Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
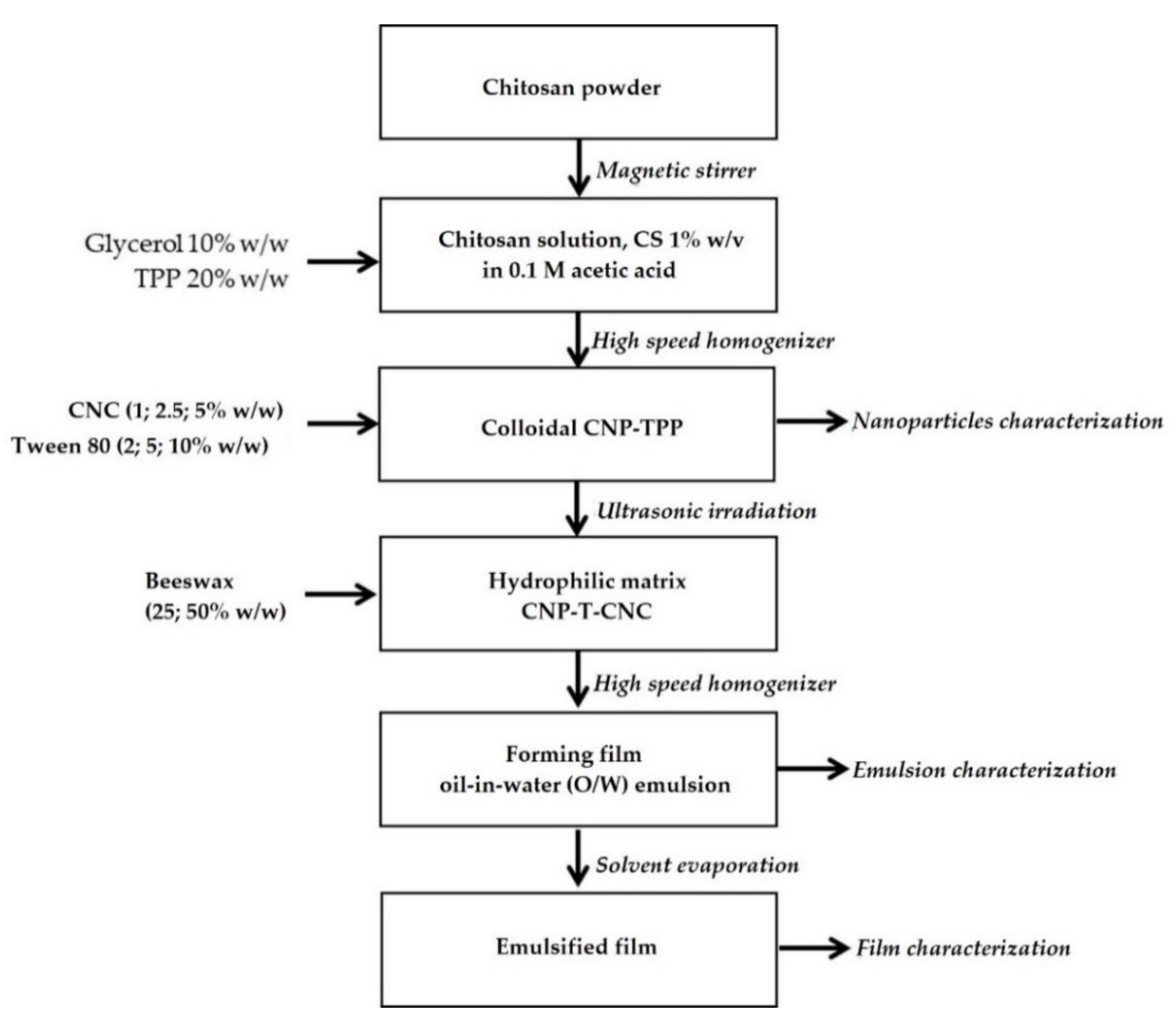
2.2. Preparation of Hydrophilic Matrix CNP–TPP–CNC
Preparation of Hydrophilic Matrix CNP–TPP–CNC
- Chitosan solution, CS (1% w/v), was prepared by dispersing chitosan powder to 0.1 M of the acetic acid solution while continuously stirring at 300 rpm using magnetic stirrer at room temperature for 60 min. The resulting CS was then filtered using Whatman filter paper no. 1 to remove the impurities.
- Colloidal CNP–TPP was synthesized by adaptation ionic cross-linking method as described by Bao et al., 2008 [51,52]. The process was carried out under constant high-speed stirring at 10,000 rpm rate, 50 °C for 5 min using rotor-stator (POLYTRON PT-3100D-Kinematica, Swiss, Kloten, Switzerland). Glycerol, 10% w/w on a dry basis, was added previously to the CS as a plasticizer. The CNP was spontaneously obtained when TPP solution, 20% w/w on a dry basis of chitosan, was added drop-wise into the CS. Upon completion of the reaction, the cross-linked CNP–TPP was cooled at ambient temperature.
- Hydrophilic matrix CNP–T–CNC was carried out under ultrasonic irradiation using an ultrasonic processor (Vibra Cell, Type 72434, 100 Watts, horn diameter: 1.0 mm, Fisher Scientific, Illkirch-Graffenstaden, France). The CNC solution, 10% w/w on a dry basis of chitosan, was introduced into the colloidal CNP, and then kept at room temperature for 30 min under magnetic stirring (1000 rpm).
