Core Nanoparticle Engineering for Narrower and More Intense Band-Edge Emission from AgInS2/GaSx Core/Shell Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumental
2.3. Synthesis of AgInS2 Core Nanoparticles by the Immediate Injection Method
2.4. Synthesis of AgInS2 Core NPs by the Dropwise Injection Method
2.5. GaSx Shell Formation on AgInS2 Core NPs
3. Results and Discussion
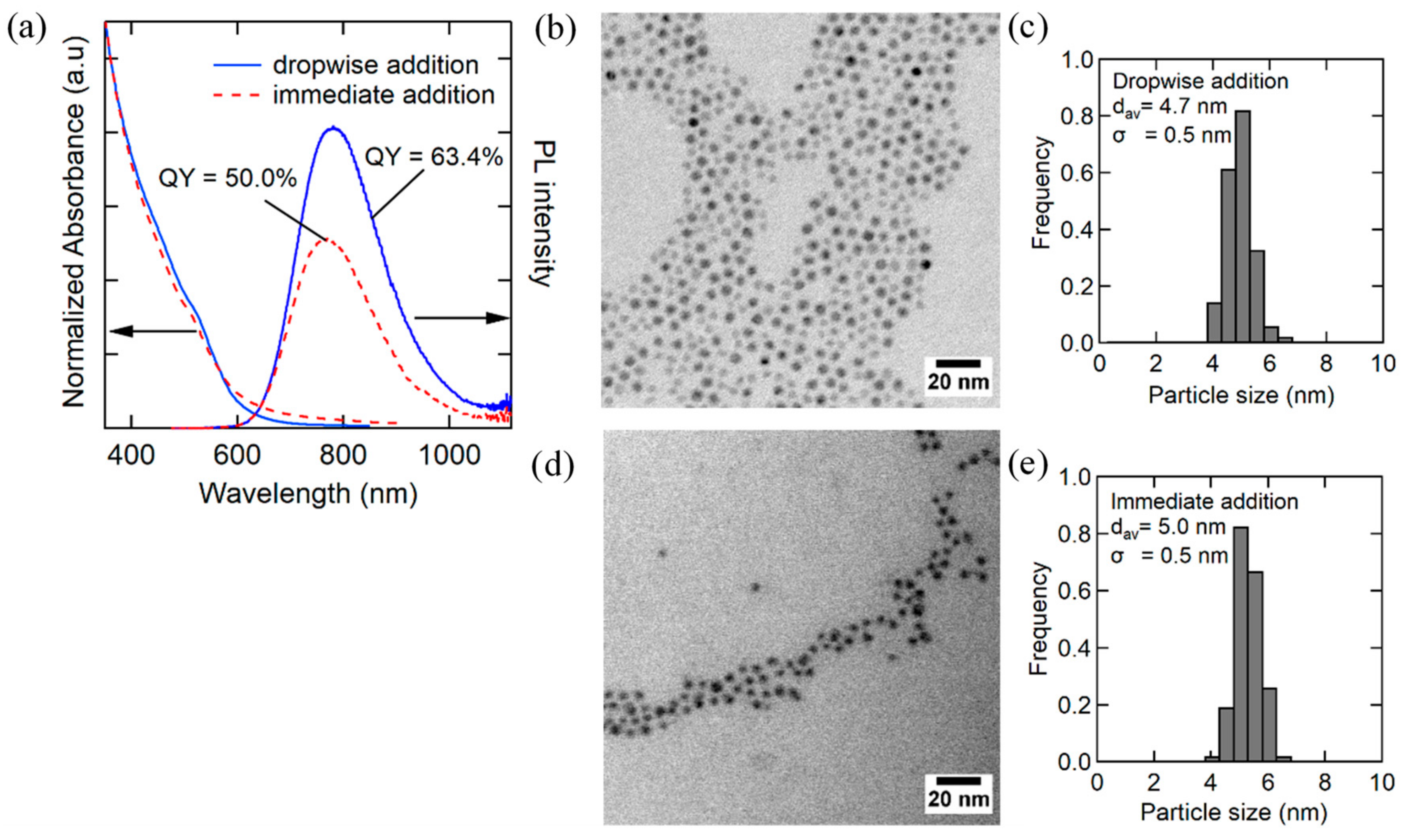
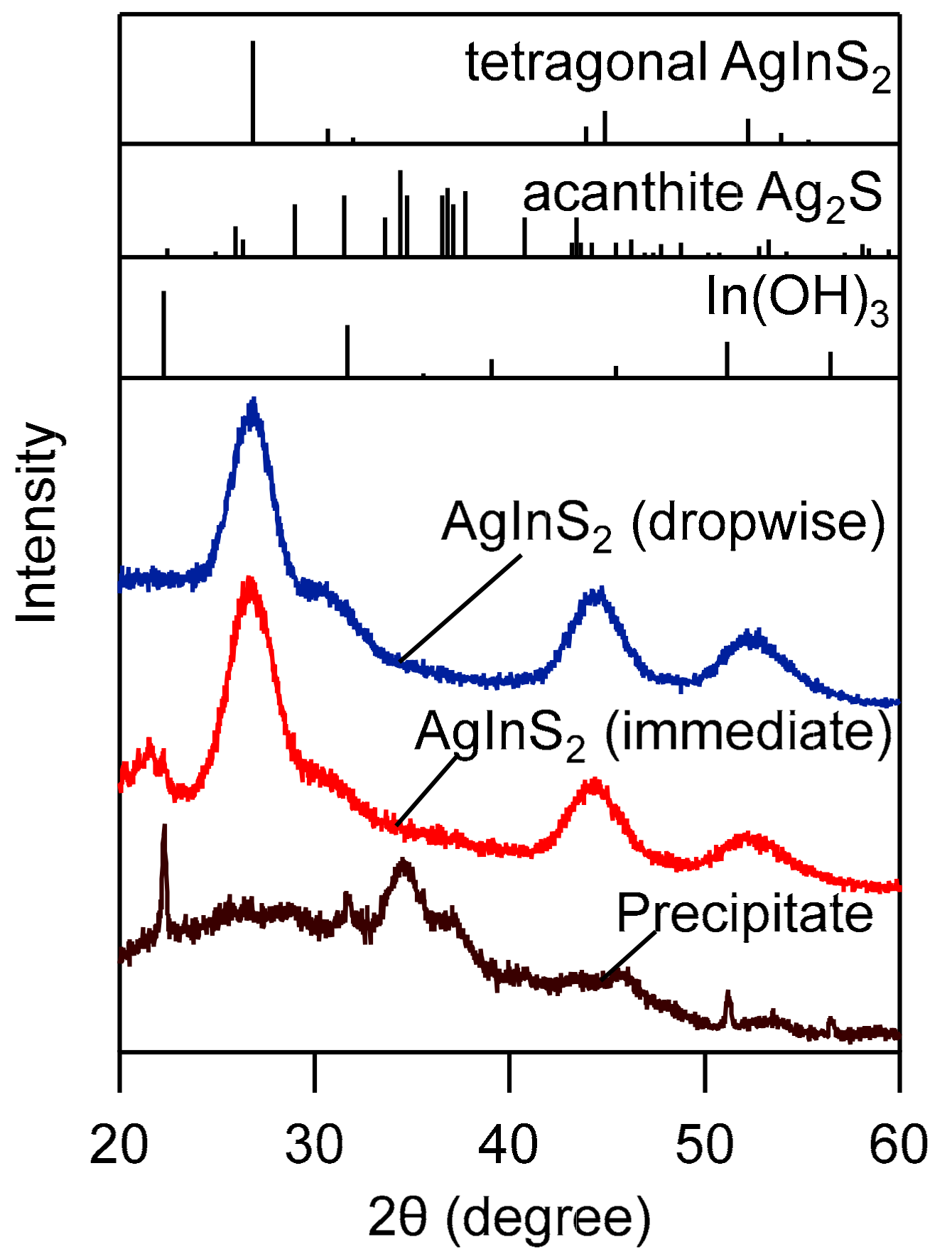
3.1. Synthesis of High-Quality AgInS2 Quantum Dots
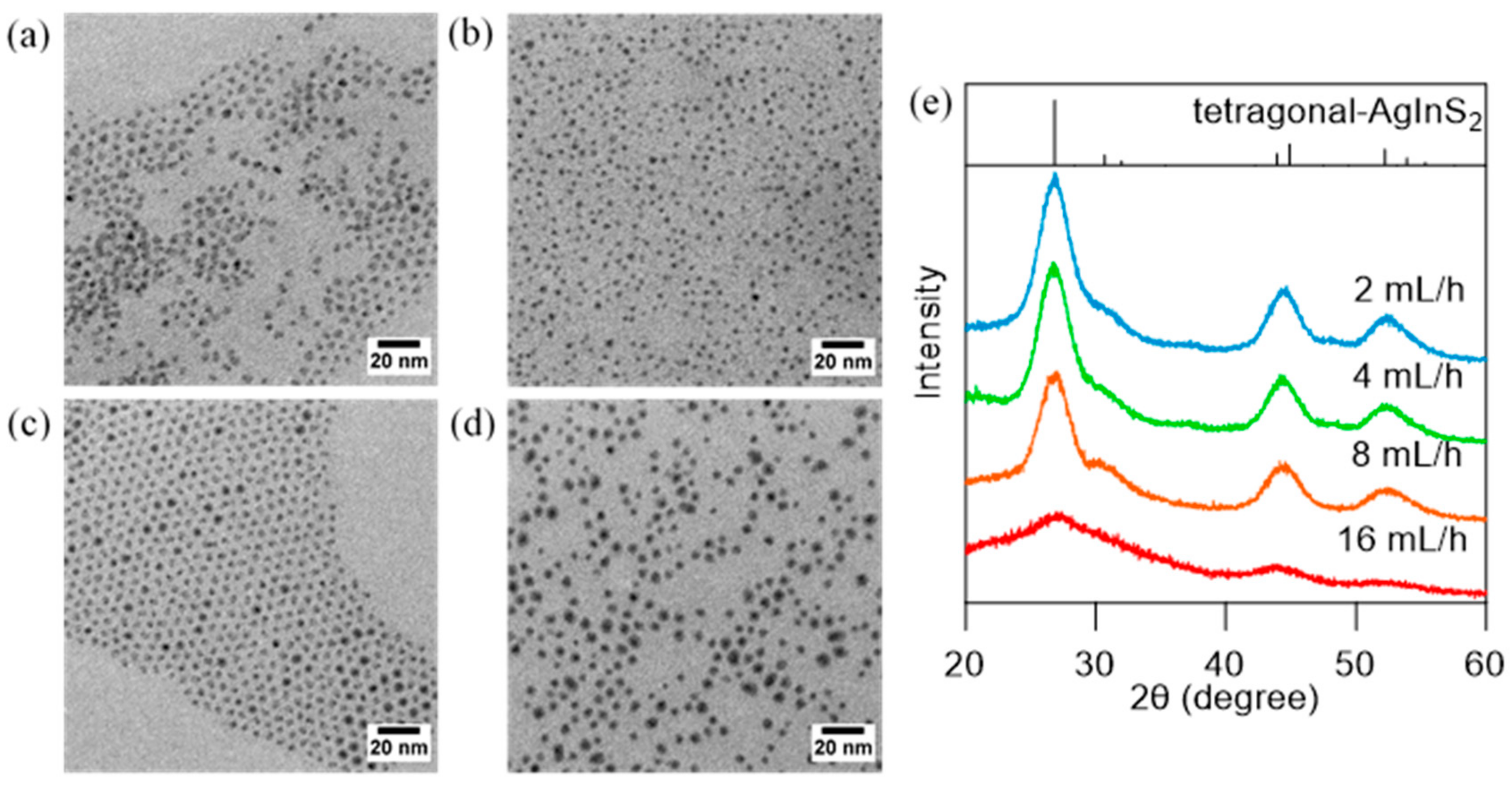
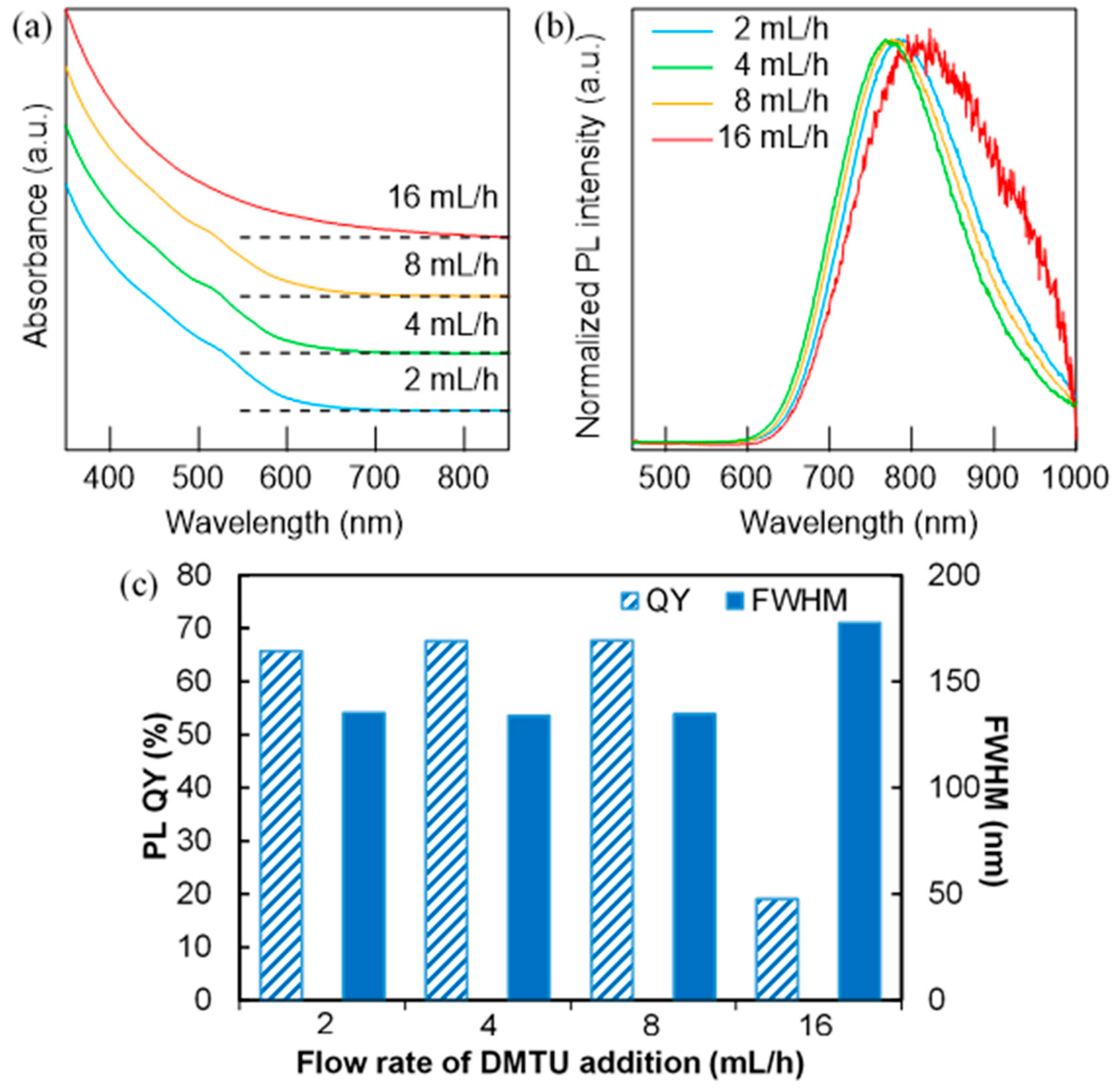
3.2. Effect of Injection Rate on the Dropwise Injection Method
3.3. Effect of Temperature on the Generation of AgInS2 QDs by DMTU Injection
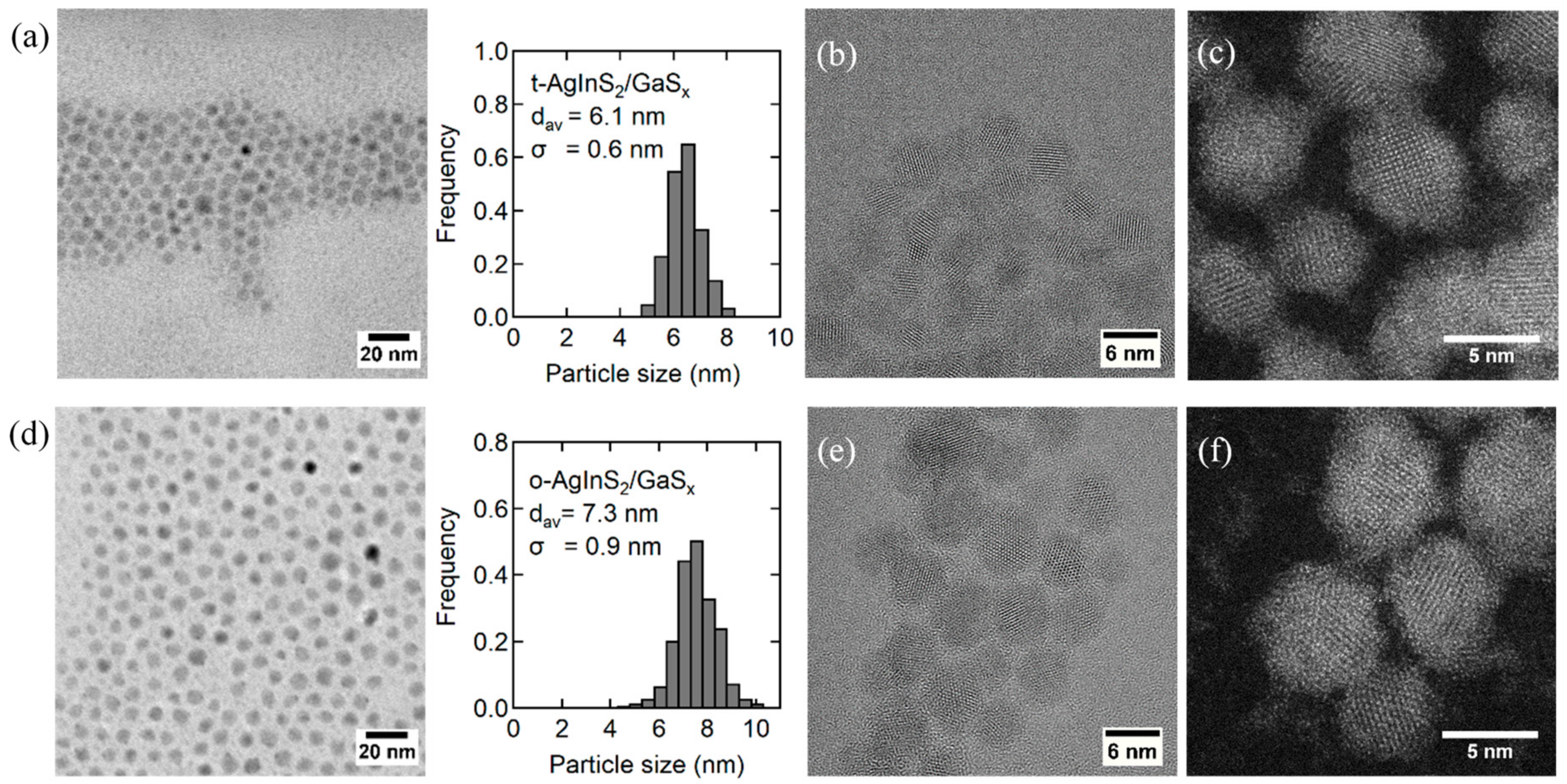
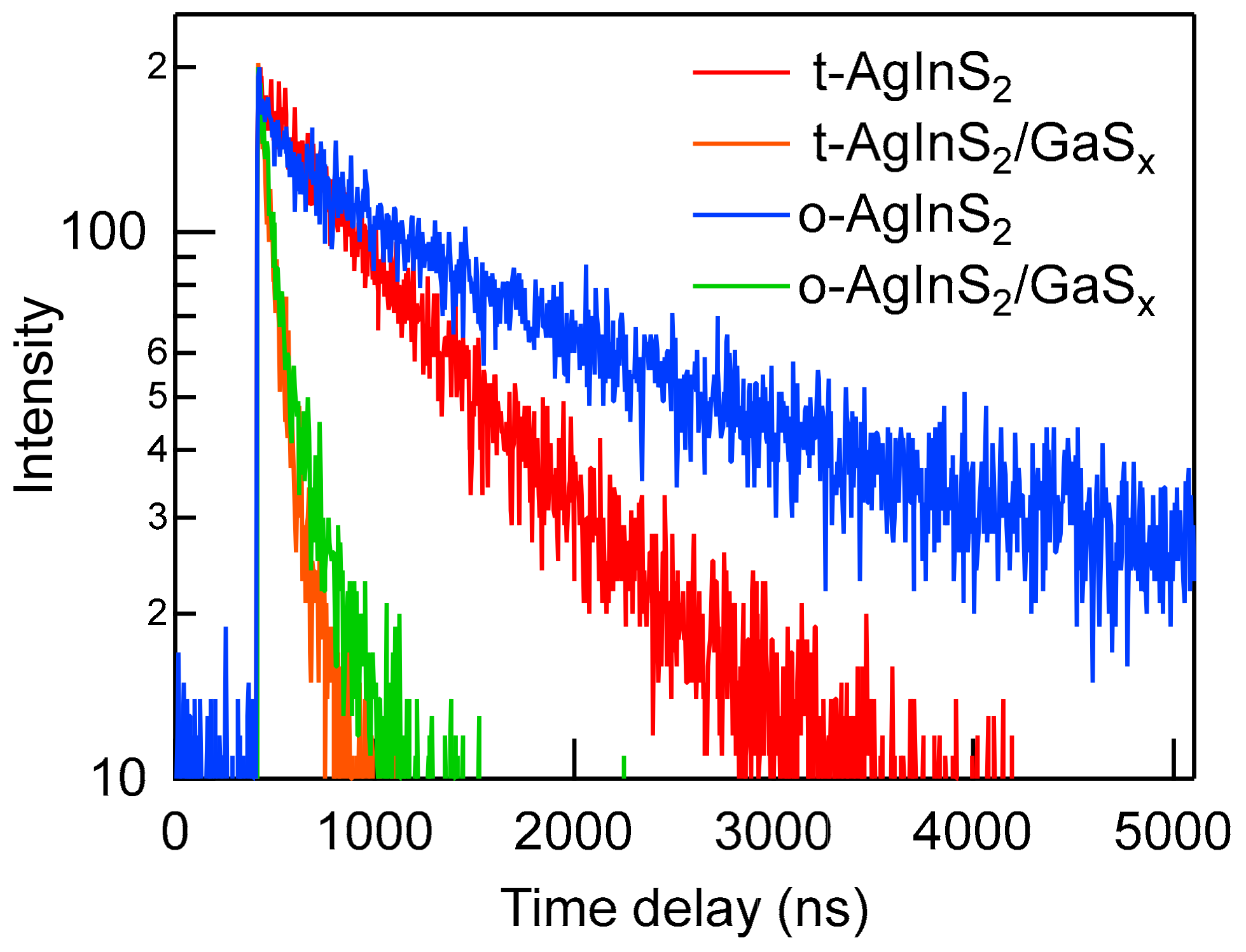
3.4. Band-Edge Emission by Surface Passivation with Gallium Sulfides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Zlateva, G.; Zhelev, Z.; Bakalova, R.; Kanno, I. Precise size control and synchronized synthesis of zhelev six colors of CdSe quantum dots in a slow-increasing temperature gradient. Inorg. Chem. 2007, 46, 6212–6214. [Google Scholar] [CrossRef] [PubMed]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Stewart, M.H.; Trammell, S.A.; Susumu, K.; Delehanty, J.B.; Mei, B.C.; Melinger, J.S.; Blanco-Canosa, J.B.; Dawson, P.E.; Mattoussi, H. Quantum-dot/dopamine bioconjugates function as redox coupled assemblies for in vitro and intracellular pH sensing. Nat. Mater. 