The Effect of Electrode Topography on the Magnetic Properties and MRI Application of Electrochemically-Deposited, Synthesized, Cobalt-Substituted Hydroxyapatite
Abstract
1. Introduction
2. Materials and Methods
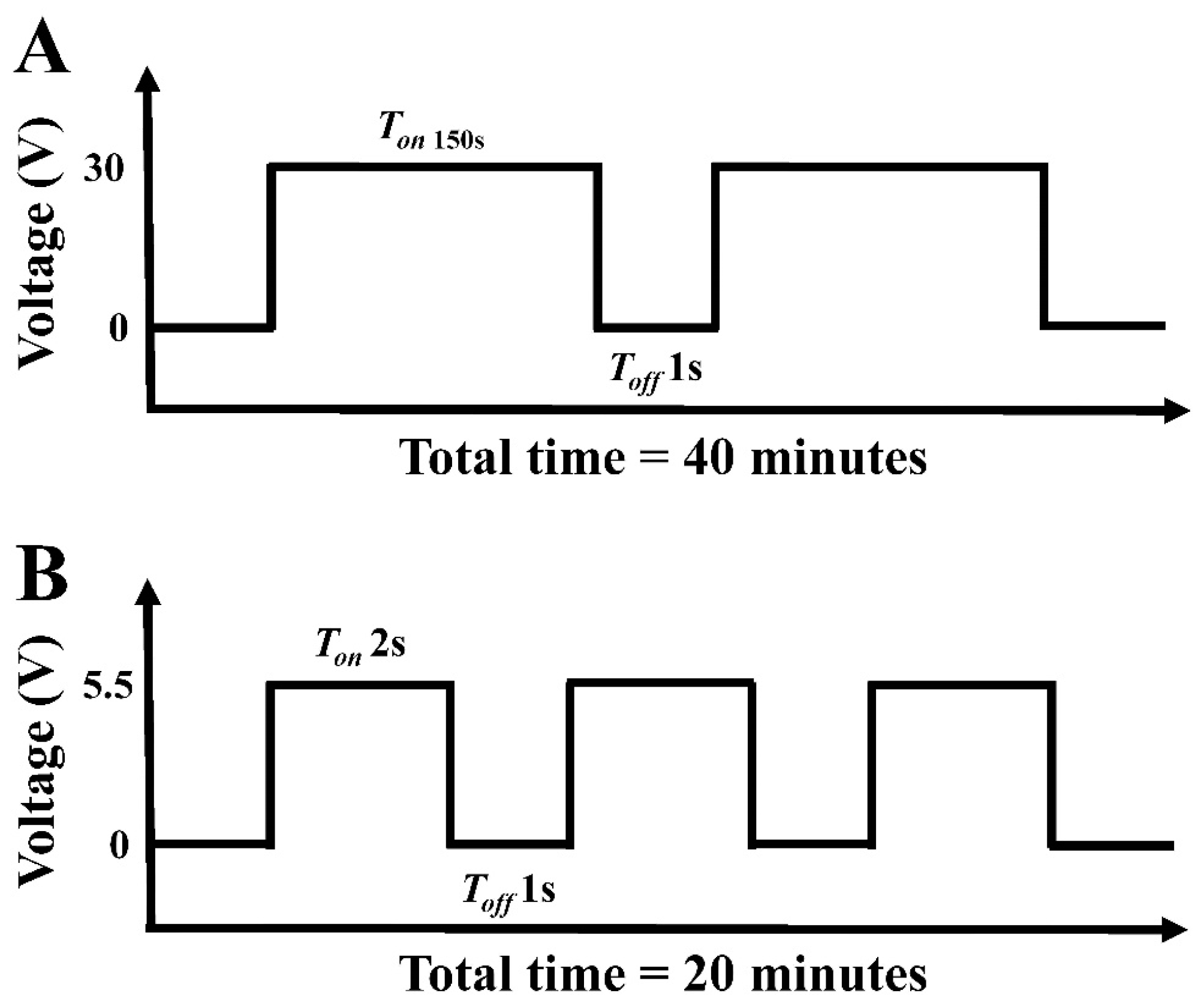
2.1. Preparation of Titanium Dioxide Nanotubes (TNT)
2.2. Preparation of Cobalt-Substituted Hydroxyapatite
2.3. Characterization
2.4. In Vitro MRI Experiments of CoHA and TNT-CoHA
2.5. Biocompatibility
2.5.1. Cytotoxicity
2.5.2. Osteogenic Differentiation
2.5.3. Antibacterial
2.5.4. In Vitro Biodegradation
2.6. Statistical Analysis
3. Results and Discussion
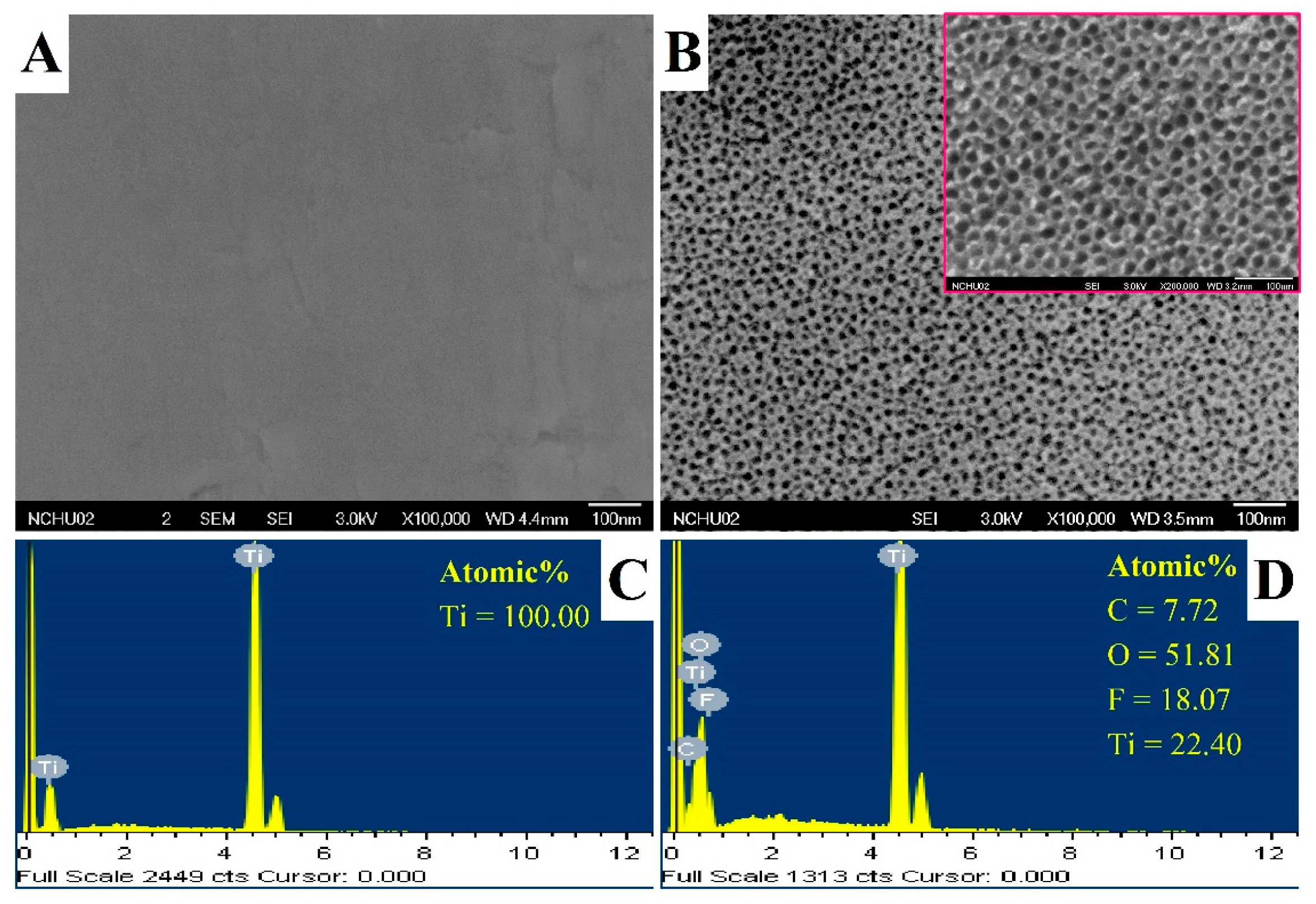
3.1. Characterization of Titanium Dioxide Nanotubes (TNT)
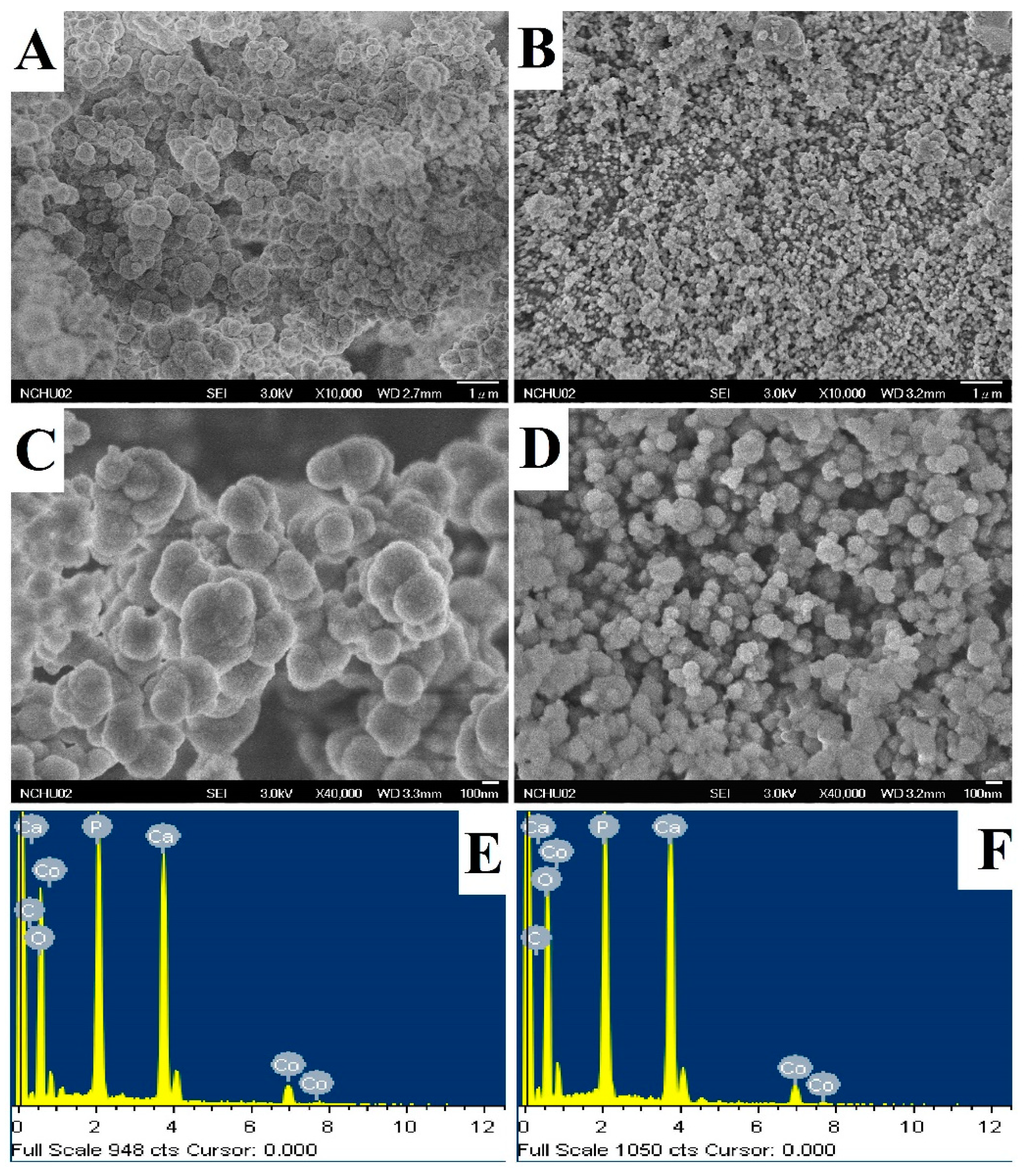
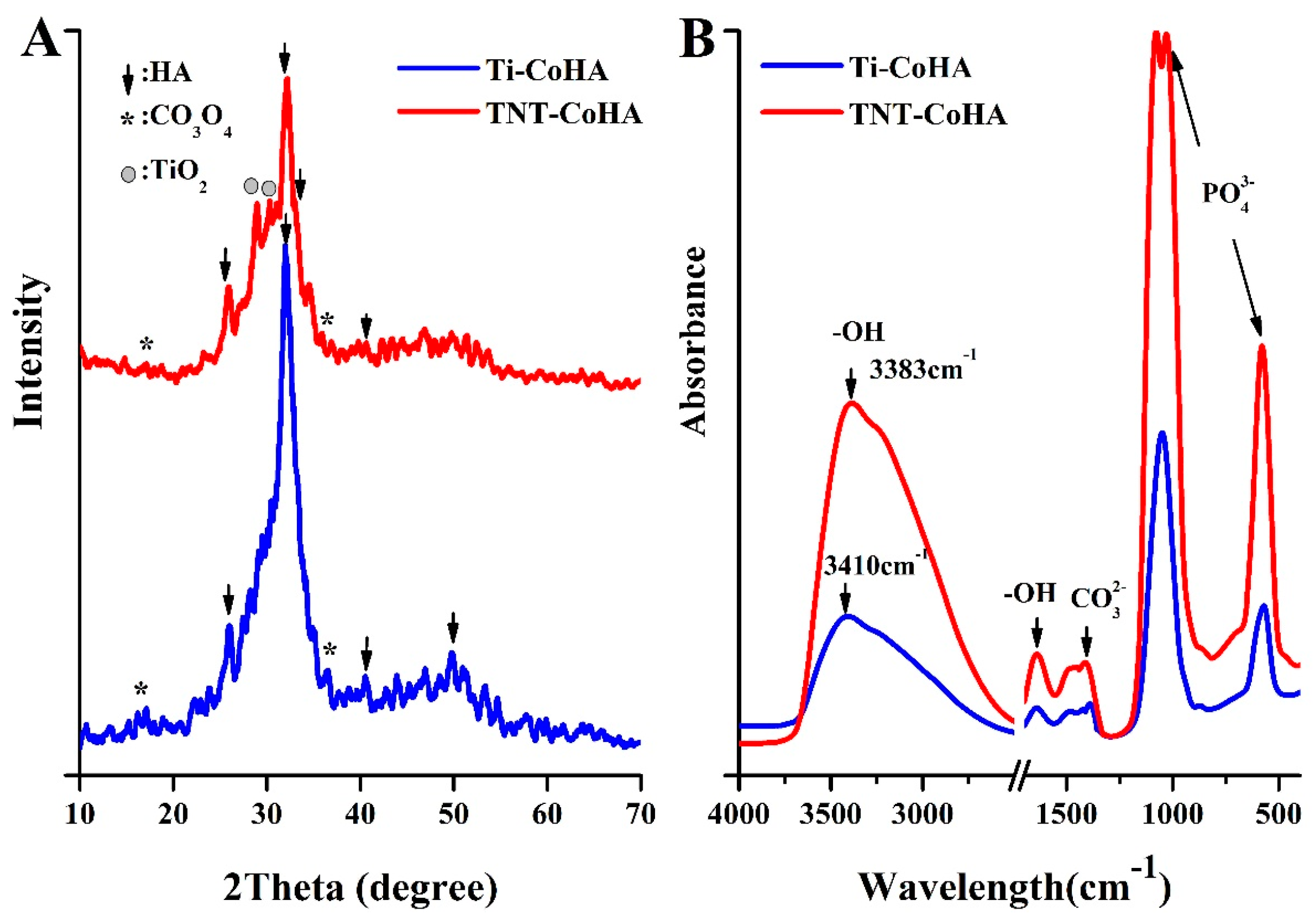
3.2. Characterization of Cobalt-Substituted Hydroxyapatite (CoHA)
3.3. Magnetic Analysis
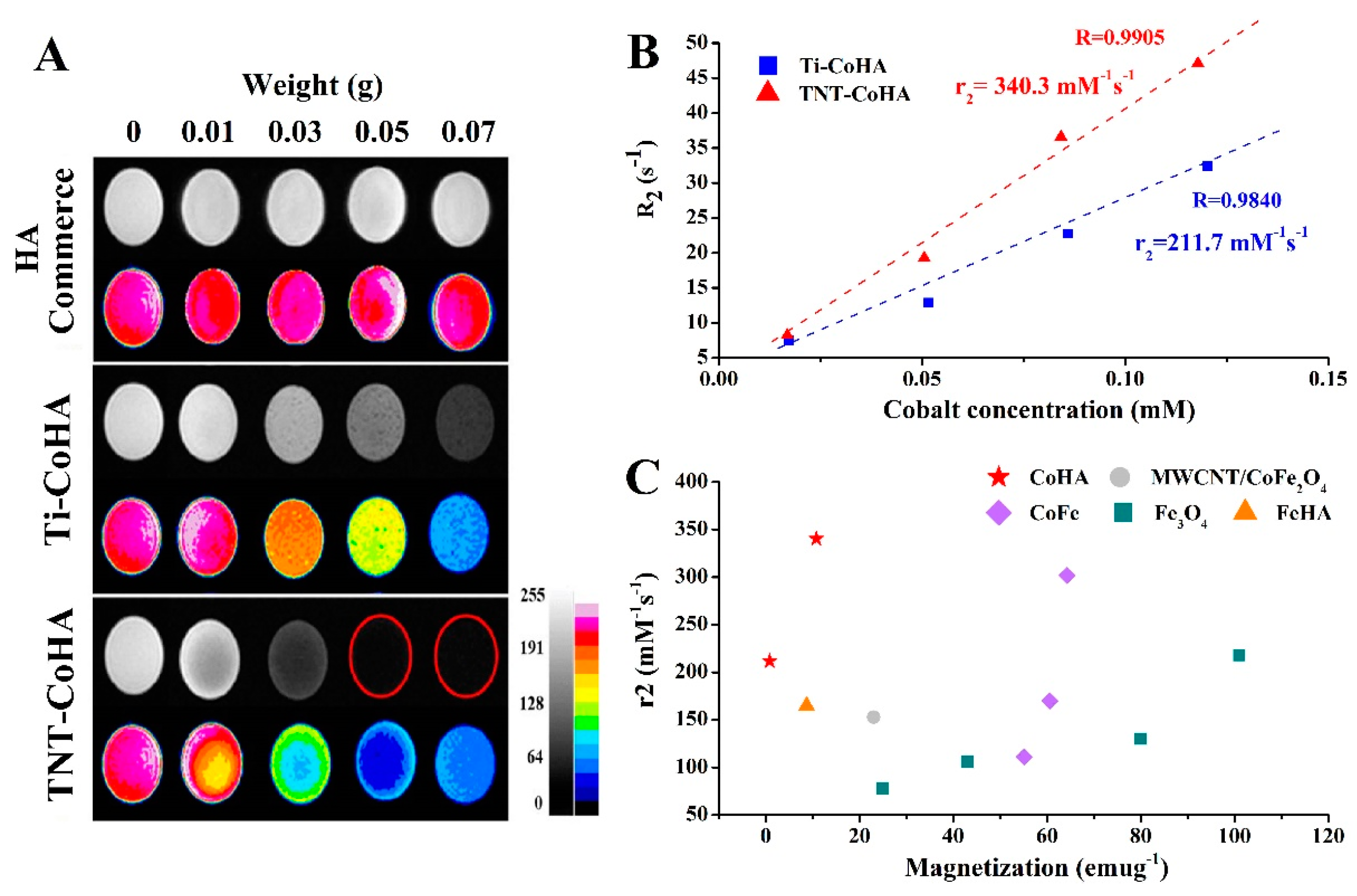
3.4. In Vitro MRI Examination
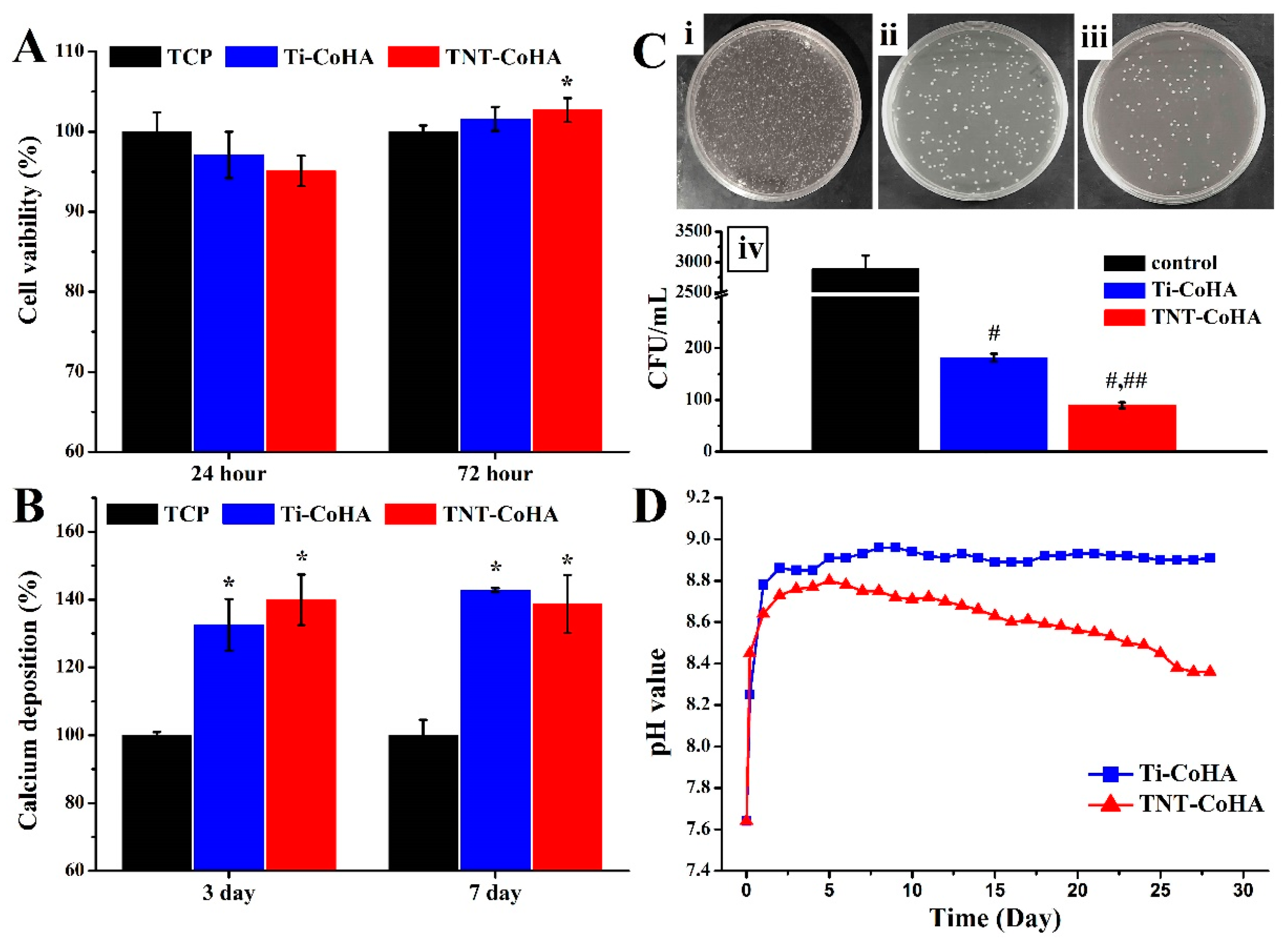
3.5. Biocompatibility
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shakir, S.I.; Udrescu, C.; Enachescu, C.; Rouviere, O.; Arion, S.; Caraivan, I.; Chapet, O. Transrectal implantation and stability of gold markers in prostate bed for salvage radiotherapy of macroscopic recurrences. Phys. Med. 2016, 32, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Laranjeira, M.S.; Moco, A.; Ferreira, J.; Coimbra, S.; Costa, E.; Santos-Silva, A.; Ferreira, P.J.; Monteiro, F.J. Different hydroxyapatite magnetic nanoparticles