2.3. Preparation of Film Forming Emulsions
2.4. Nanoparticles and Film Characterizations
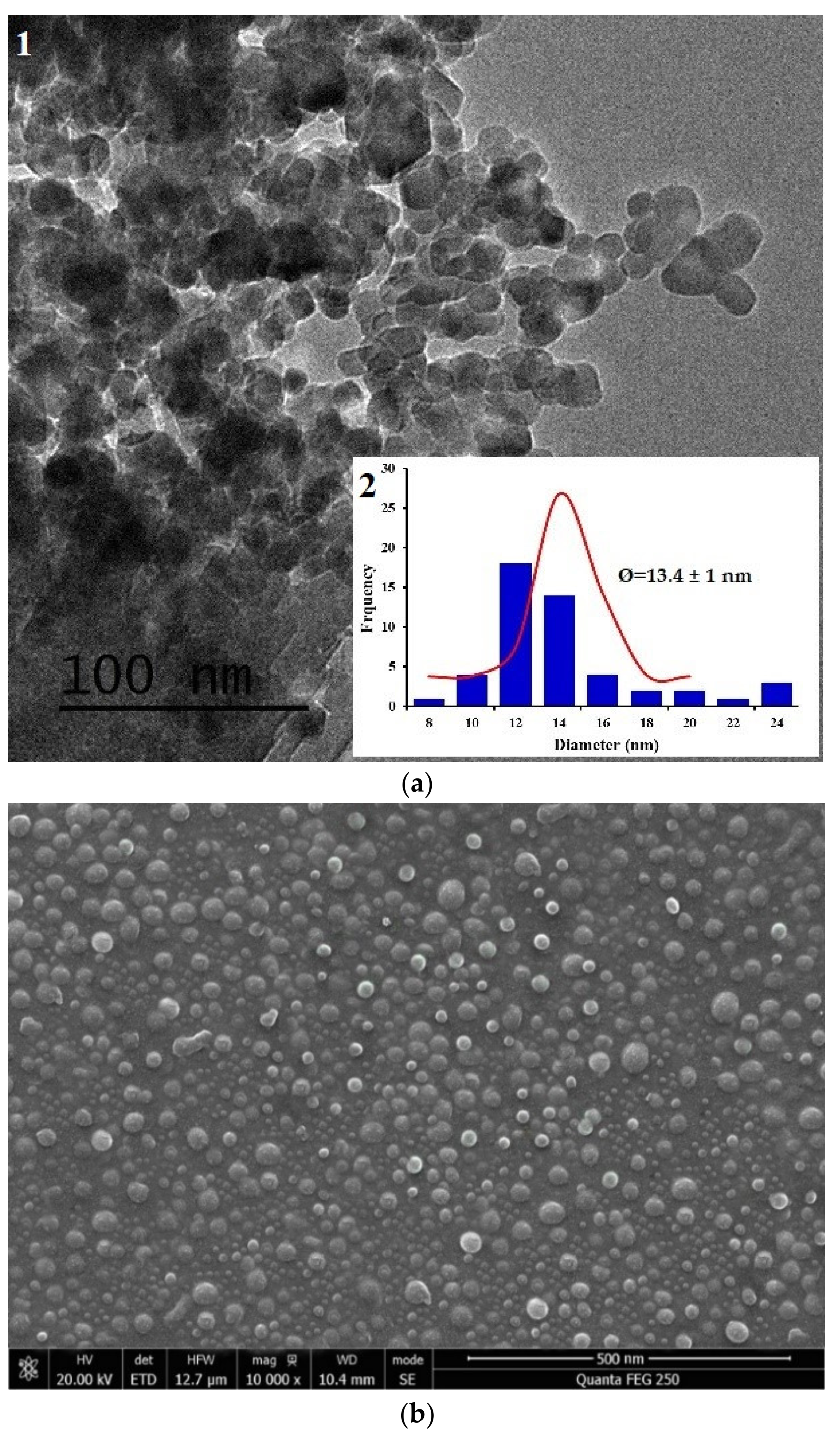
- Morphology of the chitosan nanoparticle, CNP–TPP was observed using high-resolution transmission electron microscopy, TEM (JEOL-2100F, JEOL Ltd, Tokyo, Japan) and scanning electron microscopy, SEM (JEOL-2100F, JEOL Ltd, Japan). The sample was deposited on carbon-coated copper grids and the negative staining was achieved using uranyLess solution (Delta Microscopies, Mauressac, France). The size and diameter of the particles were measured by Image J (version 1.41 h) and origin pro-8 software.
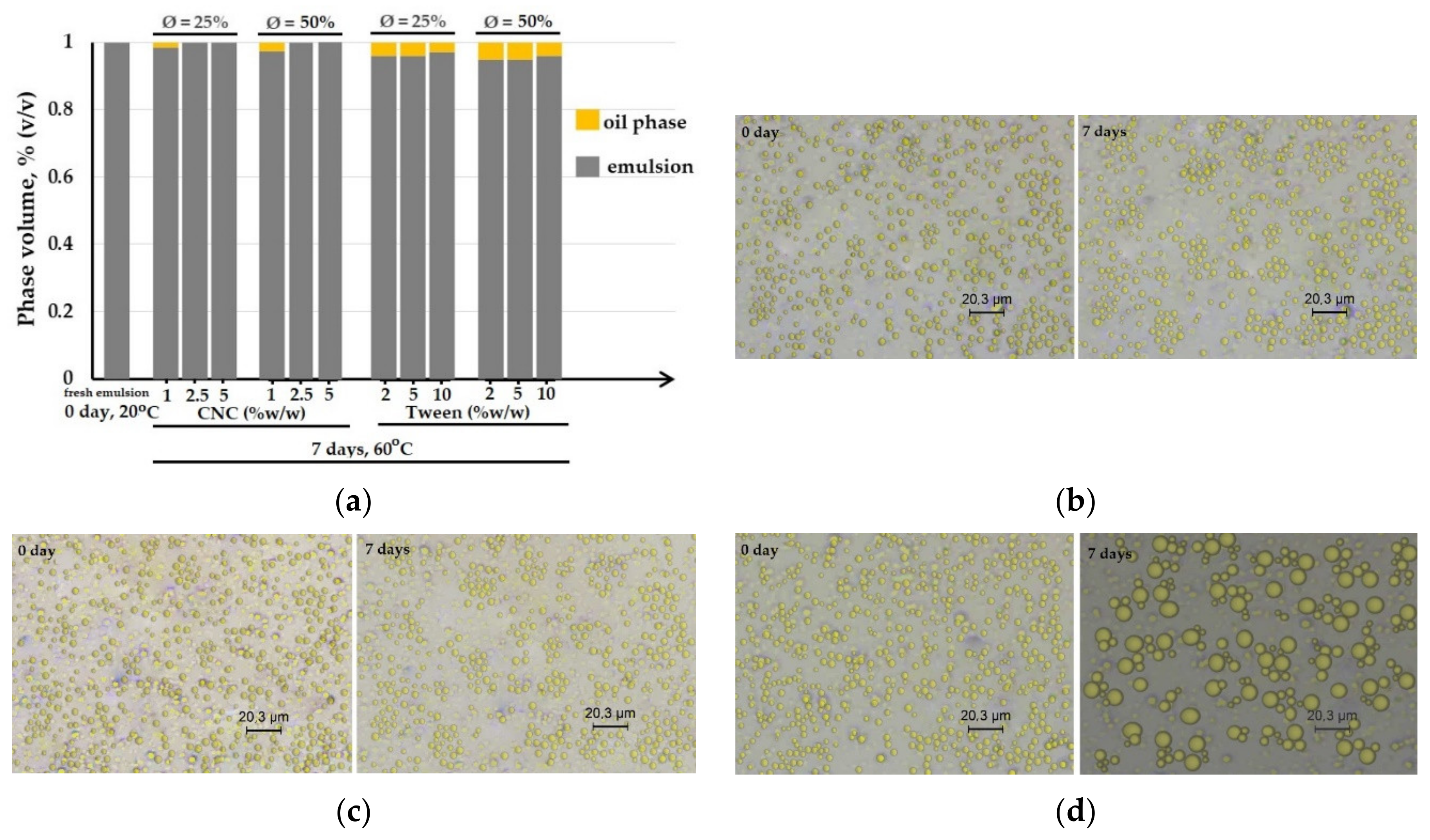
- The stability of the oil-in-water emulsion was observed by bottle test for monitoring the extent of phase separation with time. The samples were introduced into glass tubes and then stored at elevated temperature (50 °C, 7 days) for the accelerated aging test. The volume percentage of the emulsion phase was reported as a function of time.