2010, 9, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Nozik, A.J.; Beard, M.C.; Luther, J.M.; Law, M.; Ellingson, R.J.; Johnson, J.C. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem. Rev. 2010, 110, 6873–6890. [Google Scholar] [CrossRef]
- Neves, M.C.; Martins, M.A.; Soares-Santos, P.C.R.; Rauwel, P.; Ferreira, R.A.S.; Monteiro, T.; Carlos, L.D.; Trindade, T. Photoluminescent, transparent and flexible di-ureasil hybrids containing CdSe/ZnS quantum dots. Nanotechnology 2008, 19, 155601. [Google Scholar] [CrossRef]
- Pereira, A.S.; Rauwel, P.; Reis, M.S.; Oliveira Silva, N.J.; Barros-Timmons, A.; Trindade, T. Polymer encapsulation effects on the magnetism of EuS nanocrystals. J. Mater. Chem. 2008, 18, 4572–4578. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, B.-Y.; Jang, E.-P.; Yoon, S.-Y.; Kim, K.-H.; Do, Y.R.; Yang, H. Synthesis of widely emission-tunable Ag–Ga–S and its quaternary derivative quantum dots. Chem. Eng. J. 2018, 347, 791–797. [Google Scholar] [CrossRef]
- Xie, R.; Rutherford, M.; Peng, X. Formation of high-quality I−III−VI Semiconductor nanocrystals by tuning relative reactivity of cationic precursors. J. Am. Chem. Soc. 2009, 131, 5691–5697. [Google Scholar] [CrossRef]
