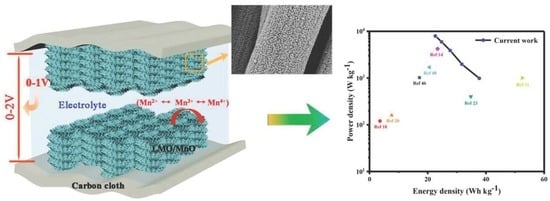
Flexible Supercapacitor Electrodes Based on Carbon Cloth-Supported LaMnO3/MnO Nano-Arrays by One-Step Electrodeposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
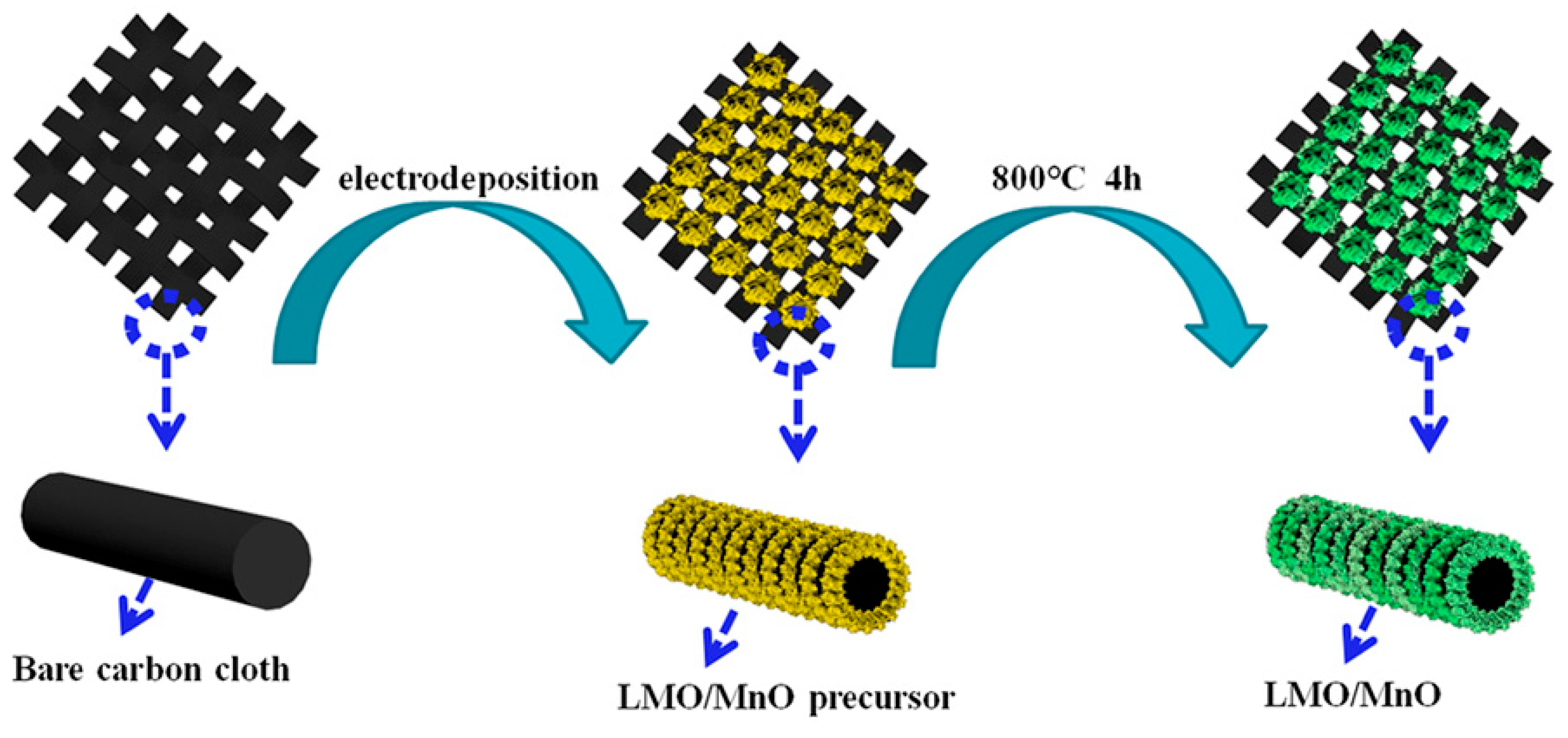
2.2. Materials Preparation
