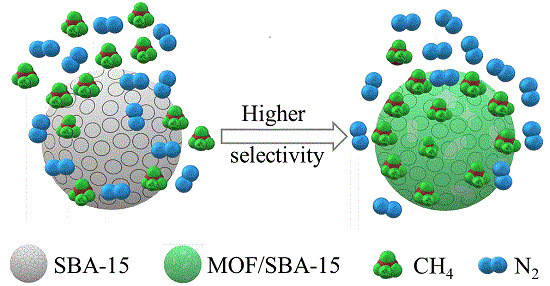
Selective Adsorption of CH4/N2 on Ni-based MOF/SBA-15 Composite Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Spherical SBA-15
2.2. Synthesis of MOF-1/SBA-15
2.3. Synthesis of MOF-2/SBA-15
2.4. Characterization
2.5. Adsorption Performance
3. Results and Discussion
3.1. Scanning electron microscopy (SEM) Images
3.2. X-ray fluorescence (XRF) and Elemental Analysis
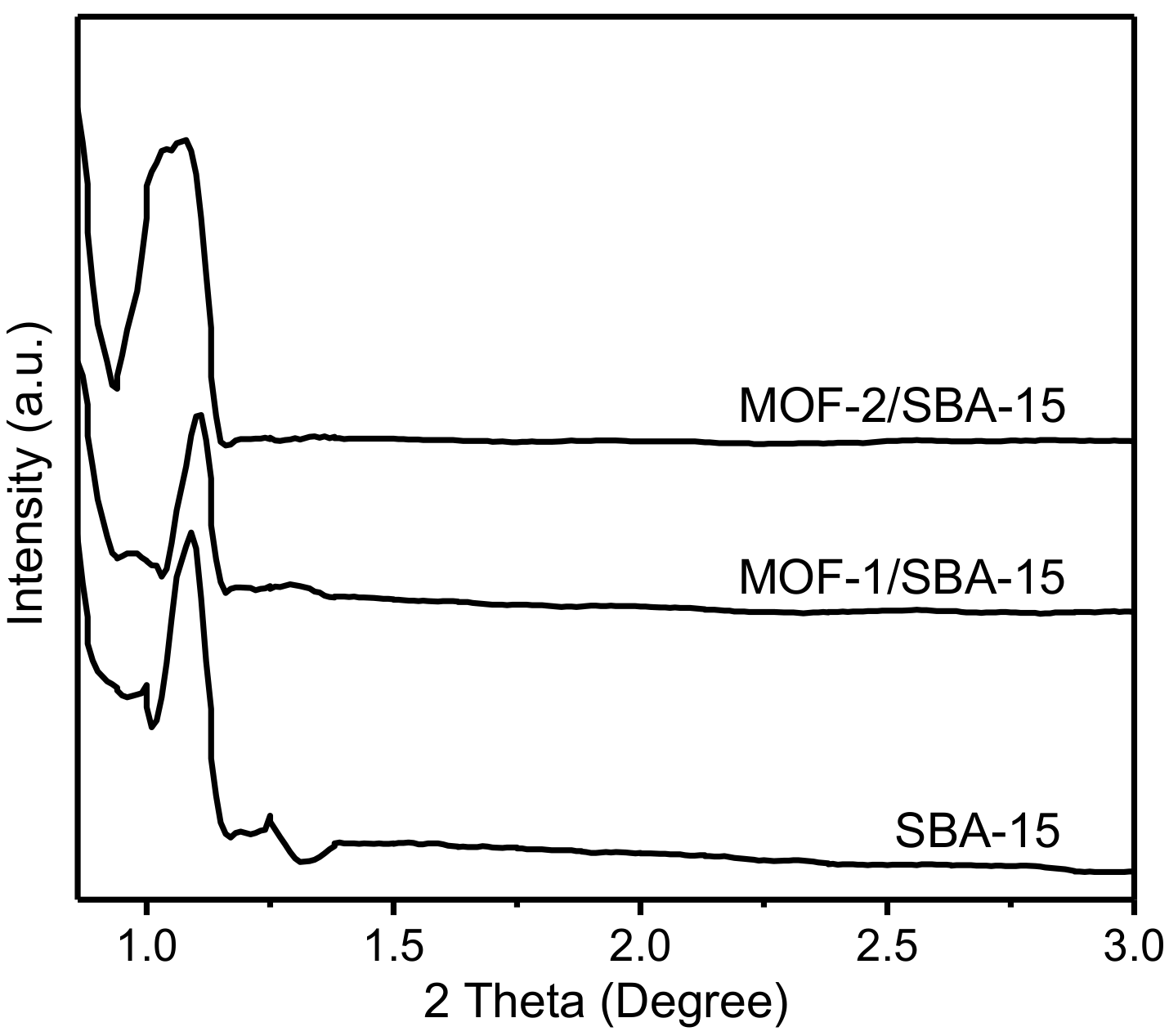
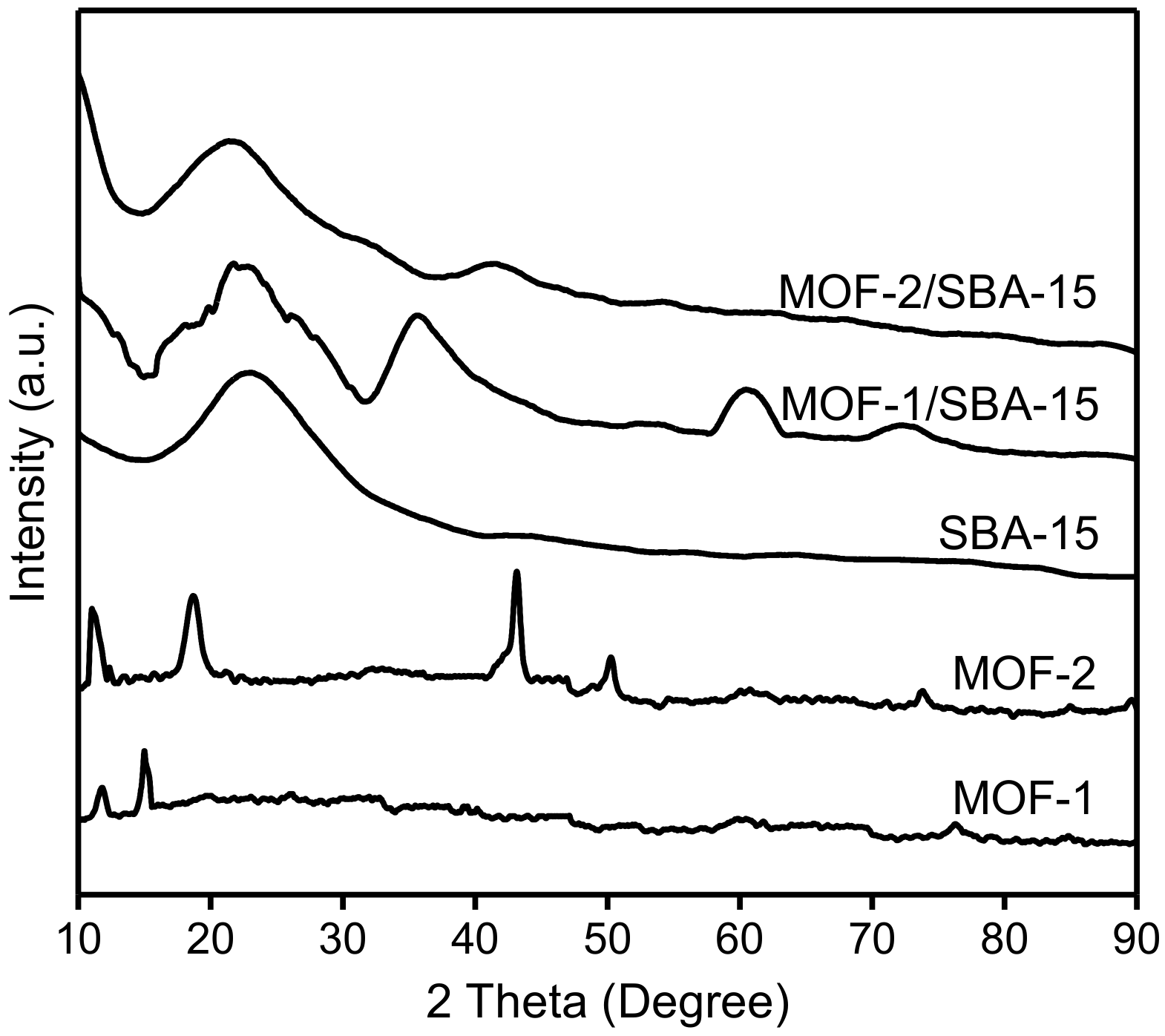
3.3. X-ray powder diffraction (XRD) Patterns
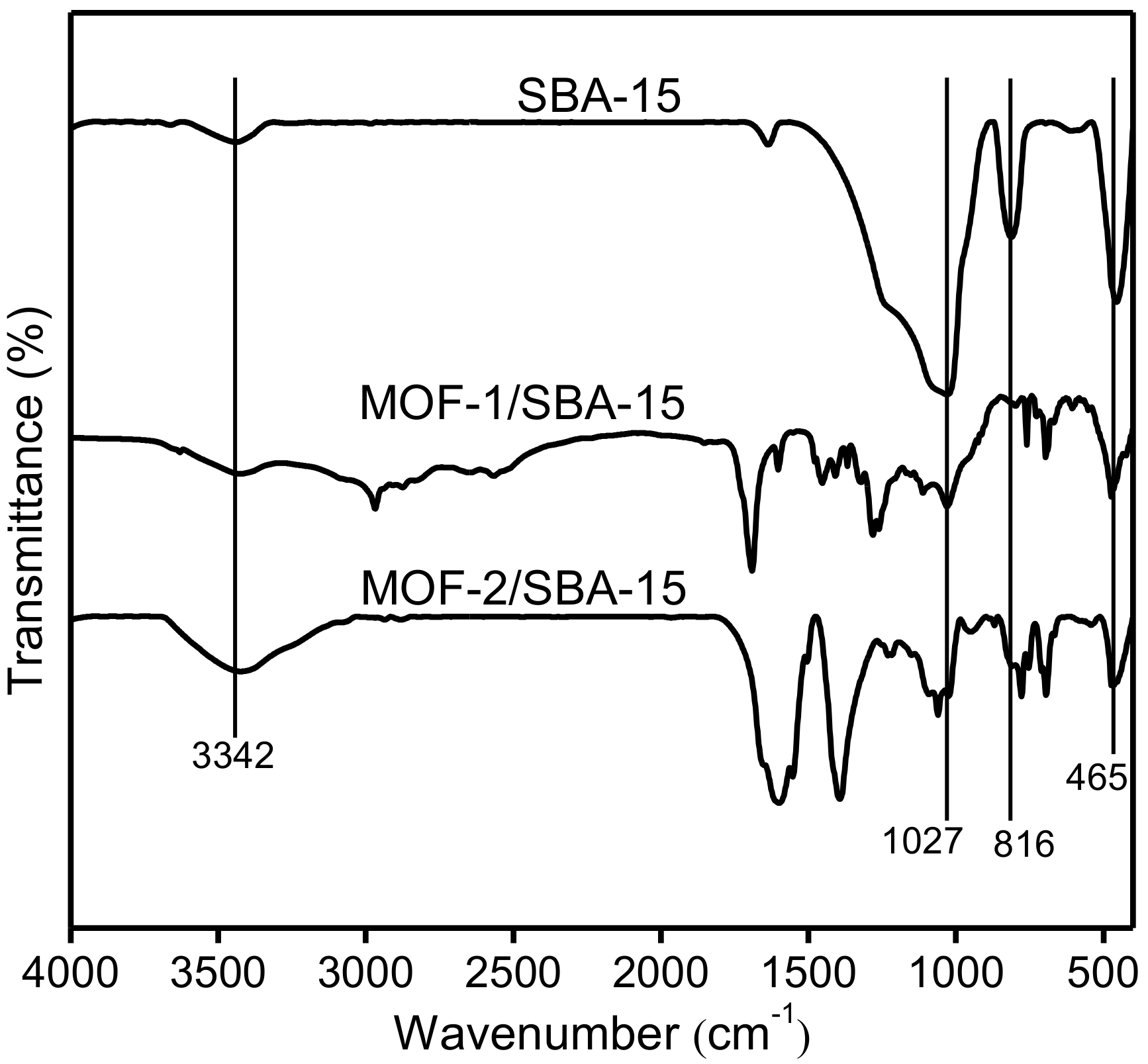
3.4. Fourier-transform infrared spectroscopy (FT-IR)
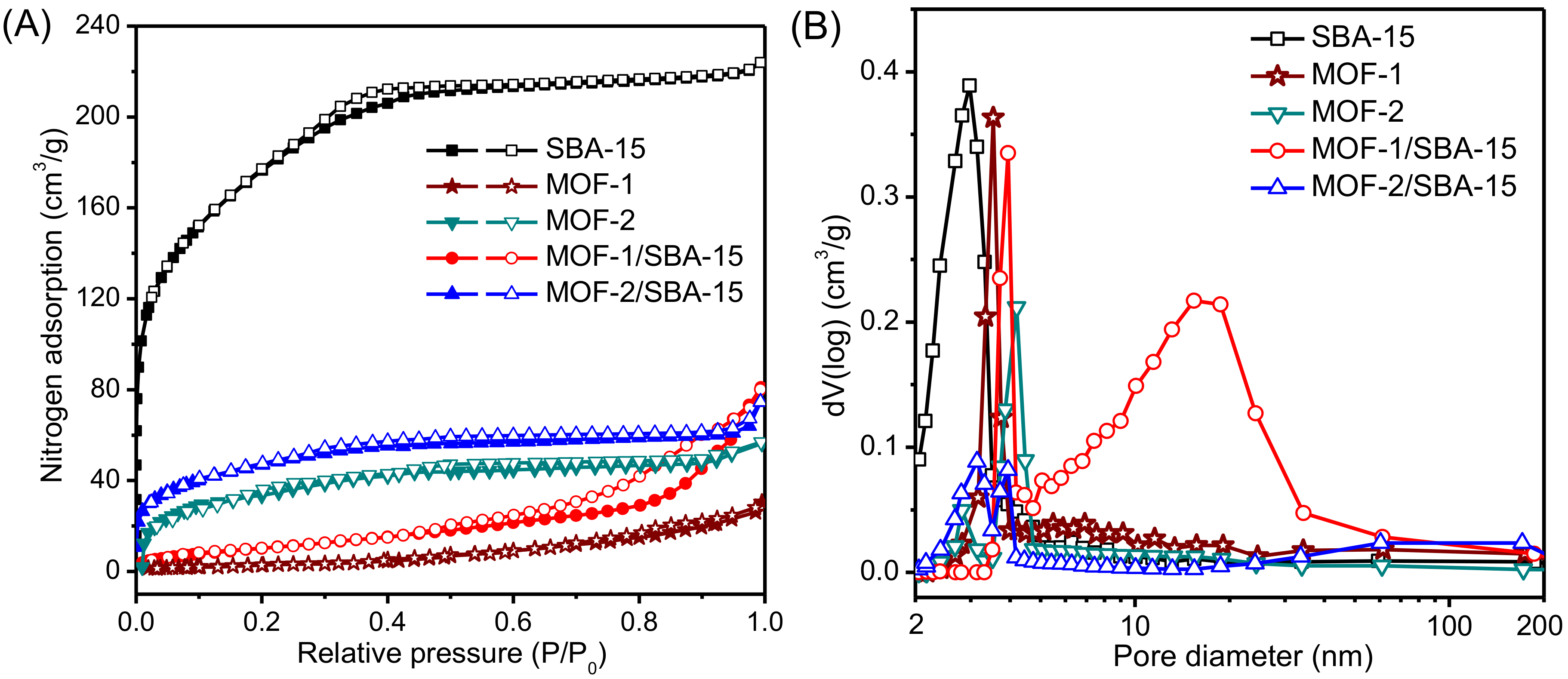
3.5. Nitrogen Adsorption–Desorption
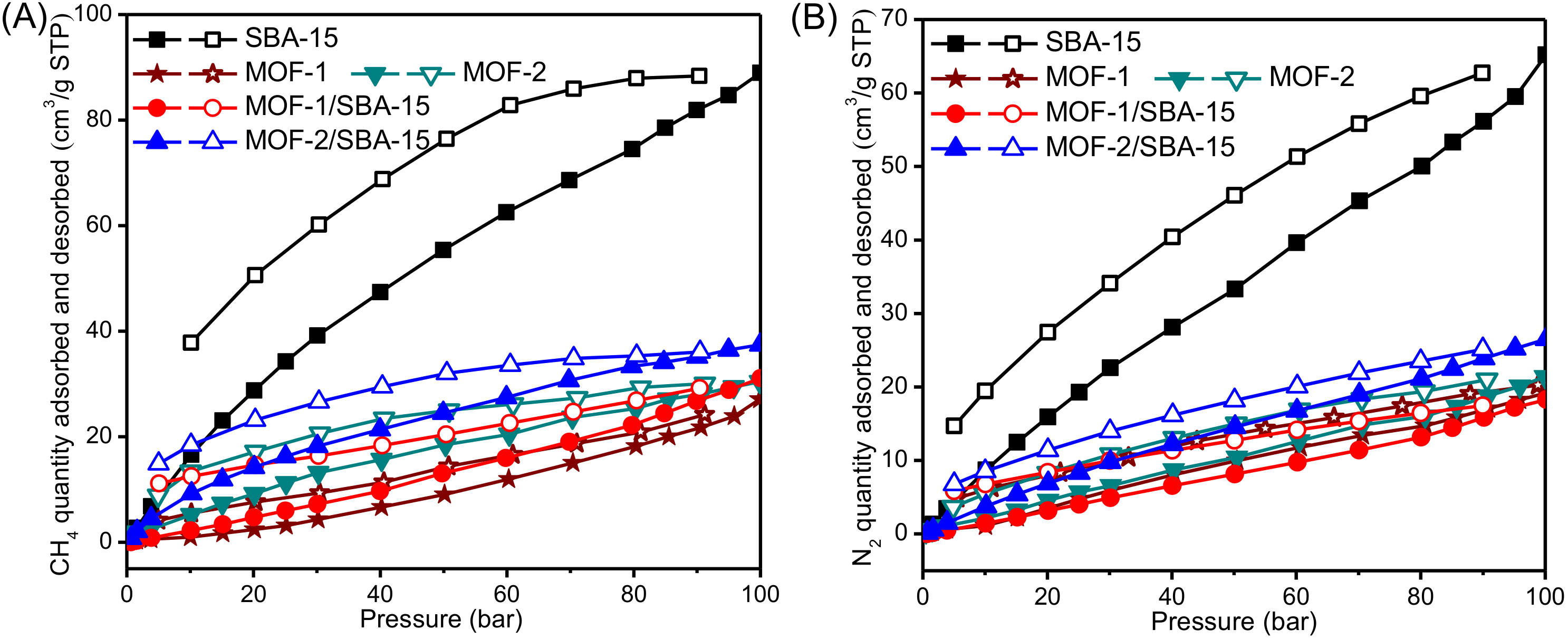
3.6. High Pressure Adsorption Isotherm
3.7. Determination of Adsorption Selectivity Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simon, C.M.; Kim, J.; Gomez-Gualdron, D.A.; Camp, J.S.; Chung, Y.G.; Martin, R.L.; Mercado, R.; Deem, M.W.; Gunter, D.; Haranczyk, M. The materials genome in action: Identifying the performance limits for methane storage. Energy Environ. Sci. 2015, 8, 1190–1199. [Google Scholar] [CrossRef]
- Kim, S.; Ko, D.; Row, S.; Kim, J. Techno-economic evaluation of hybrid systems of pressure swing adsorption and membrane processes for coalbed methane separation. Chem. Eng. Res. Des. 2016, 115, 230–240. [Google Scholar] [CrossRef]
- Zou, W.F. Proceedings of the 2016 6th International Conference on Applied Science, Engineering and Technology (ICASET). Adv. Eng. Res. 2016, 77, 359–362. [Google Scholar]
- Karakurt, I.; Aydin, G.; Aydiner, K. Mine ventilation air methane as a sustainable energy source. Renew. Sustain. Energy Rev. 2011, 15, 1042–1049. [Google Scholar] [CrossRef]
