Enhancing PD-1 Gene Silence in T Lymphocytes by Comparing the Delivery Performance of Two Inorganic Nanoparticle Platforms
Abstract
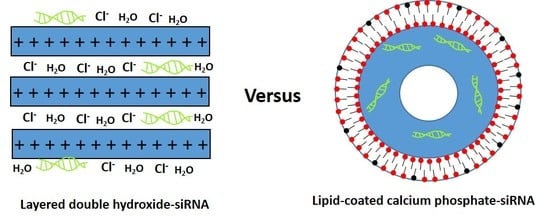
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. LDH NP Preparation
2.3. LCP NPs Preparation
2.4. Characterization of LDH, BSA-LDH and LCP NPs
2.5. Cell Culture and FACS Analysis
2.6. Tumor infiltrating lymphocyte Isolation and Characterization
2.7. Delivery of dsDNA and siRNA to EL4 and TILs
2.8. Western Blotting
2.9. Real-Time PCR
2.10. Statistical Analysis
3. Results
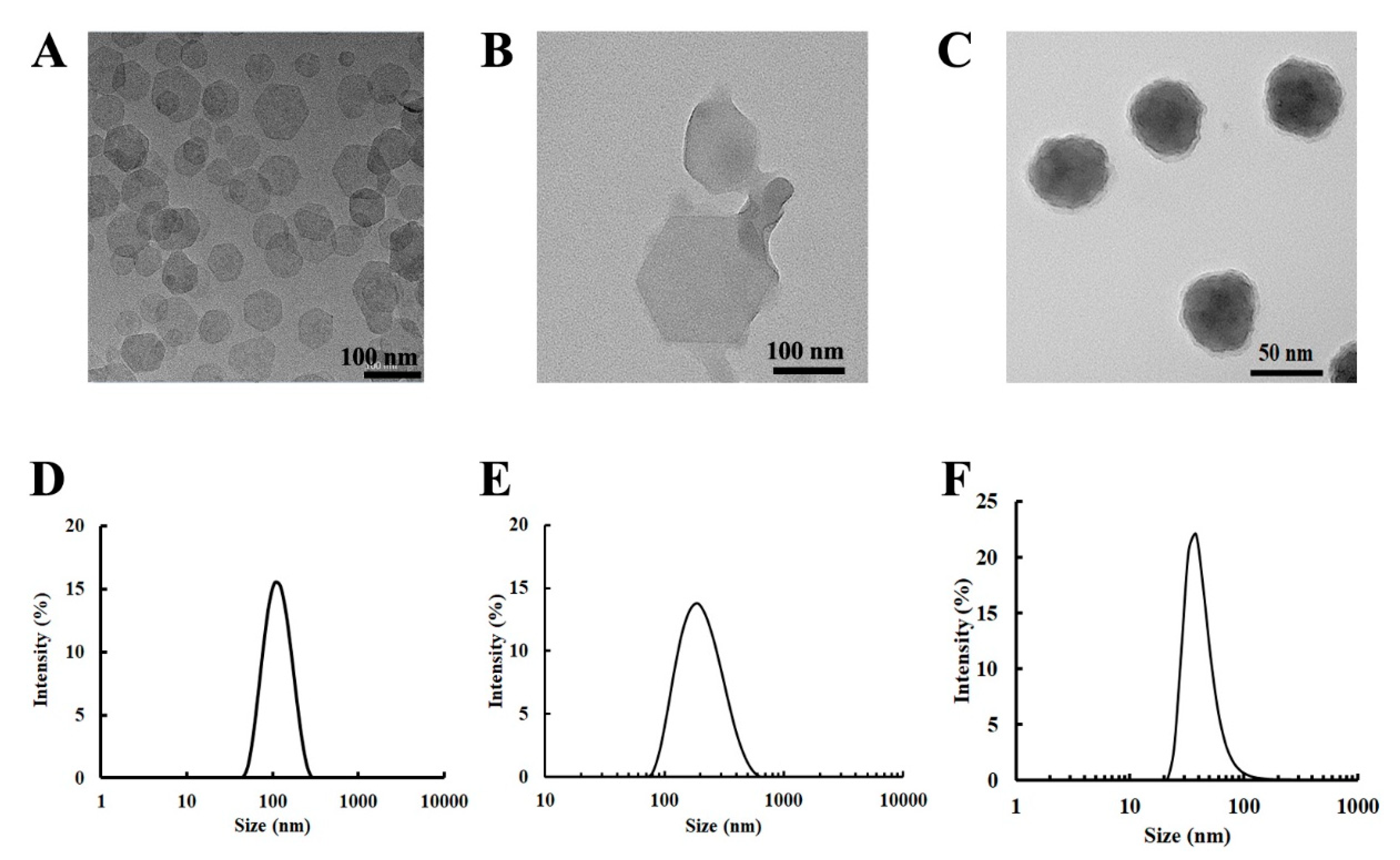
3.1. Physicochemical Properties of LDH and LCP NPs
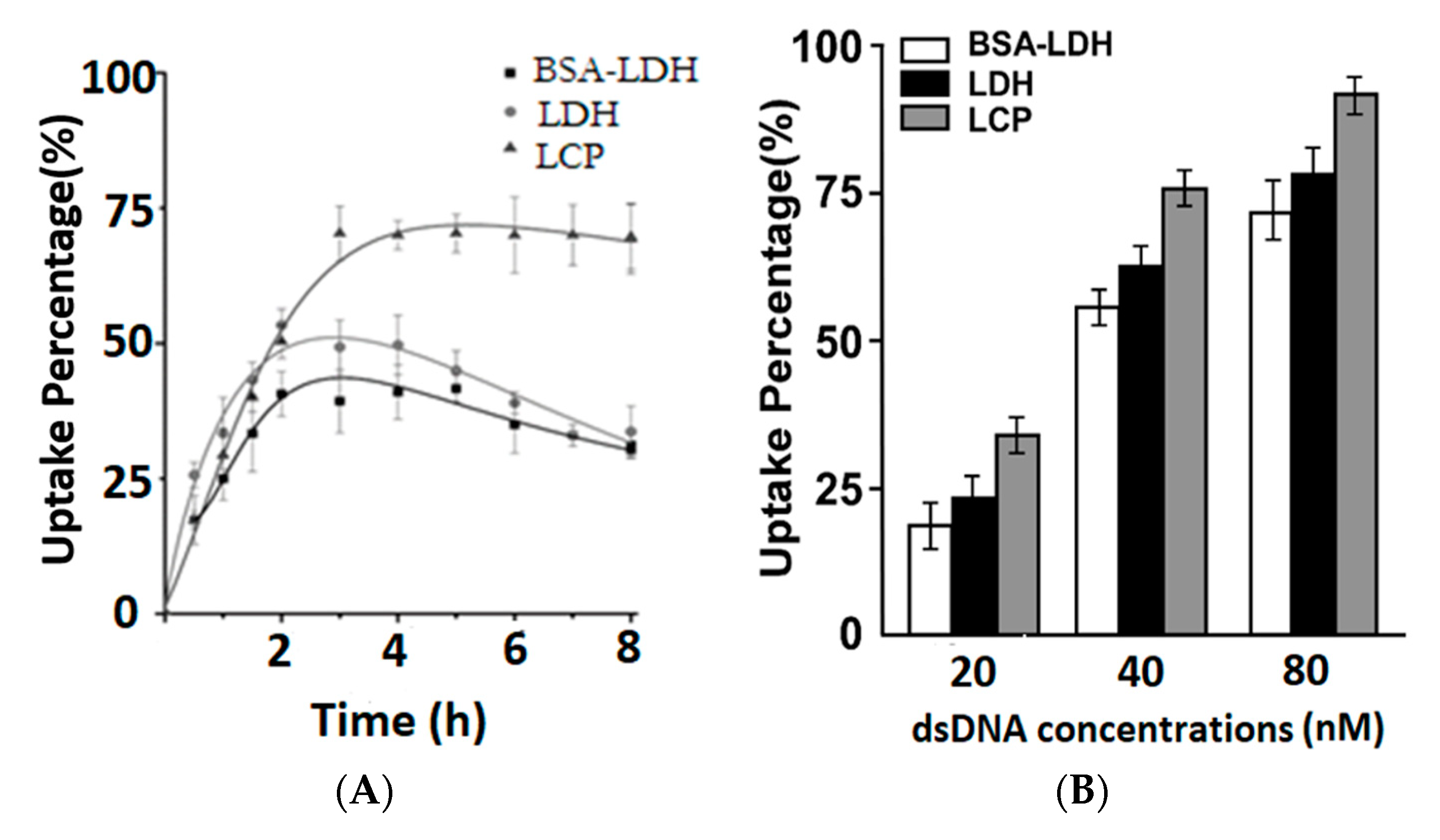
3.2. Cellular Uptake of LDH, BSA-LDH and LCP NPs
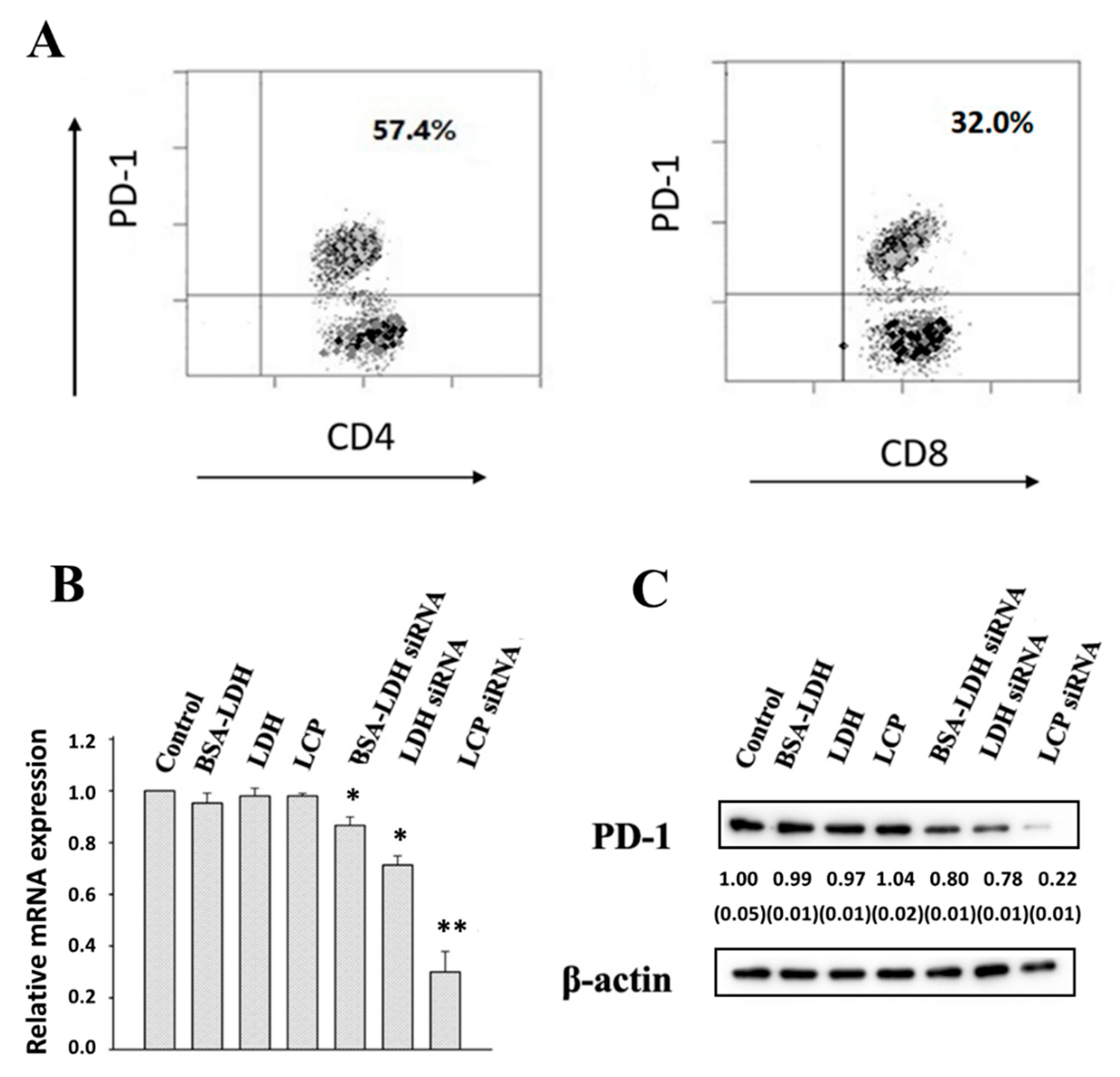
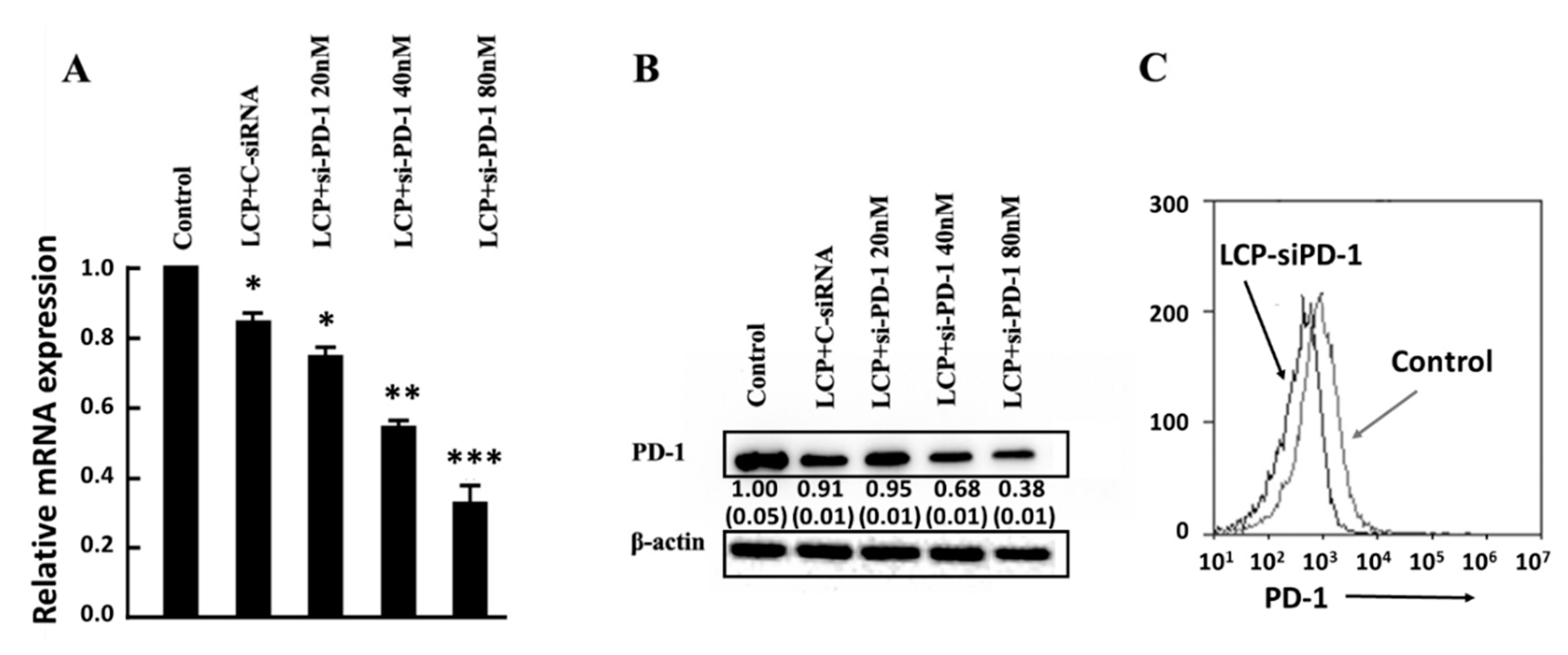
3.3. PD-1 Expression and Gene Silence in EL4 Cells
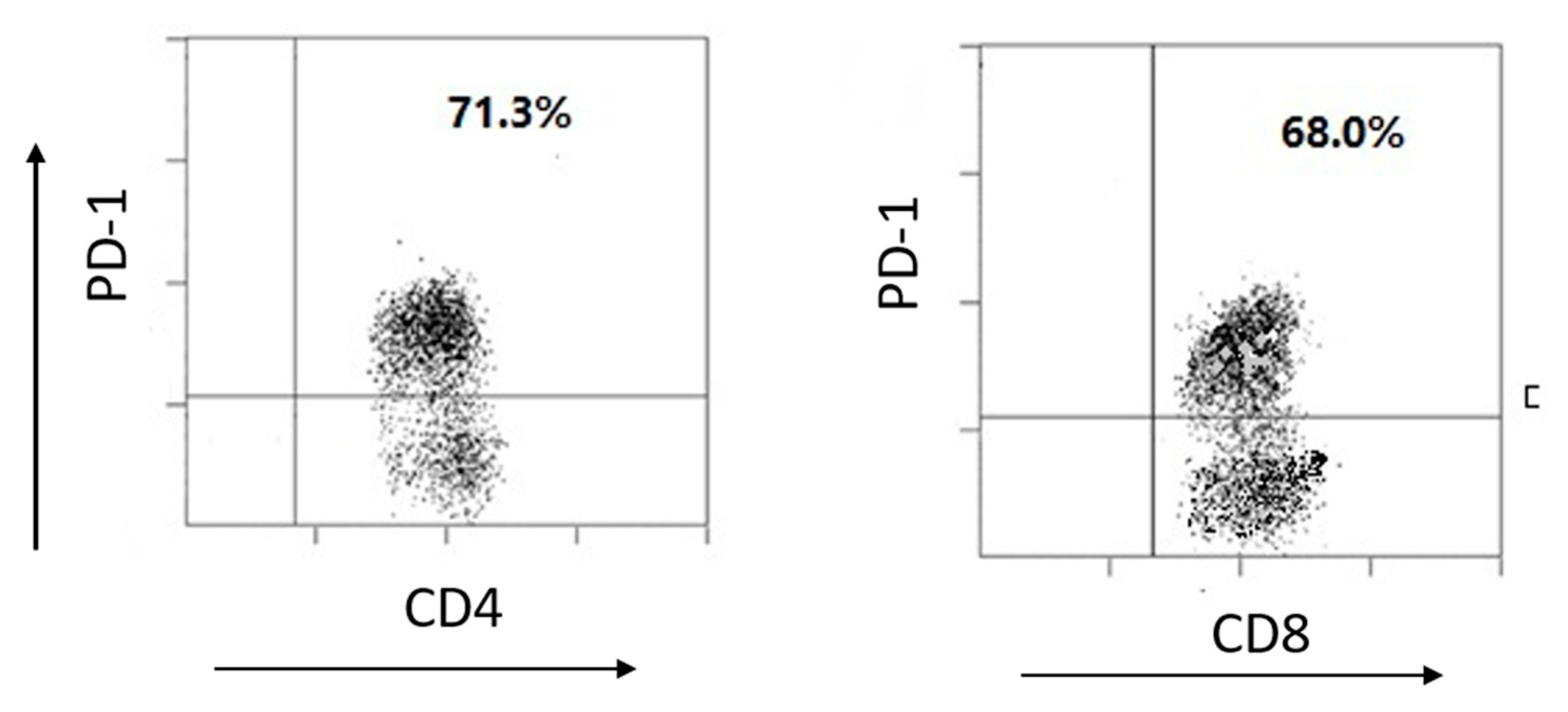
3.4. PD-1 Expression and Gene Silence in Human TILs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| TIL | tumor infiltrating lymphocyte |
| LCP | lipid-coated calcium phosphate |
| LDH | layered double hydroxide |
| NP | Nanoparticle |
| PD-1 | programmed cell death protien-1 |
| PDI | polydispersity index |
| PD-L1 | programmed cell death protien-1 ligand |
| TEM | transmission electron microscope |
Appendix A
References