- Yarema, O.; Yarema, M.; Wood, V. Tuning the composition of multicomponent semiconductor nanocrystals: The case of I–III–VI materials. Chem. Mater. 2018, 30, 1446–1461. [Google Scholar] [CrossRef]
- Allen, P.M.; Bawendi, M.G. Ternary I−III−VI quantum dots luminescent in the red to near-infrared. J. Am. Chem. Soc. 2008, 130, 9240–9241. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Daou, T.J.; Texier, I.; Kim Chi, T.T.; Liem, N.Q.; Reiss, P. Highly luminescent CuInS2/ZnS core/shell nanocrystals: Cadmium-free quantum dots for in vivo imaging. Chem. Mater. 2009, 21, 2422–2429. [Google Scholar] [CrossRef]
- Yang, W.; Gong, X.; Chang, J. Development of novel cadmium-free AgInS2 semiconductor nanoparticles. J. Nanosci. Nanotechnol. 2016, 16, 2172–2183. [Google Scholar] [CrossRef]
- Torimoto, T.; Adachi, T.; Okazaki, K.-I.; Sakuraoka, M.; Shibayama, T.; Ohtani, B.; Kudo, A.; Kuwabata, S. Facile synthesis of ZnS−AgInS2 solid solution nanoparticles for a color-adjustable luminophore. J. Am. Chem. Soc. 2007, 129, 12388–12389. [Google Scholar] [CrossRef]
- Regulacio, M.D.; Win, K.Y.; Lo, S.L.; Zhang, S.-Y.; Zhang, X.; Wang, S.; Han, M.-Y.; Zheng, Y. Aqueous synthesis of highly luminescent AgInS2-ZnS quantum dots and their biological applications. Nanoscale 2013, 5, 2322–2327. [Google Scholar] [CrossRef]