- Hao, X.F.; Hu, H.J.; Li, Z.; Wu, L.M.; Liu, X.Q.; Zhang, Y.N. Adsorption properties of modified clinoptilolite for methane and nitrogen. Materials 2018, 11, 2024. [Google Scholar] [CrossRef]
- Mehra, Y.R. Utilizing the Mehra Process for Processing and BTU Upgrading of Nitrogen-Rich Natural Gas Streams. US Patent 4623371, 18 November 1986. [Google Scholar]
- Li, P.Y.; Tezel, F.H. Adsorption separation of N2, O2, CO2 and CH4 gases by beta-zeolite. Microporous Mesoporous Mater. 2007, 98, 94–101. [Google Scholar] [CrossRef]
- Huang, Y.; Paul, D.R. Effect of film thickness on the gas-permeation characteristics of glassy polymer membranes. Ind. Eng. Chem. Res. 2007, 46, 2342–2347. [Google Scholar] [CrossRef]
- Mulgundmath, V.P.; Tezel, F.H.; Hou, F.; Golden, T.C. Binary adsorption behaviour of methane and nitrogen gases. J. Porous Mater. 2012, 19, 455–464. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006, 61, 3893–3906. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, Y.S.; Yang, X. Proportion pressure swing adsorption for low concentration coal mine methane enrichment. Sep. Sci. Technol. 2013, 48, 1201–1210. [Google Scholar] [CrossRef]
- Yi, H.H.; Li, F.R.; Ning, P.; Tang, X.L.; Peng, J.H.; Li, Y.D.; Deng, H. Adsorption separation of CO2, CH4, and N2 on microwave activated carbon. Chem. Eng. J. 2013, 215–216, 635–642. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of methane and nitrogen by adsorption on carbon molecular sieve. Sep. Sci. Technol. 2005, 40, 2721–2743. [Google Scholar] [CrossRef]
