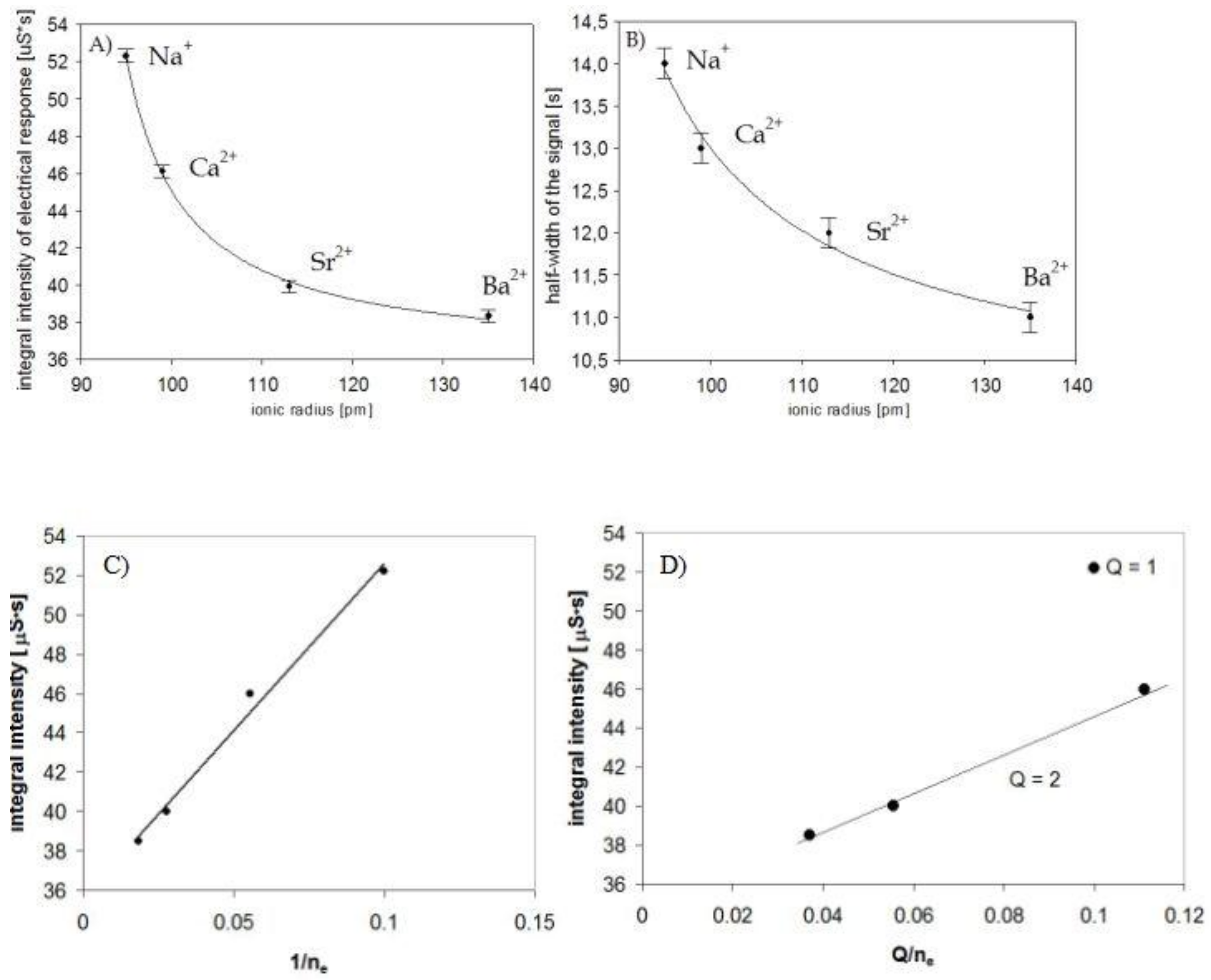
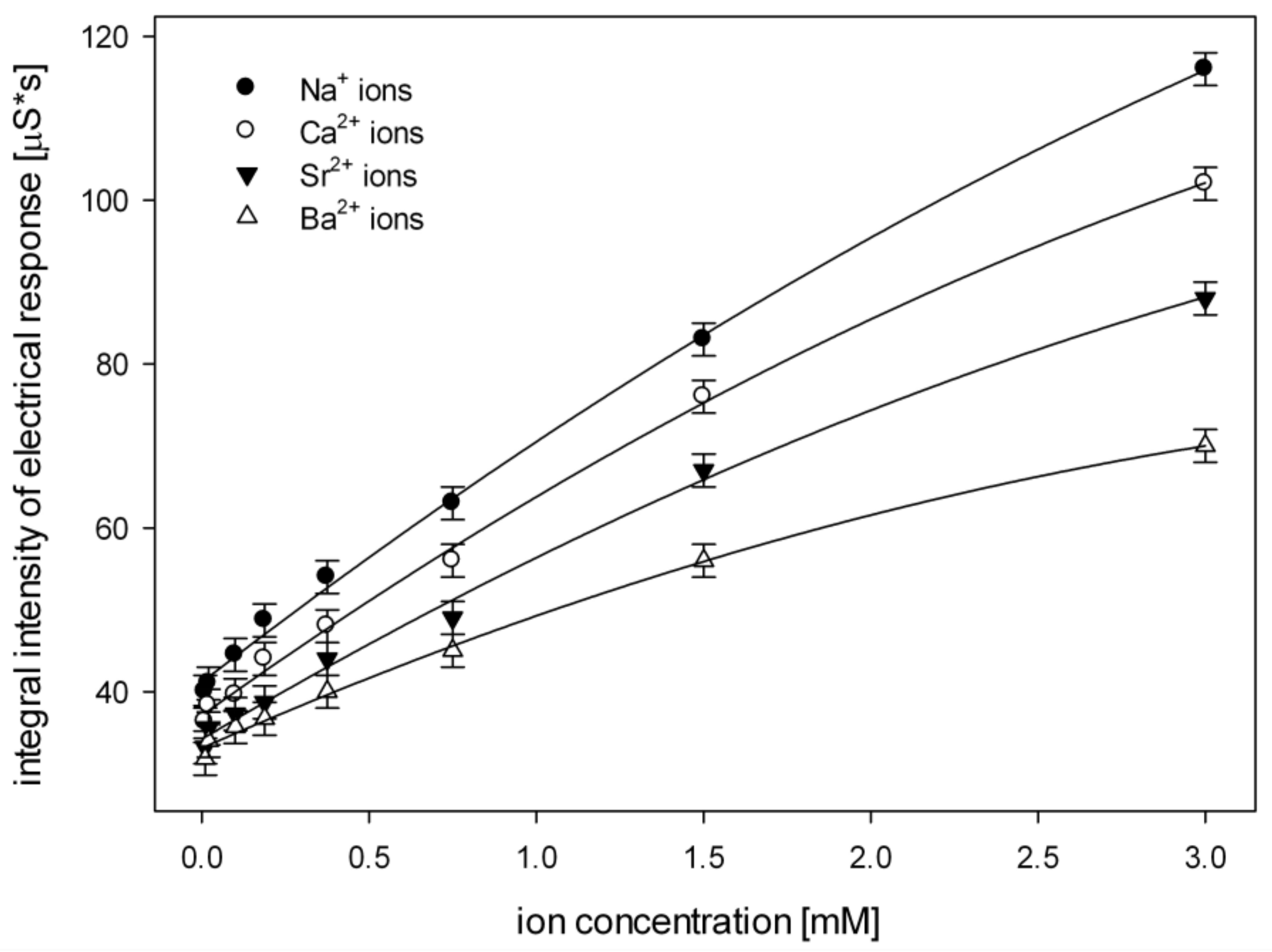
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


Appendix B

References
- Vaseashtaa, A.; Dimova-Malinovska, D. Nanostructured and nanoscale devices, sensors and detectors. Sci. Technol. Adv. Mater. 2005, 6, 312–318. [Google Scholar] [CrossRef]
- Stoal, J.T.; Trogler, W.C. Polymer sensors for nitroaromatic explosives detection. J. Mater. Chem. 2006, 16, 2871–2883. [Google Scholar]
- Shin, J.H.; Schoenfisch, M.H. Improving the Biocompatibility of in vivo Sensors via Nitric Oxide Release. Analyst 2006, 131, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Blazej, R.G.; Kumaresan, P.; Mathies, R.A. Microfabricated bioprocessor for integrated nanoliter-scale Sanger DNA sequencing. Proc. Natl. Acad. Sci. USA 2006, 103, 7240–7245. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.; Barczynski, P.; Baksalary, K.; Filipiak, M.; Golczak, S.; Langer, J.J. A fast and sensitive continuous flow nanobiodetector based on polyaniline nanofibrils. Microchim. Acta 2007, 159, 201–206. [Google Scholar] [CrossRef]
- Langer, J.J.; Langer, K. Polyaniline Nanobiodetector. Rev. Adv. Mater. Sci. 2005, 5, 434–436. [Google Scholar]
- Radke, S.M.; Alocilja, E.C. A high density microelectrode array biosensor for detection of E. coli O157:H7. Biosens. Bioelectron. 2005, 20, 1662–1667. [Google Scholar] [CrossRef]
- Cullum, B.; Griffin, G.D.; Miller, G.H.; Vo-Dinh, T. Intracellular Measurements in Mammary Carcinoma Cells Using Fiber-Optic Nanosensors. Anal. Biochem. 2000, 277, 25–32. [Google Scholar] [CrossRef]
- Kasili, P.M.; Cullum, B.M.; Griffin, G.D.; Vo-Dinh, T. Nanosensors for in vivo measurement of the cardinogen benzo(a)pyrene in a single cell. J. Nanosci. Nanotechnol. 2002, 2, 653–658. [Google Scholar] [CrossRef]