- Mao, C.-P.; Hung, C.-F.; Wu, T.-C. Immunotherapeutic strategies employing RNA interference technology for the control of cancers. J. Biomed. Sci. 2007, 14, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Qiao, S.; Dai, Y.; Xu, G.; Dai, B.; Lu, L.; Yu, X.; Luo, Q.; Zhang, Z. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano 2017, 11, 9536–9549. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.; Peggs, K. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br. J. Cancer 2013, 108, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, T.; Liu, J.; Yu, H.; Jiao, S.; Feng, B.; Zhou, F.; Fu, Y.; Yin, Q.; Zhang, P. Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy. Nano Lett. 2016, 16, 5503–5513. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zeng, Q.; Lu, G.M.; Yu, A. Inorganic Nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Ladewig, K.; Niebert, M.; Xu, Z.P.; Gray, P.P.; Lu, G.Q.M. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomaterials 2010, 31, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gu, W.; Chen, J.; Chen, W.; Xu, Z. Co-delivery of siRNAs and Anti-cancer Drugs Using Layered Double Hydroxide Nanoparticles. Biomaterials 2014, 35, 3331–3339. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Shan, Z.; Andrea, K.; MacDonald, B.; Beale, S.; Curry, D.E.; Wang, L.; Wang, S.; Oakes, K.D.; Bennett, C. Chemisorption Mechanism of DNA on Mg/Fe Layered Double Hydroxide Nanoparticles: Insights into Engineering Effective SiRNA Delivery Systems. Langmuir 2016, 32, 2659–2667. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-H.; Cho, J.; Kwon, O.-J.; Yun, C.-O.; Choy, J.-H. Biodegradable Inorganic Nanovector: Passive versus Active Tumor Targeting in siRNA Transportation. Angew. Chem. Intern. Ed. 2016, 55, 4582–4586. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Markham, K.; Xu, Z.P.; Chen, M.; Lu, G.Q.; Bartlett, P.F.; Cooper, H.M. Efficient delivery of siRNA to cortical neurons using layered double hydroxide nanoparticles. Biomaterials 2010, 31, 8770–8779. [Google Scholar] [CrossRef]
- Huang, J.-L.; Chen, H.-Z.; Gao, X.-L. Lipid-coated calcium phosphate nanoparticle and beyond: A versatile platform for drug delivery. J. Drug Target. 2018, 26, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.-C.; Tseng, Y.-C.; Mozumdar, S.; Huang, L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control Release 2010, 142, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Huang, L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J. Control Release 2012, 158, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Howard, C.B.; Mahler, S.M.T.; Thurecht, K.J.; Huang, L.; Xu, Z.P. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale 2018, 10, 4258–4266. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gu, W.; Tang, J.; Xu, Z.P. Devising new lipid-coated calcium phosphate/carbonate hybrid nanoparticles for controlled release in endosomes for efficient gene delivery. J. Mater. Chem. B 2017, 5, 7194–7203. [Google Scholar] [CrossRef]