- Liu, B.; Smit, B. Comparative molecular simulation study of CO2/N2 and CH4/N2 separation in zeolites and metal-organic frameworks. Langmuir 2009, 25, 5918–5926. [Google Scholar] [CrossRef] [PubMed]
- Rufford, T.E.; Watson, G.C.Y.; Saleman, T.L.; Hofman, P.S.; Jensen, N.K.; May, E.F. Adsorption equilibria and kinetics of methane plus nitrogen mixtures on the activated carbon norit RB3. Ind. Eng. Chem. Res. 2013, 52, 14270–14281. [Google Scholar] [CrossRef]
- Saleman, T.L.H.; Watson, G.C.Y.; Rufford, T.E.; Hofman, P.S.; Chan, K.I.; May, E.F. Capacity and kinetic measurements of methane and nitrogen adsorption on H+-mordenite at 243–303 K and pressures to 900 kPa using a dynamic column breakthrough apparatus. Adsorption 2013, 19, 1165–1180. [Google Scholar] [CrossRef]
- Lu, W.G.; Wei, Z.W.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.W.; Zhang, Q.; Gentle, T.; et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.B.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef]
- Ma, S.Q.; Sun, D.F.; Wang, X.S.; Zhou, H.C. A mesh-adjustable molecular sieve for general use in gas separation. Angew. Chem. Int. Edit. 2007, 46, 2458–2462. [Google Scholar] [CrossRef]
- Senkovska, I.; Kaskel, S. High pressure methane adsorption in the metal-organic frameworks Cu3(btc)2, Zn2(bdc)2dabco, and Cr3F(H2O)2O(bdc)3. Microporous Mesoporous Mater. 2008, 112, 108–115. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, K.; Yang, Y.; Cui, Y.J.; Chen, B.L.; Qian, G.D. A novel Zn-based heterocycle metal-organic framework for high C2H2/C2H4, CO2/CH4 and CO2/N2 separations. J. Solid State Chem. 2017, 255, 102–107. [Google Scholar] [CrossRef]