- Microstructural observations of the emulsions were visualized by an optical microscope (Leica DM2700, Leica Microsystems, Wetzlar, Germany), which was equipped with 3 magnification objectives 10 ×, 40 ×, and 100 ×. The sample was spread on a thin flat glass then observed under a high power objective of 100 × and then switched to the high power of 100×. The image captured was copied by the software Leica Application Suite, LAS.
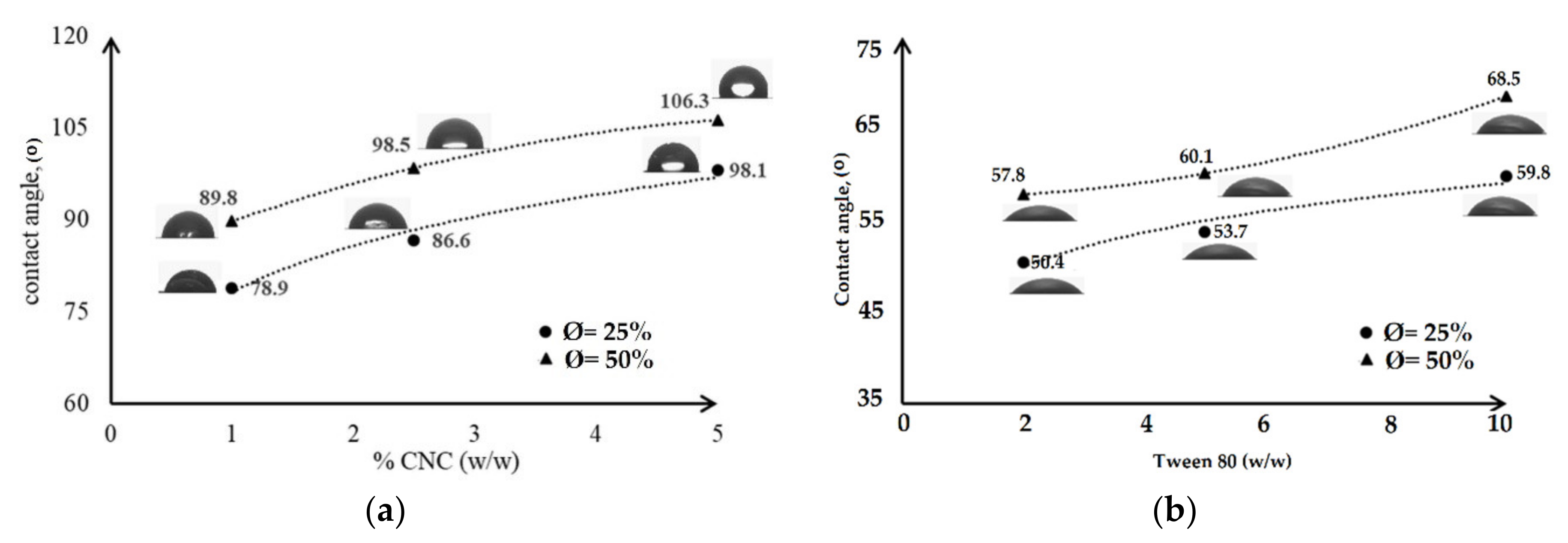
- Wetting agent analysis on a solid surface was carried out by contact angle, CA, measurement using Drop Shape Analyzer (DSA 100; Kruss GmbH, Hamburg, Germany) with the static droplet method. One drop of deionized water was dropped on the surface of the film. The image was taken by a camera and analysed by software to obtain the CA.
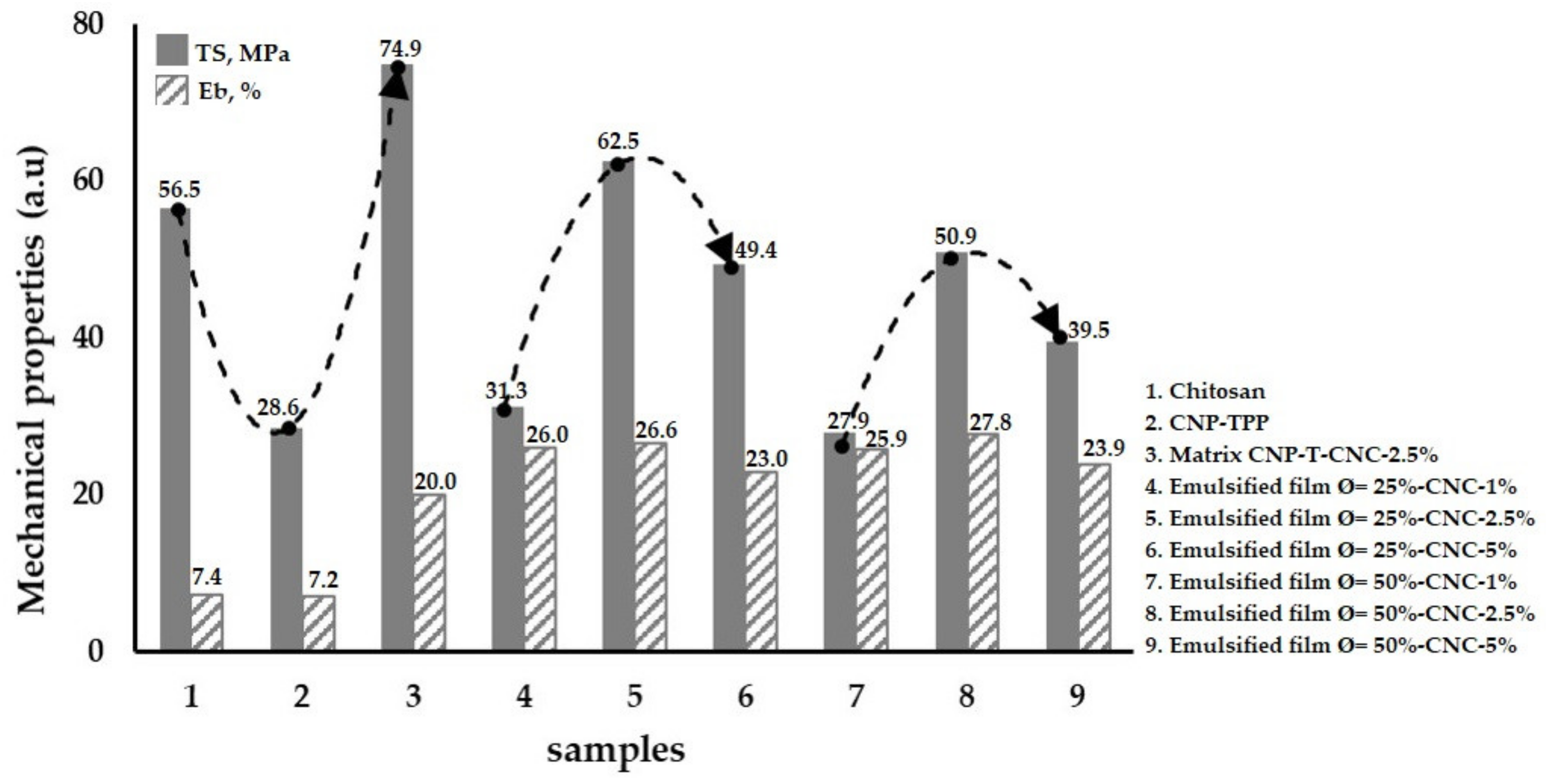
- Mechanical testing was performed with ASTM D882-02. A tensile test machine (Strograph, Toyoseiki, Tokyo, Japan) was used to measure the tensile strength, TS, and elongation at the break, EB, of the film specimens (10 cm × 4 cm × 0.03 mm). The initial grip separation was set at 100 mm and cross-head speed at 50 mm/min. The TS was calculated by dividing the maximum load on the film before failure by the cross-sectional area of the initial specimen, while the EB was defined as the percentage change in the length of the specimen compared to the original length between the grips.