- Choi, S.-J.; Choy, J.H. Layered double hydroxide nanoparticles as target-specific delivery carriers: Uptake mechanism and toxicity. Nanomedicine 2011, 6, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Niebert, M.; Porazik, K.; Walker, T.; Cooper, H.; Middelberg, A.; Gray, P.; Bartlett, P.; Lu, G. Subcellular compartment targeting of layered double hydroxide nanoparticles. J. Control Release 2008, 130, 86–94. [Google Scholar] [CrossRef]
- Li, X.; Ke, M.; Huang, W.; Ye, C.; Huang, J. A pH-Responsive Layered Double Hydroxide (LDH)–Phthalocyanine Nanohybrid for Efficient Photodynamic Therapy. Chemistry 2015, 21, 3310–3317. [Google Scholar] [CrossRef]
- Hu, H.; Xiu, K.; Xu, S.; Yang, W.; Xu, F. Functionalized Layered Double Hydroxide Nanoparticles Conjugated with Disulfide-Linked Polycation Brushes for Advanced Gene Delivery. Bioconj. Chem. 2013, 24, 968–978. [Google Scholar] [CrossRef]
- Gu, Z.; Zuo, H.; Li, L.; Wu, A.; Xu, Z.P. Pre-coating layered double hydroxide nanoparticles with albumin to improve colloidal stability and cellular uptake. J. Mater. Chem. B 2015, 3, 3331–3339. [Google Scholar] [CrossRef]