- Sumer, Z.; Keskin, S. Adsorption- and membrane-based CH4/N2 separation performances of MOFs. Ind. Eng. Chem. Res. 2017, 56, 8713–8722. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, D.H.; Xu, J.; Hu, T.L.; Bu, X.H. Flexible metal-organic frameworks: Recent advances and potential applications. Adv. Mater. 2015, 27, 5432–5441. [Google Scholar] [CrossRef] [PubMed]
- Sayari, A.; Han, B.H.; Yang, Y. Simple synthesis route to monodispersed SBA-15 silica rods. J. Am. Chem. Soc. 2004, 10, 14348–14349. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.H.; Liu, L.; Li, C.M.; Xue, X.Y.; Liang, X.M. Facile synthesis of mesoporous SBA-15 silica spheres and its application for high-performance liquid chromatography. J. Colloid Interface Sci. 2009, 337, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Song, S.W.; Hidajat, K.; Kawi, S. Functionalized SBA-15 materials as carriers for controlled drug delivery: Influence of surface properties on matrix-drug interactions. Langmuir 2005, 21, 9568–9575. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.J. Synthesis, Characterization and Gas Adsorption Behaviors of Meso- and Microporous Molecular Sieves and Metal-Organic Framework Materials. Ph.D. Thesis, Beijing University of Technology, Beijing, China, 2012. [Google Scholar]
- Usami, Y.; Hongo, T.; Yamazaki, A. Thermal stability and behavior of platelet-shaped SBA-15 containing Zr. J. Porous Mater. 2012, 19, 897–902. [Google Scholar] [CrossRef]
- Wang, J.H.; Ge, H.G.; Bao, W.R. Synthesis and characteristics of SBA-15 with thick pore wall and high hydrothermal stability. Mater. Lett. 2015, 145, 312–315. [Google Scholar] [CrossRef]
- Yang, H.; Vovk, G.; Coombs, N.; Sokolov, I.; Ozin, G.A. Synthesis of mesoporous silica spheres under quiescent aqueous acidic conditions. J. Mater. Chem. 1998, 8, 743–750. [Google Scholar] [CrossRef]