- Hafeman, D.G.; Parce, J.W.; McConnell, H.M. Light-addressable potentiometric sensor for biochemical systems. Science 1988, 240, 1182–1185. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Franklin, N.R.; Zhou, C.W.; Chapline, M.G.; Peng, S.; Cho, K.J.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Shi Kam, N.W.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Appl. Phys. Lett. 2003, 100, 4984–4989. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. An improved optical pH sensor based on polyaniline. Sens. Actuat. B-Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Riul, A.; Gallardo Soto, A.M.; Mello, S.V.; Bone, S.; Taylor, D.M.; Mattoso, L.H.C. An electronic tongue using polypyrrole and polyaniline. Synth. Met. 2003, 132, 109–116. [Google Scholar] [CrossRef]
- Adhikari, B.; Majumdar, S. Polymers in sensor applications. Prog. Polym. Sci. 2004, 29, 699–766. [Google Scholar] [CrossRef]
- Langer, J.J.; Filipiak, M.; Kęcińska, J.; Jasnowska, J.; Włodarczak, J.; Miładowski, B. Polyaniline biosensor for choline determination. Surf. Sci. 2004, 573, 140–145. [Google Scholar] [CrossRef]
- Mazur, M.; Predeep, P. Surface selective chemical deposition of polyanilines. Polymer 2005, 46, 1724–1730. [Google Scholar] [CrossRef]
- Mazur, M.; Krysinski, P. Polymer sandwiches: polyaniline films deposited on thiol-coated gold by chemical in situ method. Thin Solid Films 2001, 396, 131–137. [Google Scholar] [CrossRef]
- Mazur, M.; Krysinski, P.; Pałys, B. Preparation of ultrathin films of polyaniline and its derivatives by electrochemical deposition on thiol modified gold. J. Electroanal. Chem. 2002, 533, 145–152. [Google Scholar] [CrossRef]