2.3. Phase and Structural Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
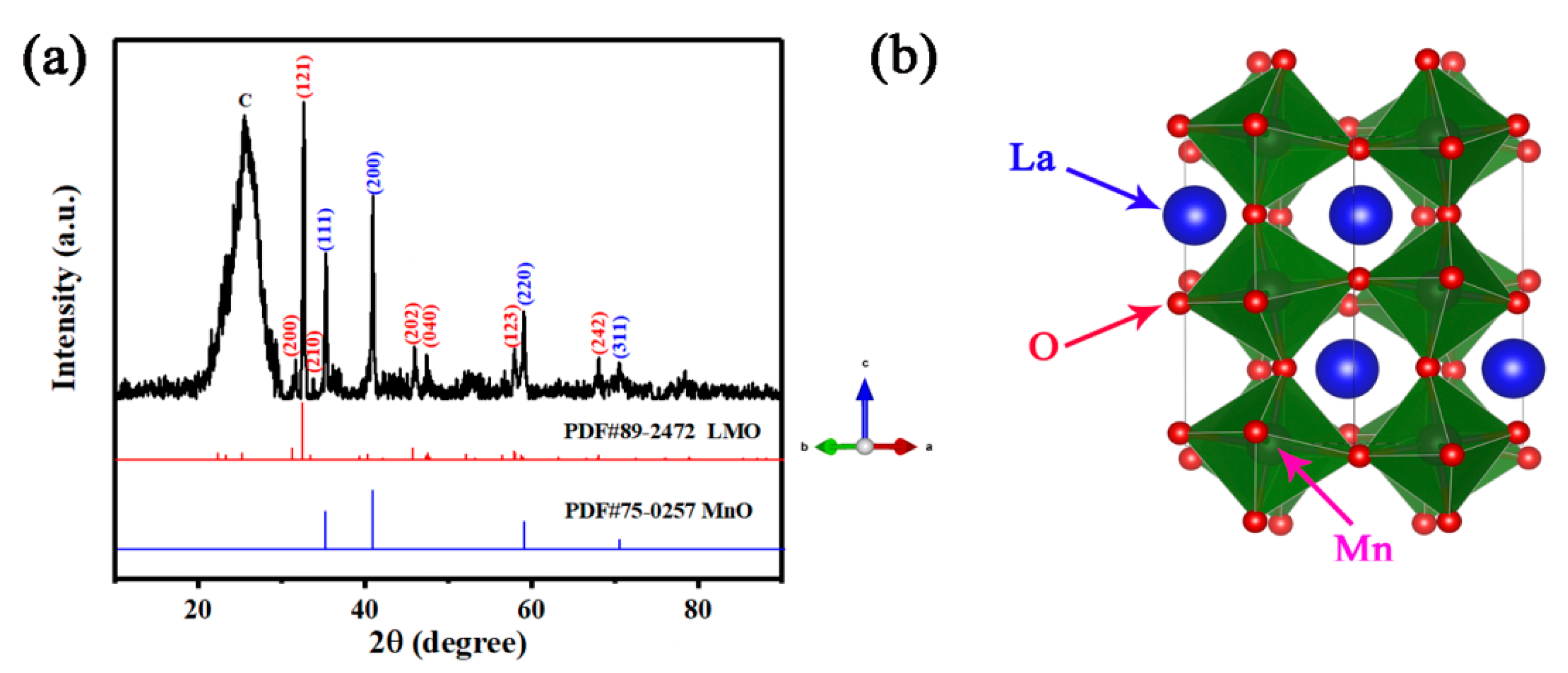