for medical imaging: Its effects on hemostatic, hemolytic activity and cellular cytotoxicity. Colloids Surf. B Biointerfaces 2016, 146, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, D.; Foran, P.; MacMahon, P.; Shelly, M.J.; Eustace, S.; O’Kennedy, R. Molecular and magnetic resonance imaging: The value of immunoliposomes. Adv. Drug Deliv. Rev. 2009, 61, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Hamilton, B.E.; Varallyay, C.G.; Rooney, W.R.; Edelman, R.D.; Jacobs, P.M.; Watnick, S.G. Ultrasmall superparamagnetic iron oxides (USPIOS): A future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int. 2009, 75, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.W.; Lee, J.H.; Cheon, J. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew Chem. Int. Ed. Engl. 2008, 47, 5122–5135. [Google Scholar] [CrossRef] [PubMed]
- Kalambur, V.S.; Han, B.; Hammer, B.E.; Shield, T.W.; Bischof, J.C. In vitro characterization of movement, heating and visualization of magnetic nanoparticles for biomedical applications. Nanotechnology 2005, 16, 1221. [Google Scholar] [CrossRef]
- Key, J.; Cooper, C.; Kim, A.Y.; Dhawan, D.; Knapp, D.W.; Kim, K.; Park, J.H.; Choi, K.; Kwon, I.C.; Park, K.; et al. In vivo nirf and mr dual-modality imaging using glycol chitosan nanoparticles. J. Control. Release 2012, 163, 249–255. [Google Scholar] [CrossRef]