- Uematsu, T.; Doi, T.; Torimoto, T.; Kuwabata, S. Preparation of luminescent AgInS2−AgGaS2 solid solution nanoparticles and their optical properties. J. Phys. Chem. Lett. 2010, 1, 3283–3287. [Google Scholar] [CrossRef]
- Torimoto, T.; Ogawa, S.; Adachi, T.; Kameyama, T.; Okazaki, K.-I.; Shibayama, T.; Kudo, A.; Kuwabata, S. Remarkable photoluminescence enhancement of ZnS–AgInS2 solid solution nanoparticles by post-synthesis treatment. Chem. Commun. 2010, 46, 2082–2084. [Google Scholar] [CrossRef]
- Feng, Z.; Dai, P.; Ma, X.; Zhan, J.; Lin, Z. Monodispersed cation-disordered cubic AgInS2 nanocrystals with enhanced fluorescence. Appl. Phys. Lett. 2010, 96, 013104. [Google Scholar] [CrossRef]
- Castro, S.L.; Bailey, S.G.; Raffaelle, R.P.; Banger, K.K.; Hepp, A.F. Synthesis and characterization of colloidal CuInS2 nanoparticles from a molecular single-source precursor. J. Phys. Chem. B 2004, 108, 12429–12435. [Google Scholar] [CrossRef]
- Chen, S.; Ahmadiantehrani, M.; Zhao, J.; Zhu, S.; Mamalis, A.G.; Zhu, X. Heat-up synthesis of Ag–In–S and Ag–In–S/ZnS nanocrystals: Effect of indium precursors on their optical properties. J. Alloy. Compd. 