- Martins, A.R.; Cunha, I.T.; Oliveira, A.A.S.; Moura, F.C.C. Highly ordered spherical SBA-15 catalysts for the removal of contaminants from the oil industry. Chem. Eng. J. 2017, 318, 189–196. [Google Scholar] [CrossRef]
- Sherino, B.; Mohamad, S.; Halim, S.N.A.; Manan, N.S.A. Electrochemical detection of hydrogen peroxide on a new microporous Ni–metal organic framework material-carbon paste electrode. Sens. Actuators B Chem. 2018, 254, 1148–1156. [Google Scholar] [CrossRef]
- Nakatsuka, K.; Yoshii, T.; Kuwahara, Y.; Mori, K.; Yamashita, H. Controlled pyrolysis of Ni-MOF-74 as a promising precursor for the creation of highly active Ni nanocatalysts in size-selective hydrogenation. Chem. Eur. J. 2018, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, S.H.; Zhu, K.; Kim, J.Y.; Neale, N.R.; Frank, A.J. Ni-NiO core-shell inverse opal electrodes for supercapacitors. Chem. Commun. 2011, 47, 5214–5216. [Google Scholar] [CrossRef] [PubMed]
- Arul, P.; John, S.A. Size controlled synthesis of Ni-MOF using polyvinylpyrrolidone: New electrode material for the trace level determination of nitrobenzene. J. Electroanal. Chem. 2018, 829, 168–176. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, Z.; Zhang, Y.; Lin, H.; Xu, Q. Highly selective detection of Pb2+ by a nanoscale Ni-based metal-organic framework fabricated through one-pot hydrothermal reaction. Sens. Actuators B Chem. 2017, 248, 430–436. [Google Scholar] [CrossRef]
- Liang, R.; Shen, L.; Jing, F.; Wu, W.; Qin, N.; Lin, R.; Wu, L. NH2-mediated indium metal-organic framework as a novel visible-light-driven photocatalyst for reduction of the aqueous Cr(VI). Appl. Catal. B Environ. 2015, 162, 245–251. [Google Scholar] [CrossRef]