- Adamiano, A.; Iafisco, M.; Sandri, M.; Basini, M.; Arosio, P.; Canu, T.; Sitia, G.; Esposito, A.; Iannotti, V.; Ausanio, G.; et al. On the use of superparamagnetic hydroxyapatite nanoparticles as an agent for magnetic and nuclear in vivo imaging. Acta Biomater. 2018, 73, 458–469. [Google Scholar] [CrossRef]
- Shokrollahi, H. Contrast agents for MRI. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4485–4497. [Google Scholar] [CrossRef]
- Shin, J.; Anisur, R.M.; Ko, M.K.; Im, G.H.; Lee, J.H.; Lee, I.S. Hollow manganese oxide nanoparticles as multifunctional agents for magnetic resonance imaging and drug delivery. Angew. Chem. 2009, 121, 327–330. [Google Scholar] [CrossRef]
- Wu, H.; Liu, G.; Wang, X.; Zhang, J.; Chen, Y.; Shi, J.; Yang, H.; Hu, H.; Yang, S. Solvothermal synthesis of cobalt ferrite nanoparticles loaded on multiwalled carbon nanotubes for magnetic resonance imaging and drug delivery. Acta Biomater. 2011, 7, 3496–3504. [Google Scholar] [CrossRef]
- Kramer, E.; Itzkowitz, E.; Wei, M. Synthesis and characterization of cobalt-substituted hydroxyapatite powders. Ceram. Int. 2014, 40, 13471–13480. [Google Scholar] [CrossRef]
- Frank, S.J.; Johansen, M.J.; Martirosyan, K.S.; Gagea, M.; Van Pelt, C.S.; Borne, A.; Carmazzi, Y.; Madden, T. A biodistribution and toxicity study of cobalt dichloride-N-acetyl cysteine in an implantable MRI marker for prostate cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Crawford, R.; Xiao, Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials 2010, 31, 3580–3589. [Google Scholar] [CrossRef] [PubMed]