- Lukkari, J.; Kleemola, K.; Meretoja, M.; Ollonqvist, T.; Kankare, J. Electrochemical Post-Self-Assembly Transformation of 4-Aminothiophenol Monolayers on Gold Electrodes. Langmuir 1998, 14, 1705–1715. [Google Scholar] [CrossRef]
- Batz, V.; Schneeweiss, M.A.; Kramer, D.; Hagenstrom, H.; Kolb, D.M.; Mandler, D. Electrochemistry and structure of the isomers of aminothiophenol adsorbed on gold. J. Electroanal. Chem. 2000, 491, 55–68. [Google Scholar] [CrossRef]
- Andrianova, M.S.; Gubanova, O.V.; Komarova, N.V.; Kuznetsov, E.V.; Kuznetsov, A.E. Development of a Biosensor Based on Phosphotriesterase and n-Channel ISFET for Detection of Pesticides. Electroanalysis 2016, 28, 1311–1321. [Google Scholar] [CrossRef]
- Moschou, E.; Chaniotakis, N.A. Potassium selective CHEMFET based on an ion-partitioning membrane. Anal. Chim. Acta 2001, 445, 183–190. [Google Scholar] [CrossRef]
- Bergveld, B. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuat. B-Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Reinhoudt, D.N.; Engbersen, J.F.J.; Brzozka, Z. Development of Durable K+-Selective Chemically Modified Field Effect Transistors with Functionalized Polysiloxane Membranes. Anal. Chem. 1994, 66, 3618–3623. [Google Scholar] [CrossRef]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef]
- Łęska, B.; Schroeder, G.; Łuczak, T.; Przybylski, P.; Pankiewicz, R.; Bełtowska-Brzezinska, M.; Brzezinski, B. Structure and electrochemical reactivity of 3-[tris(2-methoxyethoxy)silyl]-propanethiol adsorbed on silver surface. Thin Solid Films 2006, 515, 152–157. [Google Scholar] [CrossRef]
- Langer, J.J.; Czajkowski, I. Polyaniline microrods. Adv. Mater. Opt. Electron. 1997, 7, 149–156. [Google Scholar] [CrossRef]