- The molecular structures of the film samples were studied by Fourier transform infrared spectroscopy, FTIR. The functional groups present in the nanoparticle CNP–TPP and its compatibility with CNC was determined using a Nicolet iS5 spectrometer (Thermo Scientific, Waltham, MA, USA). The measurements were taking 32 scans for each sample with a resolution of 4 cm−1, ranging from 400 cm−1 to 4000 cm−1 and a scanning speed of 20 mm/sat.
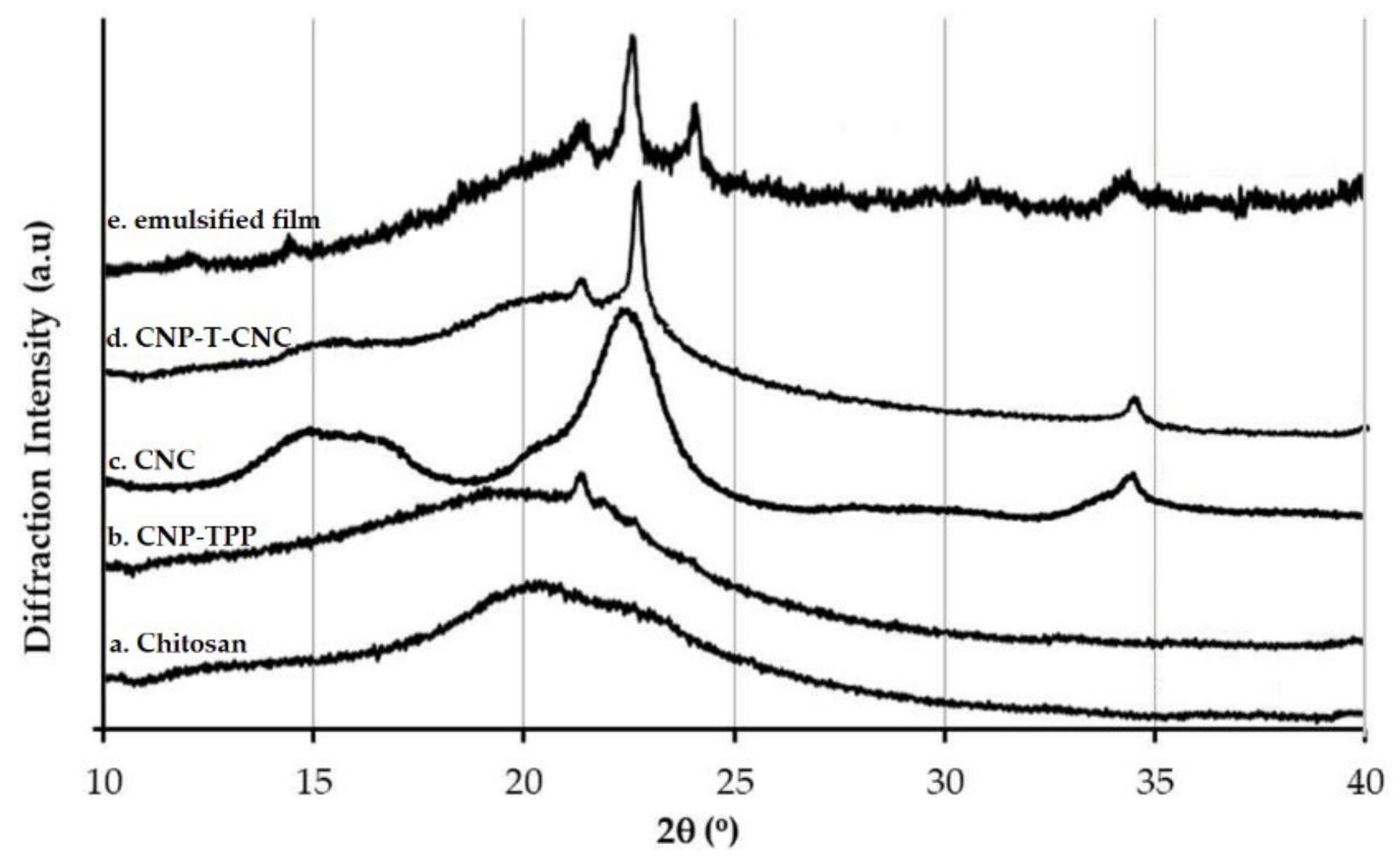
- The diffraction pattern of the films was investigated using X-Ray Diffraction, XRD, in a D8 Advance (Bruker, Billeric, MA, USA). Samples were examined with a scanning angle of 2θ from 10° to 40° at a rate of 1°/min with the CuKα filtered radiation.