2016, 665, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Reiss, P.; Carrière, M.; Lincheneau, C.; Vaure, L.; Tamang, S. Synthesis of semiconductor nanocrystals, focusing on nontoxic and earth-abundant materials. Chem. Rev. 2016, 116, 10731–10819. [Google Scholar] [CrossRef] [PubMed]
- Brichkin, S.B.; Razumov, V.F. Colloidal quantum dots: Synthesis, properties and applications. Russ. Chem. Rev. 2016, 85, 1297. [Google Scholar] [CrossRef]
- Chang, J.; Waclawik, E.R. Colloidal semiconductor nanocrystals: Controlled synthesis and surface chemistry in organic media. RSC Adv. 2014, 4, 23505–23527. [Google Scholar] [CrossRef]
- Gabka, G.; Bujak, P.; Giedyk, K.; Ostrowski, A.; Malinowska, K.; Herbich, J.; Golec, B.; Wielgus, I.; Pron, A. A simple route to alloyed quaternary nanocrystals Ag–In–Zn–S with shape and size control. Inorg. Chem. 2014, 53, 5002–5012. [Google Scholar] [CrossRef]
- Gabka, G.; Bujak, P.; Kotwica, K.; Ostrowski, A.; Lisowski, W.; Sobczak, J.W.; Pron, A. Luminophores of tunable colors from ternary Ag-In-S and quaternary Ag-In-Zn-S nanocrystals covering the visible to near-infrared spectral range. Phys. Chem. Chem. Phys. 2017, 19, 1217–1228. [Google Scholar] [CrossRef]
- Uematsu, T.; Wajima, K.; Sharma, D.K.; Hirata, S.; Yamamoto, T.; Kameyama, T.; Vacha, M.; Torimoto, T.; Kuwabata, S. Narrow band-edge photoluminescence from AgInS2 semiconductor nanoparticles by the formation of amorphous III–VI semiconductor shells. NPG Asia Mater. 2018, 10, 713–726. [Google Scholar] [CrossRef]