- Ignjatovic, N.; Ajdukovic, Z.; Rajkovic, J.; Najman, S.; Mihailovic, D.; Uskokovic, D. Enhanced osteogenesis of nanosized cobalt-substituted hydroxyapatite. J. Bionic Eng. 2015, 12, 604–612. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chuang, C.-C.; Wang, P.-T.; Tang, C.-M. A comparative study on the direct and pulsed current electrodeposition of cobalt-substituted hydroxyapatite for magnetic resonance imaging application. Materials 2018, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.A.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 1125–1142. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef]
- Rath, P.C.; Besra, L.; Singh, B.P.; Bhattacharjee, S. Titania/hydroxyapatite bi-layer coating on Ti metal by electrophoretic deposition: Characterization and corrosion studies. Ceram. Int. 2012, 38, 3209–3216. [Google Scholar] [CrossRef]
- Joshi, H.M.; Lin, Y.P.; Aslam, M.; Prasad, P.V.; Schultz-Sikma, E.A.; Edelman, R.; Meade, T.; Dravid, V.P. Effects of shape and size of cobalt ferrite nanostructures on their MRI contrast and thermal activation. J. Phys. Chem. C 2009, 113, 17761–17767. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L. Hypoxia-mimicking co doped TiO2 microporous coating on titanium with enhanced angiogenic and osteogenic activities. Acta Biomater. 2016, 43, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J.P. Influence of metal oxide nanoparticles concentration on their zeta potential. J Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, T.; Haider, S.; Kamal, T.; Ul-Islam, M. Thermal decomposition of metal complex precursor as route to the synthesis of Co3O4 nanoparticles: Antibacterial activity and mechanism. J. Alloys Compd. 2017, 704, 296–302. [Google Scholar] [CrossRef]