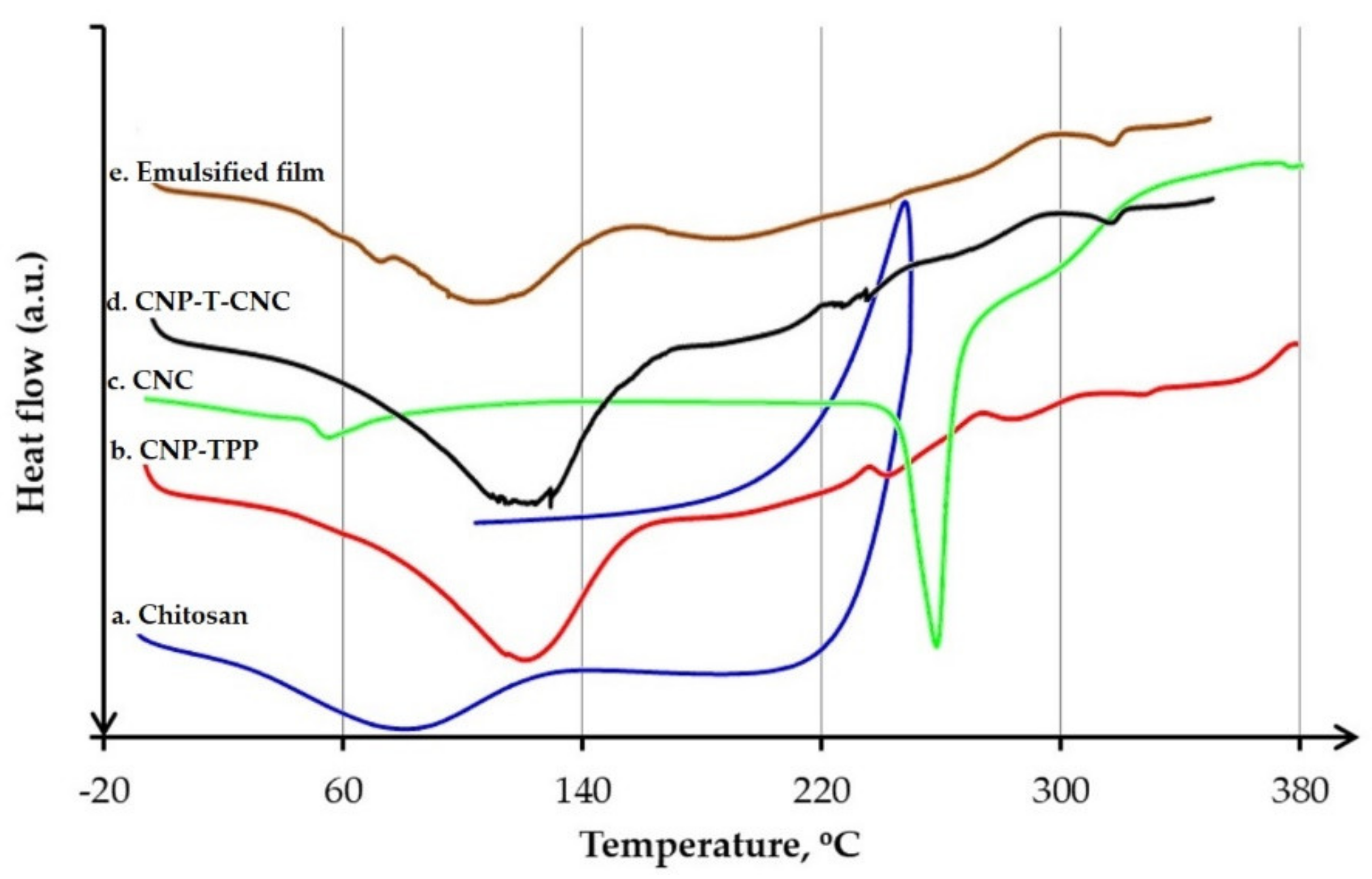
- Thermal behaviour was characterized by differential scanning calorimetry, (DSC, Q100, TA Instruments, New Castle, DE, USA). In total, 30 mg of sample was placed in an aluminium crucible then inserted into the sample pan holder directly after weighing. The analysis was carried under constant nitrogen flow (50 mL/min), from −30 to 170 °C, at a heating rate of 5 °C/min.
3. Results and Discussion
3.1. Synthesis and Characteristization of COLLOIDAL CNP–TPP
3.2. Stability Study
3.2.1. Forming Film Emulsion
3.2.2. Dried Emulsified-Film
3.3. Mechanical Properties of the Emulsified Films
3.4. Characterization of Emulsified Film
3.4.1. FT-IR Analysis
3.4.2. XRD Measurement
3.4.3. DSC Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsh, K.; Bugusu, B. Food packaging-roles, materials, and environmental issues. J. Food Sci. 2007, 72, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.A.P.R.; Pereira, R.N.C.; Da Silva Ramos, O.L.; Teixeira, J.A.C.; Vicente, A.A. Edible Food Packaging: Materials and Processing Technologies; CRC Press: Boca Ratton, FL, USA, 2016. [Google Scholar]
- Lamberti, M.; Escher, F. Aluminium foil as a food packaging material in comparison with other materials. Food Rev. Int. 2007, 23, 407–433. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Effect of γ-irradiation on the physical and mechanical properties of kefiran biopolymer film. Int. J. Biol. Macromol. 2015, 74, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Sathyanarayana, S.; Swan, S.H. Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. B 2009, 364, 2097–2113. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef]
- Guilbert, S.; Cuq, B.; Gontard, N. Recent innovations in edible and/or biodegradable packaging materials. Food Addit. Contam. 1997, 14, 741–751. [Google Scholar] [CrossRef]
- Ruzaina, I.; Zhong, F.; Rashid, N.A.; Jia, W.; Li, Y.; Zahrah, H.; Som, M.; Seng, C.C.; Sikin, A.M.; Wahab, N.A.; et al. Effect of Different Degree of Deacetylation, Molecular Weight of Chitosan and Palm Stearin and Palm Kernel Olein Concentration on Chitosan as Edible Packaging for Cherry Tomato. J. Food Process. Preserv. 2017, 41, 13090. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally Prefabricated Marine Biomaterials: Isolation and Applications of Flat Chitinous 3D Scaffolds from (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guand, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider Chitin. The biomimetic potential and applications of Caribena versicolor tubular chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef] [PubMed]
- Żółtowska-Aksamitowska, S.; Tsurkan, M.V.; Lim, S.-C.; Meissner, H.; Tabachnick, K.; Shaalaf, L.A.; Youssef, D.T.A.; Ivanenko, V.N.; Petrenko, I.; Wysokowski, M.; et al. The demosponge Pseudoceratina purpurea as a new source of fibrous chitin. Int. J. Biol. Macromol. 2018, 112, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, S.; Gontard, N.; Gorris, L.G. Prolongation of the shelf-life of perishable food products using biodegradable films and coatings. LWT Food Sci Technol. 1996, 29, 10–17. [Google Scholar]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef]
- Luduena, L.N.; Alvarez, V.A.; Vazquez, A. Processing and microstructure of PCL/clay nanocomposites. Mater. Sci. Eng. 2007, 460, 121–129. [Google Scholar] [CrossRef]
- De Silva, R.T.; Pasbakhsh, P.; Goh, K.L.; Chai, S.P.; Chen, J. Synthesis and characterisation of poly (lactic acid)/halloysite bionanocomposite films. J. Compos. Mater. 2014, 48, 3705–3717. [Google Scholar] [CrossRef]
- Gorrasi, G.; Pantani, R.; Murariu, M.; Dubois, P. PLA/H alloysite Nanocomposite Films: Water Vapor Barrier Properties and Specific Key Characteristics. Macromol. Mater. Eng. 2014, 299, 104–115. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Kanani, N.; Alfirano, A.; Rahmayetti, R. Development of polylactic acid (PLA) bio-composite films reinforced with bacterial cellulose nanocrystals (BCNC) without any surface modification. J. Dispers. Sci. Technol. 2019, 1–8. [Google Scholar] [CrossRef]