- Zuo, H.; Gu, Z.; Cooper, H.; Xu, Z.P. Crosslinking to enhance colloidal stability and redispersity of layered double hydroxide nanoparticles. J. Colloid Interface Sci. 2015, 459, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.; Li, J.; Wilson, M.; Rogers, T.; Close, J.; Huang, L.; Kumta, P.; Sfeir, C. Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: Influence of the synthesis parameters on transfection efficiency. Biomaterials 2007, 28, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Liu, F.; Huang, L. Systemic Delivery of siRNA via LCP Nanoparticle Efficiently Inhibits Lung Metastasis. Mol. Ther. 2012, 20, 609–615. [Google Scholar] [CrossRef]
- Tang, J.; Li, L.; Howard, C.; Mahler, S.; Huang, L.; Xu, Z. Preparation of optimized lipid-coated calcium phosphate nanoparticles for enhanced in vitro gene delivery to breast cancer cells. J. Mater. Chem. B 2015, 3, 6805–6812. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Stevenson, G.; Lu, C.; Lu, G. Stable Suspension of Layered Double Hydroxide Nanoparticles in Aqueous Solutions. J. Phys. Chem. B 2006, 110, 16923–16929. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Stevenson, G.; Lu, C.-Q.; Lu, G.Q.M.; Bartlett, P.F.; Gray, P. Stable suspension of layered double hydroxide nanoparticles in aqueous solution. J. Am. Chem. Soc. 2006, 128, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Sabatino, M.; Somerville, R.; Wilson, J.R.; Dudley, M.E.; Stroncek, D.; Rosenberg, S. Simplified method of the growth of human tumor infiltrating lymphocytes in gas-permeable flasks to numbers needed for patient treatment. J. Immunother. 