- Zhen, G.; Muir, B.W.; Moffat, B.A.; Harbour, P.; Murray, K.S.; Moubaraki, B.; Suzuki, K.; Madsen, I.; Agron-Olshina, N.; Waddington, L. Comparative study of the magnetic behavior of spherical and cubic superparamagnetic iron oxide nanoparticles. J. Phys. Chem. C 2010, 115, 327–334. [Google Scholar] [CrossRef]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M. Characterization and thermal behavior of calcium deficient hydroxyapatite whiskers with various ca/p ratios. Mater. Chem. Phys. 2011, 126, 642–648. [Google Scholar] [CrossRef]
- Kolmas, J.; Piotrowska, U.; Kuras, M.; Kurek, E. Effect of carbonate substitution on physicochemical and biological properties of silver containing hydroxyapatites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 124–130. [Google Scholar] [CrossRef]
- Suchanek, K.; Bartkowiak, A.; Gdowik, A.; Perzanowski, M.; Kac, S.; Szaraniec, B.; Suchanek, M.; Marszalek, M. Crystalline hydroxyapatite coatings synthesized under hydrothermal conditions on modified titanium substrates. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 51, 57–63. [Google Scholar] [CrossRef]
- Stojanović, Z.; Veselinović, L.; Marković, S.; Ignjatović, N.; Uskoković, D. Hydrothermal synthesis of nanosized pure and cobalt-exchanged hydroxyapatite. Mater. Manuf. Process. 2009, 24, 1096–1103. [Google Scholar] [CrossRef]