- Rexer, T.F.T.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane adsorption on shale under simulated geological temperature and pressure conditions. Energy Fuel 2013, 27, 3099–3109. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems—With special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Leona, G.C.; Mata-Padilla, J.M.; García-Uriostegui, L. Synthesis of spherical SBA-15 mesoporous silica. Influence of reaction conditions on the structural order and stability. Ceram. Int. 2016, 42, 7564–7570. [Google Scholar] [CrossRef]
- Pang, J.B.; Qiu, K.Y.; Wei, Y. A new nonsurfactant pathway to mesoporous silica materials based on tartaric acid in conjunction with metallic chloride. Chem. Mater. 2001, 13, 2361–2365. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Pang, J.B.; Qiu, K.Y.; Wei, Y. Synthesis and characterization of mesoporous titania and silica-titania materials by urea templated sol-gel reactions. Microporous Mesoporous Mater. 2001, 49, 189–195. [Google Scholar] [CrossRef]
- Yang, R.T. Adsorbents: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Wu, H.; Simmons, J.M.; Liu, Y.; Brown, C.M.; Wang, X.S.; Ma, S. Metal-organic frameworks with exceptionally high methane uptake: Where and how is methane stored? Chem. Eur. J. 2010, 16, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bao, Z.B.; Jia, F.; Deng, S.G. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and Zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.S.; Guo, G.D.; Yang, X.; Li, Y.L.; Zhang, H.; Meng, Y. Selection experiment of adsorbent for coal-bed methane enrichment by pressure swing adsorption process. Min. Saf. Environ. Prot. 2010, 37, 4–8. [Google Scholar]