- Dufresne, A. Processing of polymer nanocomposites reinforced with polysaccharide nanocrystals. Molecules 2010, 15, 4111–4128. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shankaran, D.R. Preparation and physicochemical characterization of cellulose nanocrystals from industrial waste cotton. Appl. Surf. Sci. 2017, 412, 405–416. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Wahyudi, H.; Agustina, S.; Oudet, F.; Pinem, M.P.; Clausse, D.; Saleh, K.; Guenin, E. Ultrasonic Irradiation Coupled with Microwave Treatment for Eco-friendly Process of Isolating Bacterial Cellulose Nanocrystals. Nanomaterials 2018, 8, 859. [Google Scholar] [CrossRef] [PubMed]
- Wardhono, E.Y.; Kanani, N.; Alfirano, A. A simple process of isolation microcrystalline cellulose using ultrasonic irradiation. J. Dispers. Sci. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Anker, M.; Berntsen, J.; Hermansson, A.-M.; Stading, M. Improved water vapor barrier of whey protein films by addition of an acetylated monoglyceride. Innov. Food Sci. Emerg. Technol. 2002, 3, 81–92. [Google Scholar] [CrossRef]
- Sothornvit, R. Effect of hydroxypropyl methylcellulose and lipid on mechanical properties and water vapor permeability of coated paper. Food Res. Int. 2009, 42, 307–311. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.; Guo, K.; Zhao, X. High-pressure homogenization lowers water vapor permeability of soybean protein isolate–beeswax films. J. Agric. Food Chem. 2012, 60, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Debeaufort, F.; Voilley, A. Effect of surfactants and drying rate on barrier properties of emulsified edible films. Int. J. Food Sci. Technol. 1995, 30, 183–190. [Google Scholar] [CrossRef]
- Gallo, J.-A.Q.; Debeaufort, F.; Callegarin, F.; Voilley, A. Lipid hydrophobicity, physical state and distribution effects on the properties of emulsion-based edible films. J. Membr. Sci. 2000, 180, 37–46. [Google Scholar] [CrossRef]
- Navarro-Tarazaga, M.L.; Massa, A.; Pérez-Gago, M.B. Effect of beeswax content on hydroxypropyl methylcellulose-based edible film properties and postharvest quality of coated plums (Cv. Angeleno). LWT Food Sci. Technol. 2011, 44, 2328–2334. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Zafimahova-Ratisbonne, A.; Saleh, K.; Clausse, D.; Lanoiselle, J.-L. W/O Emulsion Destabilization and Release of a Polysaccharide Entrapped in the Droplets. J. Dispers. Sci. Technol. 2016, 37, 1581–1589. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Wang, Q.; Liu, F.; Wei, Z.H. Characterization of gelatin-based edible films incorporated with olive oil. Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yang, X.-Q.; Yin, S.-W. Fabrication and characterization of kidney bean (Phaseolus vulgaris L.) protein isolate–chitosan composite films at acidic pH. Food Hydrocoll. 2013, 31, 237–247. [Google Scholar] [CrossRef]
- Clausse, D.; Wardhono, E.Y.; Lanoiselle, J.-L. Formation and determination of the amount of ice formed in water dispersed in various materials. Colloids Surf. A 2014, 460, 519–526. [Google Scholar] [CrossRef]
- Fabra, M.J.; Pérez-Masiá, R.; Talens, P.; Chiralt, A. Influence of the homogenization conditions and lipid self-association on properties of sodium caseinate based films containing oleic and stearic acids. Food Hydrocoll. 2011, 25, 1112–1121. [Google Scholar] [CrossRef]
- Zafimahova-Ratisbonne, A.; Wardhono, E.Y.; Lanoisellé, J.-L.; Saleh, K.; Clausse, D. Stability of W/O emulsions encapsulating polysaccharides. J. Dispers. Sci. Technol. 2014, 35, 38–47. [Google Scholar] [CrossRef]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Wen, C.; Yuan, Q.; Liang, H.; Vriesekoop, F. Preparation and stabilization of d-limonene Pickering emulsions by cellulose nanocrystals. Carbohydr. Polym. 2014, 112, 695–700. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir 2000, 16, 8622–8631. [Google Scholar] [CrossRef]
- Yin, D.; Zhang, Q.; Yin, C.; Jia, Y.; Zhang, H. Effect of particle coverage on morphology of SiO2-covered polymer microspheres by Pickering emulsion polymerization. Colloids Surf. A 2010, 367, 70–75. [Google Scholar] [CrossRef]
- Yin, G.; Zheng, Z.; Wang, H.; Du, Q. Slightly surface-functionalized polystyrene microspheres prepared via Pickering emulsion polymerization using for electrophoretic displays. J. Colloid Interface Sci. 2011, 361, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, C.; Li, Y.; Chen, Y.; Tong, Z. Facile fabrication of nanocomposite microspheres with polymer cores and magnetic shells by Pickering suspension polymerization. React. Funct. Polym. 2009, 69, 750–754. [Google Scholar] [CrossRef]