- Ignjatovic, N.; Ajdukovic, Z.; Savic, V.; Najman, S.; Mihailovic, D.; Vasiljevic, P.; Stojanovic, Z.; Uskokovic, V.; Uskokovic, D. Nanoparticles of cobalt-substituted hydroxyapatite in regeneration of mandibular osteoporotic bones. J. Mater. Sci. Mater. Med. 2013, 24, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Xie, S.; Yu, M.; Ci, L. Fabrication and electromagnetic properties of carbon-based iron nitride composite. J. Magn. Magn. Mater. 2018, 466, 22–27. [Google Scholar] [CrossRef]
- Mohammad, A.; Ridha, S.; Mubarak, T. Structural and magnetic properties of mg-co ferrite nanoparticles. Dig. J. Nano. Mater. Bios. 2018, 13, 615–623. [Google Scholar]
- Druc, A.; Dumitrescu, A.; Borhan, A.; Nica, V.; Iordan, A.; Palamaru, M. Optimization of synthesis conditions and the study of magnetic and dielectric properties for MgFe2O4 ferrite. Open Chem. 2013, 11. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Khansari, A. Synthesis and characterization of Co3O4 nanoparticles by a simple method. C. R. Chim. 2014, 17, 352–358. [Google Scholar] [CrossRef]
- Sarath Chandra, V.; Elayaraja, K.; Thanigai Arul, K.; Ferraris, S.; Spriano, S.; Ferraris, M.; Asokan, K.; Narayana Kalkura, S. Synthesis of magnetic hydroxyapatite by hydrothermal–microwave technique: Dielectric, protein adsorption, blood compatibility and drug release studies. Ceram. Int. 2015, 41, 13153–13163. [Google Scholar] [CrossRef]
- El-Dek, S.I.; Ali, M.A.; El-Zanaty, S.M.; Ahmed, S.E. Comparative investigations on ferrite nanocomposites for magnetic hyperthermia applications. J. Magn. Magn. Mater. 2018, 458, 147–155. [Google Scholar] [CrossRef]