| Samples | Inorganic Component (wt %) | Organic Component (wt %) | ||||
|---|---|---|---|---|---|---|
| SiO2 | NiO | C | H | N | O | |
| SBA-15 | 100 | - | - | - | - | - |
| MOF-1/SBA-15 | 47.8 ± 0.07 | 14.0 ±0.02 | 21.5 ± 0.02 | 2.8 ± 0.005 | - | 13.9 ± 0.01 |
| MOF-2/SBA-15 | 43.3 ±0.06 | 13.0 ±0.01 | 23.5 ± 0.03 | 2.8 ± 0.004 | 4.0 ± 0.003 | 13.4 ± 0.02 |
| Samples | BET Surface Area (m2/g) | Average Pore Diameter (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| SBA-15 | 638 ± 9.6 | 3.0 ± 0.15 | 1.28 ± 0.09 |
| MOF-1 | 26 ± 1.3 | 3.6 ± 0.08 | 0.25 ± 0.04 |
| MOF-2 | 57 ± 2.1 | 4.2 ± 0.21 | 0.31 ± 0.03 |
| MOF-1/SBA-15 | 43 ± 2.3 | 4.0 ± 0.33 | 0.53 ± 0.04 |
| MOF-2/SBA-15 | 171 ± 3.5 | 3.1 ± 0.17 | 0.43 ± 0.02 |
| Samples | CH4 Adsorption | N2 Adsorption | ||
|---|---|---|---|---|
| P/P0 | VCH4 (mL/g) | P/P0 | VN2 (mL/g) | |
| SBA-15 | 0.49 | 0.87 | 0.50 | 0.45 |
| 0.70 | 1.24 | 0.70 | 0.62 | |
| 1.02 | 1.78 | 1.00 | 0.88 | |
| 1.29 | 2.24 | 1.32 | 1.15 | |
| 1.64 | 2.72 | 1.64 | 1.34 | |
| MOF-1 | 0.57 | 0.20 | 0.56 | 0.17 |
| 0.71 | 0.26 | 0.76 | 0.20 | |
| 1.08 | 0.28 | 1.10 | 0.24 | |
| 1.25 | 0.32 | 1.27 | 0.27 | |
| 1.52 | 0.37 | 1.53 | 0.29 | |
| MOF-2 | 0.55 | 1.42 | 0.51 | 0.30 |
| 0.71 | 1.54 | 0.76 | 0.38 | |
| 1.10 | 1.68 | 1.12 | 0.45 | |
| 1.26 | 1.72 | 1.28 | 0.50 | |
| 1.52 | 1.89 | 1.47 | 0.57 | |
| MOF-1/SBA-15 | 0.51 | 0.09 | 0.49 | 0.04 |
| 0.72 | 0.14 | 0.71 | 0.06 | |
| 1.11 | 0.22 | 1.01 | 0.08 | |
| 1.27 | 0.23 | 1.29 | 0.11 | |
| 1.66 | 0.28 | 1.66 | 0.14 | |
| MOF-2/SBA-15 | 0.50 | 0.65 | 0.50 | 0.19 |
| 0.69 | 0.92 | 0.71 | 0.27 | |
| 1.14 | 1.39 | 1.02 | 0.38 | |
| 1.33 | 1.69 | 1.23 | 0.46 | |