- Kameyama, T.; Kishi, M.; Miyamae, C.; Sharma, D.K.; Hirata, S.; Yamamoto, T.; Uematsu, T.; Vacha, M.; Kuwabata, S.; Torimoto, T. Wavelength-tunable band-edge photoluminescence of nonstoichiometric Ag–In–S nanoparticles via Ga3+ doping. ACS Appl. Mater. Interface. 2018, 10, 42844–42855. [Google Scholar] [CrossRef]
- Shang, H.; Di, Q.; Ji, M.; Bai, B.; Liu, J.; Chen, W.; Xu, M.; Rong, H.; Liu, J.; Zhang, J. From indium-doped Ag2S to AgInS2 nanocrystals: Low-temperature in situ conversion of colloidal Ag2S nanoparticles and their nir fluorescence. Chem. Eur. J. 2018, 24, 13676–13680. [Google Scholar] [CrossRef]
- Doh, H.; Hwang, S.; Kim, S. Size-Tunable Synthesis of nearly monodisperse Ag2S nanoparticles and size-dependent fate of the crystal structures upon cation exchange to AgInS2 nanoparticles. Chem. Mater. 2016, 28, 8123–8127. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Wang, G.-Q.; Cheng, C.-Y.; Lin, W.-X.; Hsu, J.-C. Strategies for photoluminescence enhancement of AgInS2 quantum dots and their application as bioimaging probes. J. Mater. Chem. 2012, 22, 10609–10618. [Google Scholar] [CrossRef]