- Gopi, D.; Indira, J.; Kavitha, L.; Sekar, M.; Mudali, U.K. Synthesis of hydroxyapatite nanoparticles by a novel ultrasonic assisted with mixed hollow sphere template method. Spectrochim. Acta A 2012, 93, 131–134. [Google Scholar] [CrossRef]
- Lam, T.; Pouliot, P.; Avti, P.K.; Lesage, F.; Kakkar, A.K. Superparamagnetic iron oxide based nanoprobes for imaging and theranostics. Adv. Colloid Interface Sci. 2013, 199–200, 95–113. [Google Scholar] [CrossRef]
- Shin, T.H.; Choi, Y.; Kim, S.; Cheon, J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem. Soc. Rev. 2015, 44, 4501–4516. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed.-Nanotechnol. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for mr imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Wells, S. Surface-modified superparamagnetic nanoparticles for drug delivery: Preparation, characterization, and cytotoxicity studies. IEEE T. Nanobiosci. 2004, 3, 66–73. [Google Scholar] [CrossRef]
- Jun, Y.W.; Huh, Y.-M.; Choi, J.-S.; Lee, J.-H.; Song, H.-T.; Kim, S.; Kim, S.; Yoon, S.; Kim, K.-S.; Shin, J.-S. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef] [PubMed]
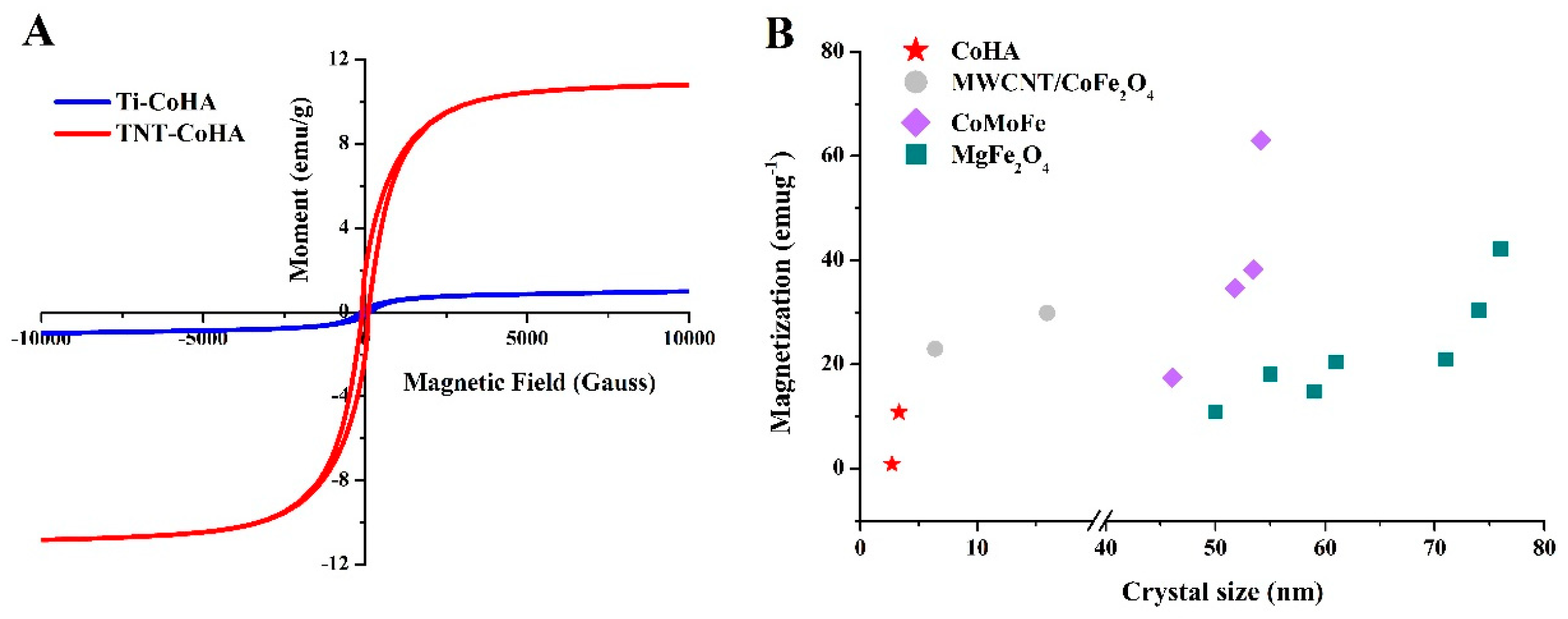
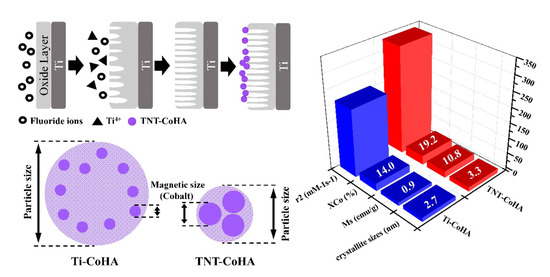
| Sample | Particle Size (nm) | Surface Composition * | Overall Composition ** | Magnetic Field Strength | ||||
|---|---|---|---|---|---|---|---|---|