- Do, T.-A.; Mitchell, J.R.; Wolf, B.; Vieira, J. Use of ethylcellulose polymers as stabilizer in fat-based food suspensions examined on the example of model reduced-fat chocolate. React. Funct. Polym. 2010, 70, 856–862. [Google Scholar] [CrossRef]
- Baudouin, A.-C.; Auhl, D.; Tao, F.; Devaux, J.; Bailly, C. Polymer blend emulsion stabilization using carbon nanotubes interfacial confinement. Polymer 2011, 52, 149–156. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Wang, L.; Li, C.; Xu, J.; Sun, D. Synergistic stabilization of emulsions by poly (oxypropylene) diamine and Laponite particles. Colloids Surf. A 2010, 353, 117–124. [Google Scholar] [CrossRef]
- Muller, F.; Salonen, A.; Glatter, O. Monoglyceride-based cubosomes stabilized by Laponite: Separating the effects of stabilizer, pH and temperature. Colloids Surf. A 2010, 358, 50–56. [Google Scholar] [CrossRef]
- Fujii, S.; Read, E.S.; Binks, B.P.; Armes, S.P. Stimulus-Responsive Emulsifiers Based on Nanocomposite Microgel Particles. Adv. Mater. 2005, 17, 1014–1018. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S.; Hua, Y.; Chen, J.; Hu, C.P. Hydrophilic porous polymers based on high internal phase emulsions solely stabilized by poly (urethane urea) nanoparticles. Polymer 2010, 51, 3612–3617. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.; Hua, Y.; Jegat, C.; Chen, J.; Taha, M. Stability of surfactant-free high internal phase emulsions and its tailoring morphology of porous polymers based on the emulsions. Polymer 2011, 52, 4881–4890. [Google Scholar] [CrossRef]
- Bao, H.; Li, L.; Zhang, H. Influence of cetyltrimethylammonium bromide on physicochemical properties and microstructures of chitosan—TPP nanoparticles in aqueous solutions. J. Colloid Interface Sci. 2008, 328, 270–277. [Google Scholar] [CrossRef]
- Kocak, N.; Sahin, M.; Akin, I.; Kus, M.; Yilmaz, M. Microwave assisted synthesis of chitosan nanoparticles. J. Macromol. Sci. 2011, 48, 776–779. [Google Scholar] [CrossRef]
- Gokce, Y.; Cengiz, B.; Yildiz, N.; Calimli, A.; Aktas, Z. Ultrasonication of chitosan nanoparticle suspension: Influence on particle size. Colloids Surf. A 2014, 462, 75–81. [Google Scholar] [CrossRef]
- Pérez-Gago, M.B.; Krochta, J.M. Lipid particle size effect on water vapor permeability and mechanical properties of whey protein/beeswax emulsion films. J. Agric. Food Chem. 2001, 49, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of cellulose nanomaterials, their capabilities and applications. Jom 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Deng, Z.; Jung, J.; Simonsen, J.; Zhao, Y. Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocoll. 2018, 84, 229–237. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Xiang, W.; Rojas, O.J. Pickering emulsions by combining cellulose nanofibrils and nanocrystals: Phase behavior and depletion stabilization. Green Chem. 2018, 20, 1571–1582. [Google Scholar] [CrossRef]
- Xiao, M.; Xu, A.; Zhang, T.; Hong, L. Tailoring the wettability of colloidal particles for Pickering emulsions via surface modification and roughness. Front. Chem. 2018, 6, 225. [Google Scholar] [CrossRef]
- Shi, H.; Fan, Z.; Ponsinet, V.; Sellier, R.; Liu, H.; Pera-Titus, M.; Clacens, J.-M. Glycerol/Dodecanol Double Pickering Emulsions Stabilized by Polystyrene-Grafted Silica Nanoparticles for Interfacial Catalysis. ChemCatChem 2015, 7, 3229–3233. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Zafimahova-Ratisbonne, A.; Lanoiselle, J.-L.; Saleh, K.; Clausse, D. Optimization of the formulation of water in oil emulsions entrapping polysaccharide by increasing the amount of water and the stability. Can. J. Chem. Eng. 2014, 92, 1189–1196. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Jiménez, A.; Talens, P.; Chiralt, A. Effect of the incorporation of surfactants on the physical properties of corn starch films. Food Hydrocoll. 2014, 38, 66–75. [Google Scholar] [CrossRef]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and Water-Resistant Properties of Eco-Friendly Chitosan Membrane Reinforced with Cellulose Nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Gontard, N.; Marchesseau, S.; CUQ, J.-L.; Guilbert, S. Water vapour permeability of edible bilayer films of wheat gluten and lipids. Int. J. Food Sci. Technol. 1995, 30, 49–56. [Google Scholar] [CrossRef]