- Du, Y.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, Q. near-infrared photoluminescent Ag2S quantum dots from a single source precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, R.; Abbastabar Ahangar, H.; Zakaria, A.; Zamiri, G.; Shabani, M.; Singh, B.; Ferreira, J.M.F. The structural and optical constants of Ag2S semiconductor nanostructure in the Far-Infrared. Chem. Cent. J. 2015, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xu, Z. Controllable growth of semiconductor heterostructures mediated by bifunctional Ag2S nanocrystals as catalyst or source-host. J. Am. Chem. Soc. 2011, 133, 148–157. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.-Y. Synthetic strategies and biomedical applications of I–III–VI ternary quantum dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
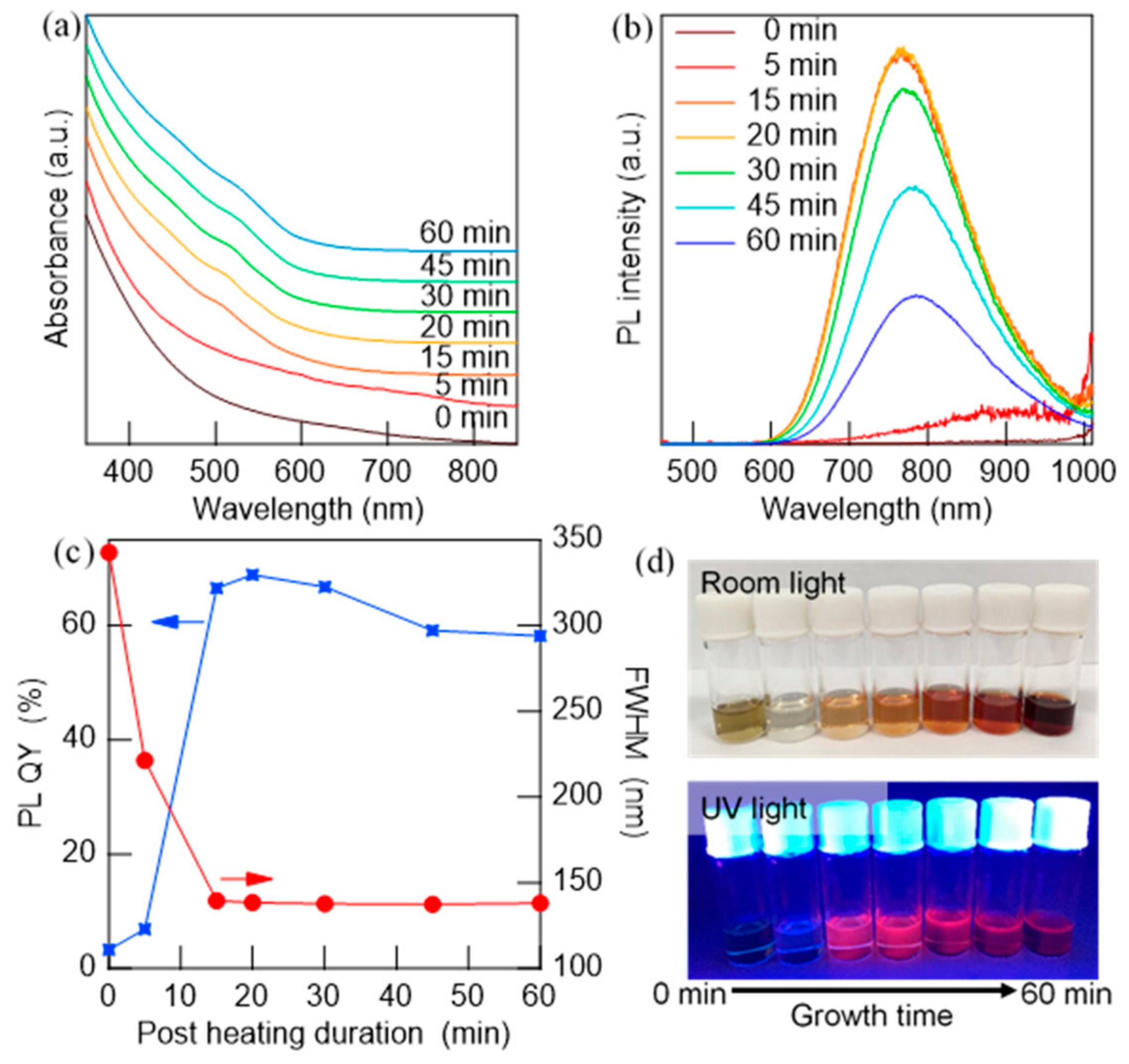
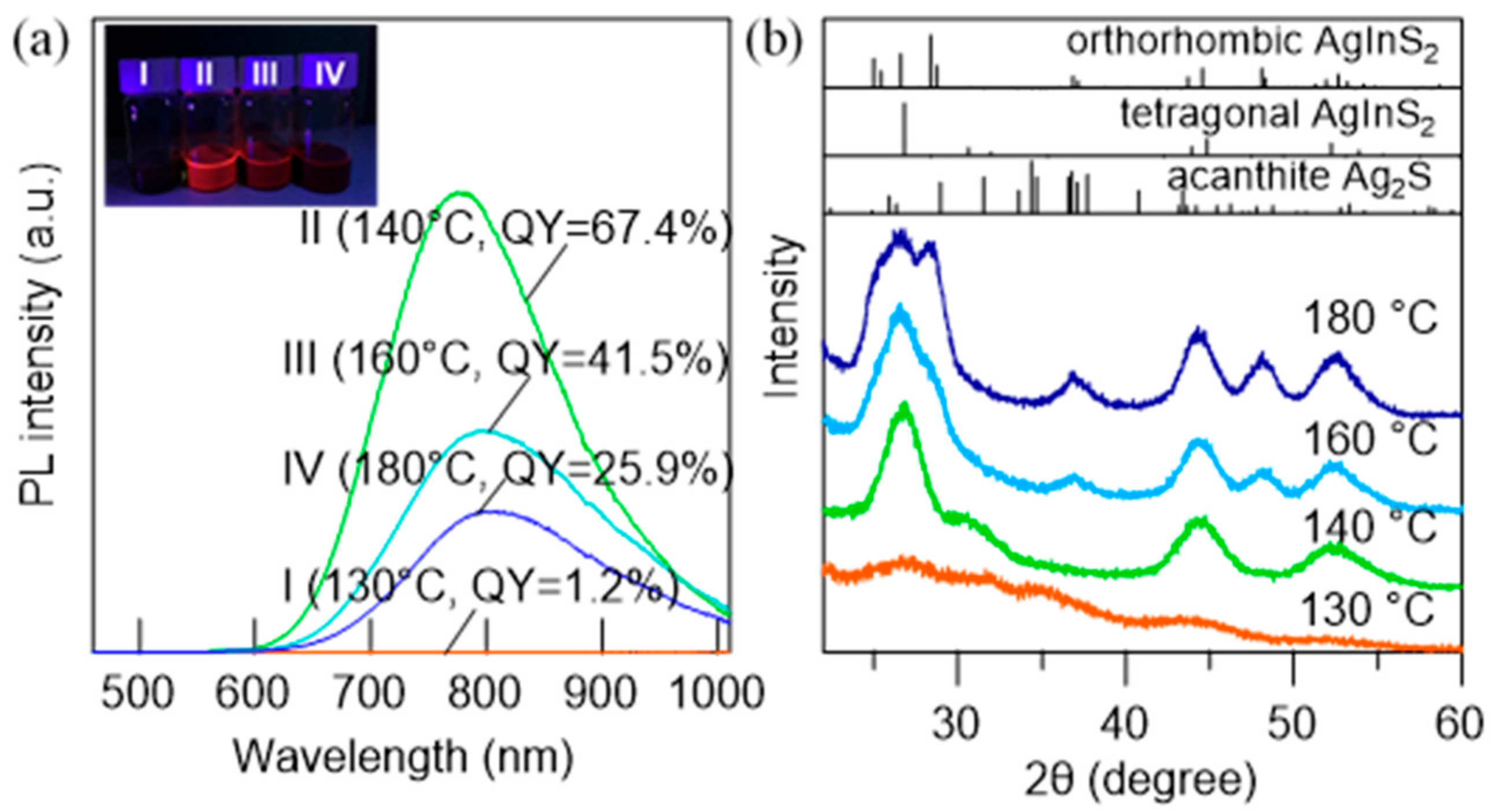
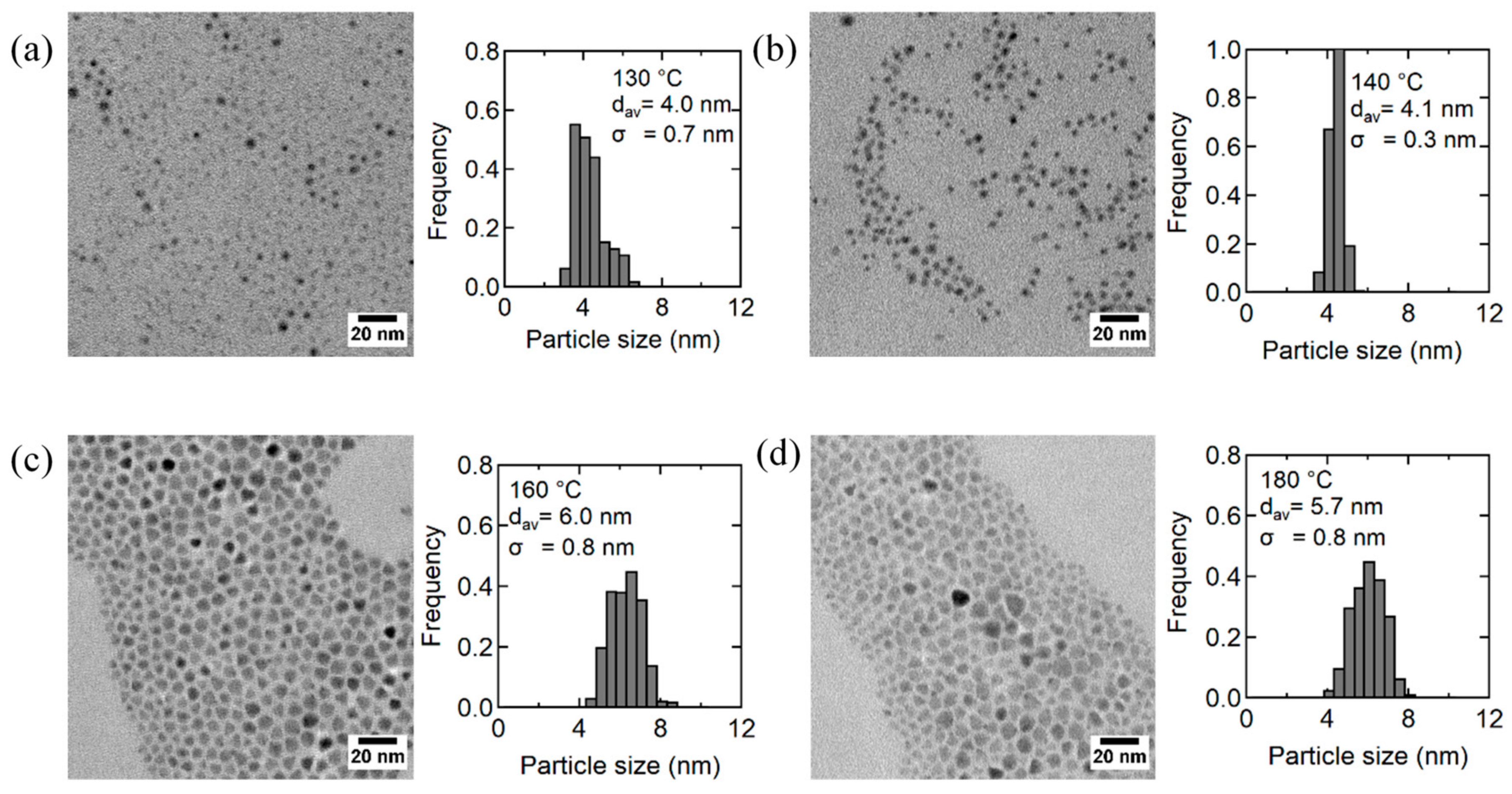
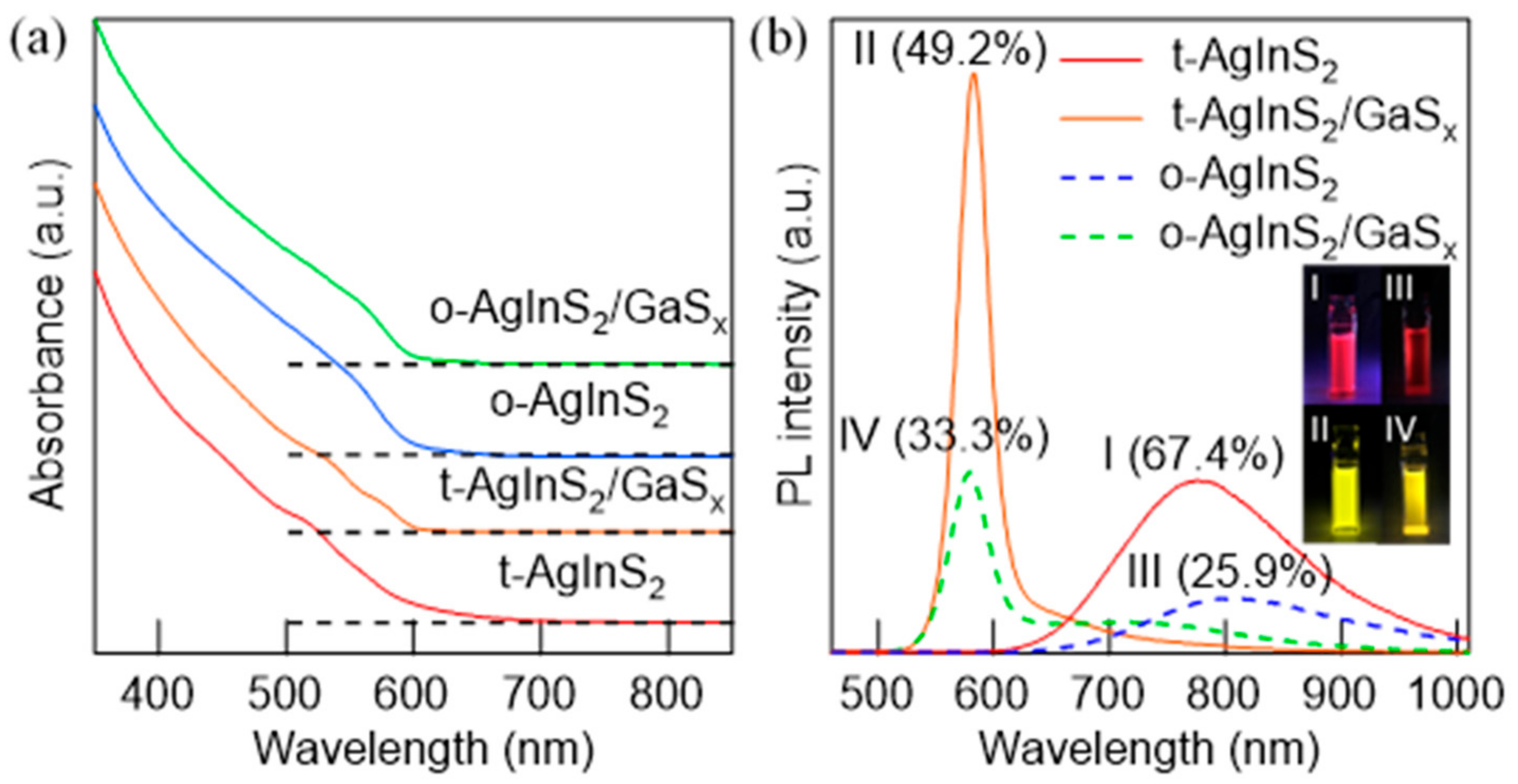
| Reaction Temperature (°C) | Measured Atomic Ratios | PL QY (%) | FWHM (nm) | ||
|---|---|---|---|---|---|
| Ag | In | S | |||
| 130 | 1 | 0.74 | 2.40 | 1.2 | 259.4 |
| 140 | 1 | 1.11 | 2.46 | 67.4 | 137.1 |
| 160 | 1 | 1.05 | 2.30 | 41.5 | 143.5 |
| 180 | 1 | 0.98 | 2.11 | 25.9 | 142.9 |
| NPs a | λPL/nm | τ1/ns | A1 | τ2/ns | A2 | χ2 |
|---|---|---|---|---|---|---|
| t-AgInS2 | 780 | 876 | 1 | – | – | 1.08 |