2012, 35, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Q.; He, J.; Li, Z.; Tang, X.; Chen, S.; Xie, C.; Li, Y.; Huang, L.; Ye, S.; et al. Phase I trial of adoptively transferred tumor-infiltrating lymphocyte immunotherapy following concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. OncoImmunology 2015, 4, e976507. [Google Scholar] [CrossRef]
- Wong, Y.; Cooper, H.M.; Zhang, K.; Chen, M.; Bartlett, P.; Xu, Z.P. Efficiency of layered double hydroxide nanoparticle-mediated delivery of siRNA is determined by nucleotide sequence. J. Colloid Interface Sci. 2012, 369, 453–459. [Google Scholar] [CrossRef]
- Gu, Z.; Rolfe, B.E.; Xu, Z.P.; Thomas, A.C.; Campbell, J.H.; Lu, G.Q. Max, Enhanced anti-proliferation, anti-migration and cellular uptake of LMWH to rat vascular smooth muscle cells via layered double hydroxide (LDH) nanoparticles. Biomaterials 2010, 31, 5455–5462. [Google Scholar] [CrossRef]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The Possible “Proton Sponge” Effect of Polyethylenimine (PEI) Does Not Include Change in Lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, B.; Mahony, T.; Gu, W.; Rolfe, B.; Xu, Z. Efficient and Durable Vaccine against Intimin β of Diarrheagenic E. Coli Induced by Clay Nanoparticles. Small 2016, 12, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-M.; Choi, S.J.; Lee, G.E.; Han, S.H.; Choy, J.H. Inorganic Drug-Delivery Nanovehicle Conjugated with Cancer-Cell-Specific Ligand. Adv. Funct. Mater. 