| 1.63 | 2.11 | 1.66 | 0.59 | |
| Samples | Adsorption Equilibrium Amount (mL/g) | |||
|---|---|---|---|---|
| At an Adsorption Pressure of 150 kPa | At a Desorption Pressure of 50 kPa | |||
| VCH4 | VN2 | VCH4 | VN2 | |
| SBA-15 | 2.56 | 1.30 | 1.23 | 0.58 |
| MOF-1 | 0.35 | 0.29 | 0.21 | 0.18 |
| MOF-2 | 1.82 | 0.54 | 0.49 | 0.41 |
| MOF-1/SBA-15 | 0.28 | 0.13 | 0.15 | 0.07 |
| MOF-2/SBA-15 | 1.89 | 0.55 | 0.90 | 0.24 |
| Parameters related to the adsorption selectivity | ||||
| αCH4/N2 | WCH4/N2 | SCH4/N2 | Reference | |
| SBA-15 | 1.99 | 1.84 | 3.66 | |
| MOF-1 | 1.12 | 1.39 | 1.56 | |
| MOF-2 | 2.66 | 1.93 | 5.13 | |
| MOF-1/SBA-15 | 2.17 | 2.19 | 4.75 | |
| MOF-2/SBA-15 | 3.44 | 3.24 | 11.1 | |
| MOF-5 | 1.13 | - | 0.67 | [46] |
| MOF-177 | 4.00 | - | 8.45 | [46] |
| Zeolite 5A | 0.94 | - | 0.81 | [46] |
| Activated carbon | 4.60 | - | 4.02 | [47] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Ding, W.; Lei, S.; Tian, X.; Zhou, F. Selective Adsorption of CH4/N2 on Ni-based MOF/SBA-15 Composite Materials. Nanomaterials 2019, 9, 149. https://doi.org/10.3390/nano9020149
Liu H, Ding W, Lei S, Tian X, Zhou F. Selective Adsorption of CH4/N2 on Ni-based MOF/SBA-15 Composite Materials. Nanomaterials. 2019; 9(2):149. https://doi.org/10.3390/nano9020149
Chicago/Turabian StyleLiu, Hong, Wei Ding, Shaohua Lei, Xupei Tian, and Fubao Zhou. 2019. "Selective Adsorption of CH4/N2 on Ni-based MOF/SBA-15 Composite Materials" Nanomaterials 9, no. 2: 149. https://doi.org/10.3390/nano9020149
APA StyleLiu, H., Ding, W., Lei, S., Tian, X., & Zhou, F. (2019). Selective Adsorption of CH4/N2 on Ni-based MOF/SBA-15 Composite Materials. Nanomaterials, 9(2), 149. https://doi.org/10.3390/nano9020149