- Langer, J.J. Polyaniline micro- and nanostructure. Adv. Mater. Opt. Electron. 1999, 9, 1–7. [Google Scholar] [CrossRef]
- Michalski, A.; Zabrocka, L.; Kocik, J.; Langer, K.; Langer, J.J. Nanobiodetector for spore forming bacteria. Final Rep. Projects 2012, 37–68. [Google Scholar]
- Zabrocka, L.; Langer, K.; Michalski, A.; Kocik, J.; Langer, J.J. A microfluidic device for real-time monitoring of Bacillus subtilis bacterial spores during germination based on non-specific physicochemical interactions on the nanoscale level. Lab Chip 2015, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, E.; Zheng, G.; Lieber, C.M. Nanowire sensors for medicine and the life sciences. Nanomedicine 2006, 1, 51–65. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska, A.; Golczak, S.; Langer, K.; Langer, J.J. Micro- and Nanostructured Polyaniline for Instant Identification of Metal Ions in Solution. Nanomaterials 2019, 9, 231. https://doi.org/10.3390/nano9020231
Michalska A, Golczak S, Langer K, Langer JJ. Micro- and Nanostructured Polyaniline for Instant Identification of Metal Ions in Solution. Nanomaterials. 2019; 9(2):231. https://doi.org/10.3390/nano9020231
Chicago/Turabian StyleMichalska, Agnieszka, Sebastian Golczak, Krzysztof Langer, and Jerzy J. Langer. 2019. "Micro- and Nanostructured Polyaniline for Instant Identification of Metal Ions in Solution" Nanomaterials 9, no. 2: 231. https://doi.org/10.3390/nano9020231
APA StyleMichalska, A., Golczak, S., Langer, K., & Langer, J. J. (2019). Micro- and Nanostructured Polyaniline for Instant Identification of Metal Ions in Solution. Nanomaterials, 9(2), 231. https://doi.org/10.3390/nano9020231