2009, 19, 1617–1624. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, W.; Chen, C.; Do, S.; Xu, Z. Optimization of formulations consisting of layered double hydroxide nanoparticles and small interfering RNA for efficient knockdown of the target gene. ACS Omega 2018, 3, 4871–4877. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Gu, W.; Li, L.; Chen, C.; Xu, Z.P. Enhancing PD-1 Gene Silence in T Lymphocytes by Comparing the Delivery Performance of Two Inorganic Nanoparticle Platforms. Nanomaterials 2019, 9, 159. https://doi.org/10.3390/nano9020159
Wu Y, Gu W, Li L, Chen C, Xu ZP. Enhancing PD-1 Gene Silence in T Lymphocytes by Comparing the Delivery Performance of Two Inorganic Nanoparticle Platforms. Nanomaterials. 2019; 9(2):159. https://doi.org/10.3390/nano9020159
Chicago/Turabian StyleWu, Yanheng, Wenyi Gu, Li Li, Chen Chen, and Zhi Ping Xu. 2019. "Enhancing PD-1 Gene Silence in T Lymphocytes by Comparing the Delivery Performance of Two Inorganic Nanoparticle Platforms" Nanomaterials 9, no. 2: 159. https://doi.org/10.3390/nano9020159
APA StyleWu, Y., Gu, W., Li, L., Chen, C., & Xu, Z. P. (2019). Enhancing PD-1 Gene Silence in T Lymphocytes by Comparing the Delivery Performance of Two Inorganic Nanoparticle Platforms. Nanomaterials, 9(2), 159. https://doi.org/10.3390/nano9020159