- Queiroz, F.M.; Melo, K.; Sabry, D.; Sassaki, G.; Rocha, H. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Dai, L.; Qi, Y.-P.; Niu, L.-N.; Liu, Y.; Pucci, C.R.; Looney, S.W.; Ling, J.-Q.; Pashley, D.H.; Tay, F.R. Inorganic–organic nanocomposite assembly using collagen as a template and sodium tripolyphosphate as a biomimetic analog of matrix phosphoprotein. Cryst. Growth Des. 2011, 11, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Gokila, S.; Gomathi, T.; Sudha, P.N.; Anil, S. Removal of the heavy metal ion chromiuim (VI) using Chitosan and Alginate nanocomposites. Int. J. Biol. Macromol. 2017, 104, 1459–1468. [Google Scholar] [CrossRef]
- Shi, G.; Sun, L.; Luo, S.L.; Sun, F.Q. Preparation and properties research of nanocomposite film based chitosan and nanocrystal cellulose. Mater. Res. Appl. 2008, 4, 41. [Google Scholar]
- Hromiš, N.M.; Lazić, V.L.; Markov, S.L.; Vaštag, Z.G.; Popović, S.Z.; Šuput, D.Z.; Džinić, N.R.; Velićanski, A.S.; Popović, L.M. Optimization of chitosan biofilm properties by addition of caraway essential oil and beeswax. J. Food Eng. 2015, 158, 86–93. [Google Scholar] [CrossRef]
- Osorio-Madrazo, A.; David, L.; Trombotto, S.; Lucas, J.-M.; Peniche-Covas, C.; Domard, A. Kinetics study of the solid-state acid hydrolysis of chitosan: Evolution of the crystallinity and macromolecular structure. Biomacromolecules 2010, 11, 1376–1386. [Google Scholar] [CrossRef]
- Anand, M.; Maruthupandy, M.; Kalaivani, R.; Suresh, S.; Kumaraguru, A.K. Larvicidal activity of chitosan nanoparticles synthesized from crab and squilla species against Aedes aegypti. J. Colloid Sci. Biotechnol. 2014, 3, 188–193. [Google Scholar] [CrossRef]
- Anand, M.; Sathyapriya, P.; Maruthupandy, M.; Beevi, A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front Lab. Med. 2018, 2, 72–78. [Google Scholar]
- Reddy, N.; Yang, Y. Characterizing natural cellulose fibers from velvet leaf (Abutilon theophrasti) stems. Bioresour. Technol. 2008, 99, 2449–2454. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, Y.; Mija, A.; Burr, A.; Darque-Ceretti, E.; Felder, E.; Sbirrazzuoli, N. Green material composites from renewable resources: Polymorphic transitions and phase diagram of beeswax/rosin resin. Thermochim. Acta 2011, 521, 90–97. [Google Scholar] [CrossRef]
- Dinker, A.; Agarwal, M.; Agarwal, G.G. Preparation, characterization, and performance study of beeswax/expanded graphite composite as thermal storage material. Exp. Heat Transf. 2017, 30, 139–150. [Google Scholar] [CrossRef]
- Kittur, F.S.; Prashanth, K.H.; Sankar, K.U.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Deng, L.; Qi, H.; Yao, C.; Feng, M.; Dong, A. Investigation on the properties of methoxy poly (ethylene glycol)/chitosan graft co-polymers. J. Biomater. Sci. Polym. 2007, 18, 1575–1589. [Google Scholar]
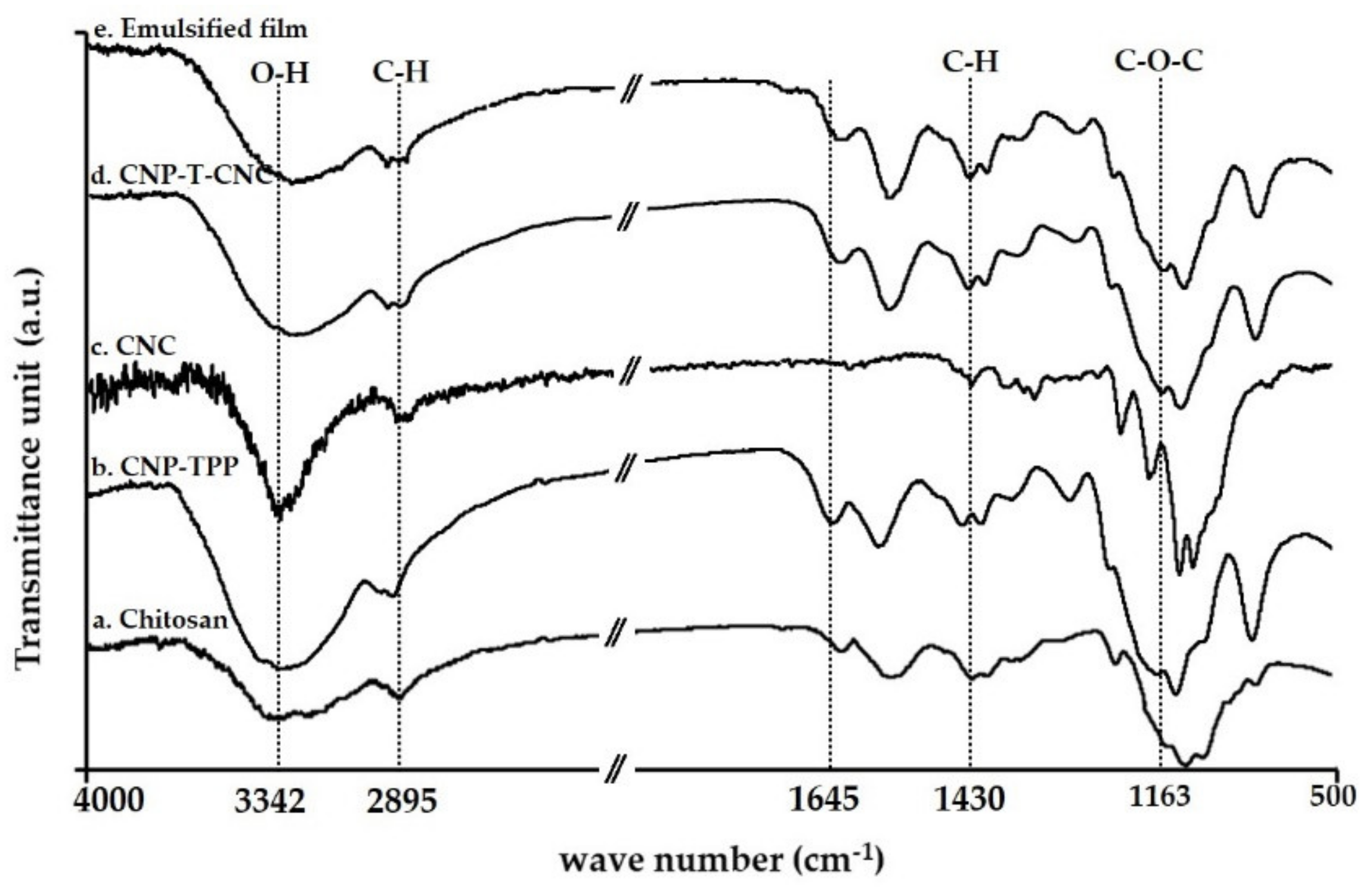
| Wave Number, cm−1 | Assignment |
|---|---|
| 3342 | Stretching of O–H bond |
| 2895 | Symmetric C–H stretching |
| 1645 | Stretching C=O amide I |
| 1550 | Bending N–H amide II |
| 1383 | Acetyl groups |
| 960–1100 | Asymmetric C–O–C stretching region |
| Temperature | Sample (°C) | ||||
|---|---|---|---|---|---|
| Chitosan | CNP–TPP | CNC | CNP-T-CNC | Emulsified Film | |
| Tg | 31.0 | 36.1 | 59.2 | 36.8 | - |
| Tm | 81.4 | 118.2 | 288.6 | 120.4 | 117.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Agustina, S.; Oudet, F.; Lefebvre, C.; Clausse, D.; Saleh, K.; Guénin, E. Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax. Nanomaterials 2019, 9, 1707. https://doi.org/10.3390/nano9121707
Wardhono EY, Pinem MP, Kustiningsih I, Agustina S, Oudet F, Lefebvre C, Clausse D, Saleh K, Guénin E. Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax. Nanomaterials. 2019; 9(12):1707. https://doi.org/10.3390/nano9121707
Chicago/Turabian StyleWardhono, Endarto Yudo, Mekro Permana Pinem, Indar Kustiningsih, Sri Agustina, François Oudet, Caroline Lefebvre, Danièle Clausse, Khashayar Saleh, and Erwann Guénin. 2019. "Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax" Nanomaterials 9, no. 12: 1707. https://doi.org/10.3390/nano9121707
APA StyleWardhono, E. Y., Pinem, M. P., Kustiningsih, I., Agustina, S., Oudet, F., Lefebvre, C., Clausse, D., Saleh, K., & Guénin, E. (2019). Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax. Nanomaterials, 9(12), 1707. https://doi.org/10.3390/nano9121707