| Co+Ca/P (%) | Co+Ca/P (%) | XCo (%) | Hc (Oe) | Ms (emu/g) | Mr (emu/g) | Squareness Ratio (Mr/Ms) | ||
| Ti-CoHA | 418.6 ± 8.0 | 1.67 | 1.67 | 14.0 | 133.98 | 0.86 | 0.15 | 0.17 |
| TNT-CoHA | 127.5 ± 2.9 | 1.43 | 1.62 | 19.2 | 89.68 | 10.82 | 2.02 | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-C.; Chuang, C.-C.; Chang, C.-J.; Chiu, Y.-H.; Yan, M.; Tang, C.-M. The Effect of Electrode Topography on the Magnetic Properties and MRI Application of Electrochemically-Deposited, Synthesized, Cobalt-Substituted Hydroxyapatite. Nanomaterials 2019, 9, 200. https://doi.org/10.3390/nano9020200
Lin W-C, Chuang C-C, Chang C-J, Chiu Y-H, Yan M, Tang C-M. The Effect of Electrode Topography on the Magnetic Properties and MRI Application of Electrochemically-Deposited, Synthesized, Cobalt-Substituted Hydroxyapatite. Nanomaterials. 2019; 9(2):200. https://doi.org/10.3390/nano9020200
Chicago/Turabian StyleLin, Wei-Chun, Chun-Chao Chuang, Chen-Jung Chang, Ya-Hsu Chiu, Min Yan, and Cheng-Ming Tang. 2019. "The Effect of Electrode Topography on the Magnetic Properties and MRI Application of Electrochemically-Deposited, Synthesized, Cobalt-Substituted Hydroxyapatite" Nanomaterials 9, no. 2: 200. https://doi.org/10.3390/nano9020200
APA StyleLin, W.-C., Chuang, C.-C., Chang, C.-J., Chiu, Y.-H., Yan, M., & Tang, C.-M. (2019). The Effect of Electrode Topography on the Magnetic Properties and MRI Application of Electrochemically-Deposited, Synthesized, Cobalt-Substituted Hydroxyapatite. Nanomaterials, 9(2), 200. https://doi.org/10.3390/nano9020200