| t-AgInS2/GaSx | 582 | 37.2 | 0.777 | 159 | 0.23 | 0.98 |
| o-AgInS2 | 803 | 278 | 0.29 | 1976 | 0.71 | 1.06 |
| o-AgInS2/GaSx | 578 | 33.4 | 0.69 | 161 | 0.31 | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoisang, W.; Uematsu, T.; Yamamoto, T.; Torimoto, T.; Kuwabata, S. Core Nanoparticle Engineering for Narrower and More Intense Band-Edge Emission from AgInS2/GaSx Core/Shell Quantum Dots. Nanomaterials 2019, 9, 1763. https://doi.org/10.3390/nano9121763
Hoisang W, Uematsu T, Yamamoto T, Torimoto T, Kuwabata S. Core Nanoparticle Engineering for Narrower and More Intense Band-Edge Emission from AgInS2/GaSx Core/Shell Quantum Dots. Nanomaterials. 2019; 9(12):1763. https://doi.org/10.3390/nano9121763
Chicago/Turabian StyleHoisang, Watcharaporn, Taro Uematsu, Takahisa Yamamoto, Tsukasa Torimoto, and Susumu Kuwabata. 2019. "Core Nanoparticle Engineering for Narrower and More Intense Band-Edge Emission from AgInS2/GaSx Core/Shell Quantum Dots" Nanomaterials 9, no. 12: 1763. https://doi.org/10.3390/nano9121763
APA StyleHoisang, W., Uematsu, T., Yamamoto, T., Torimoto, T., & Kuwabata, S. (2019). Core Nanoparticle Engineering for Narrower and More Intense Band-Edge Emission from AgInS2/GaSx Core/Shell Quantum Dots. Nanomaterials, 9(12), 1763. https://doi.org/10.3390/nano9121763