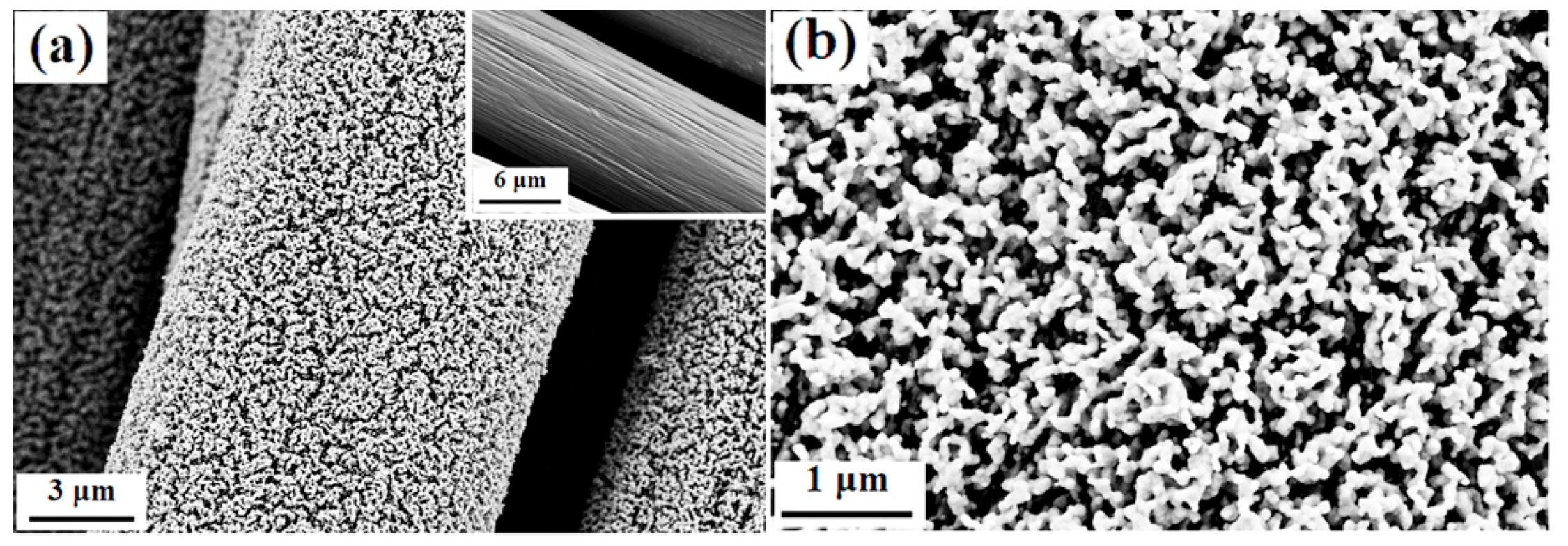
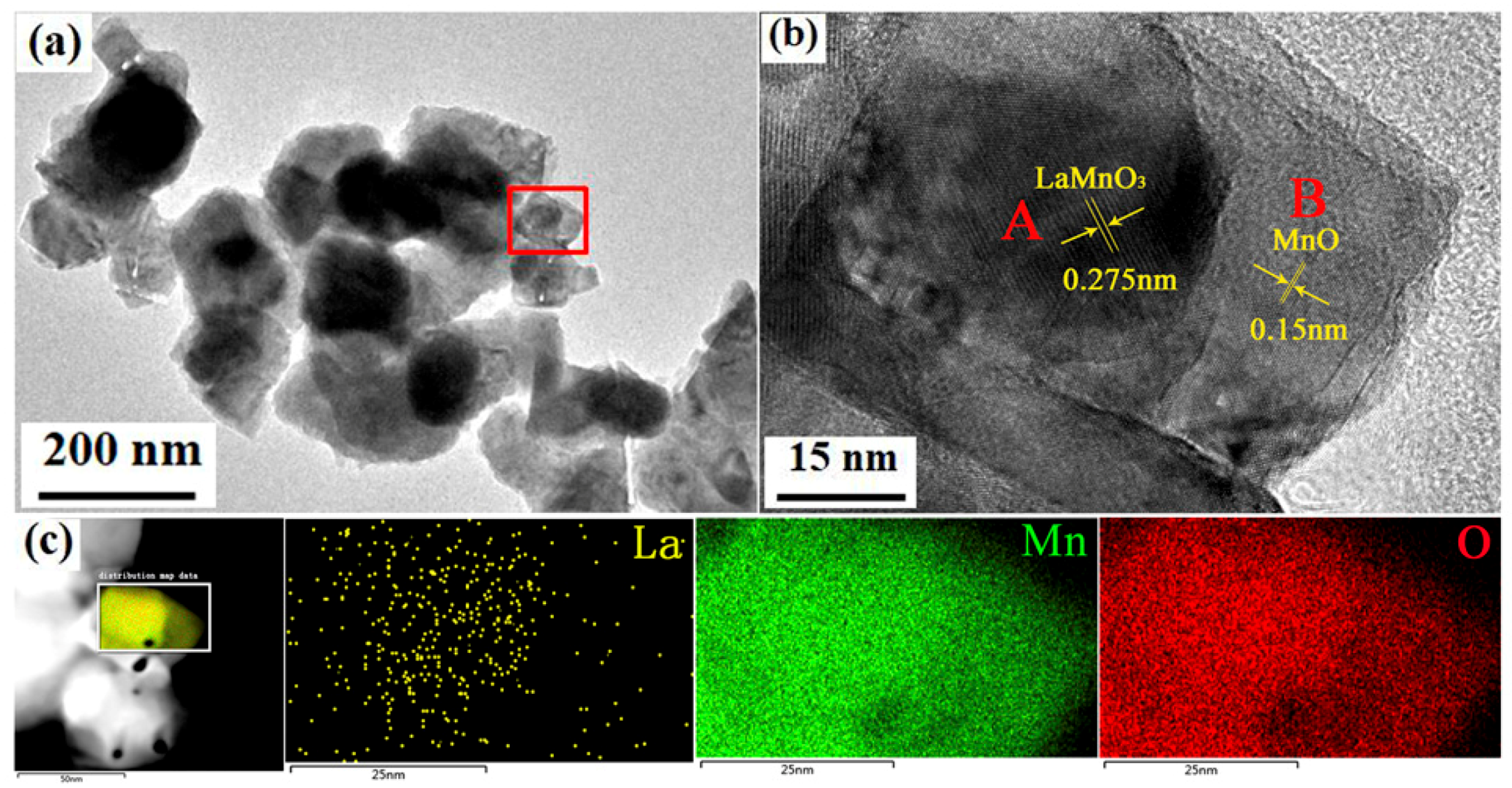
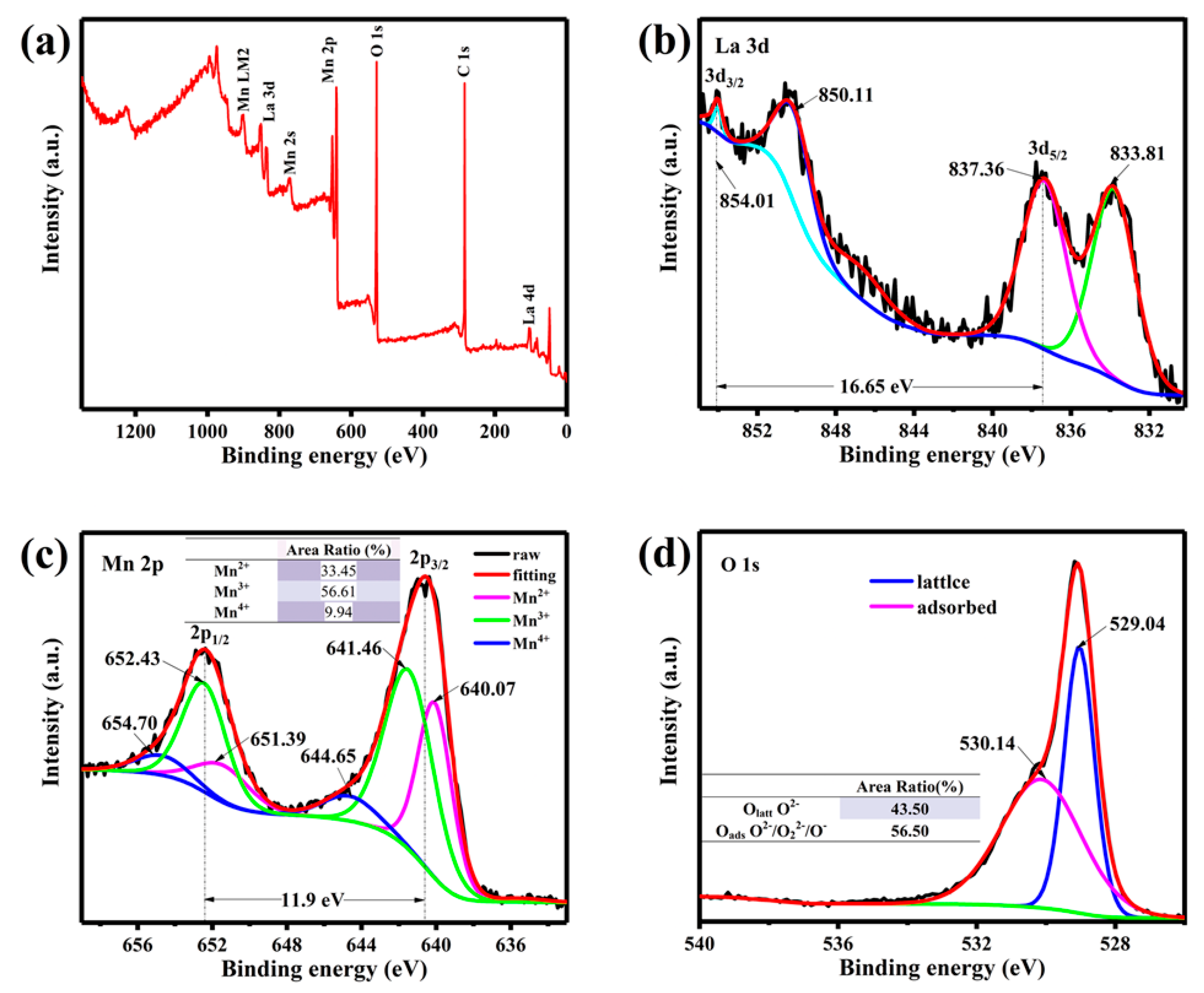
3.1. Phase Structure and Morphology
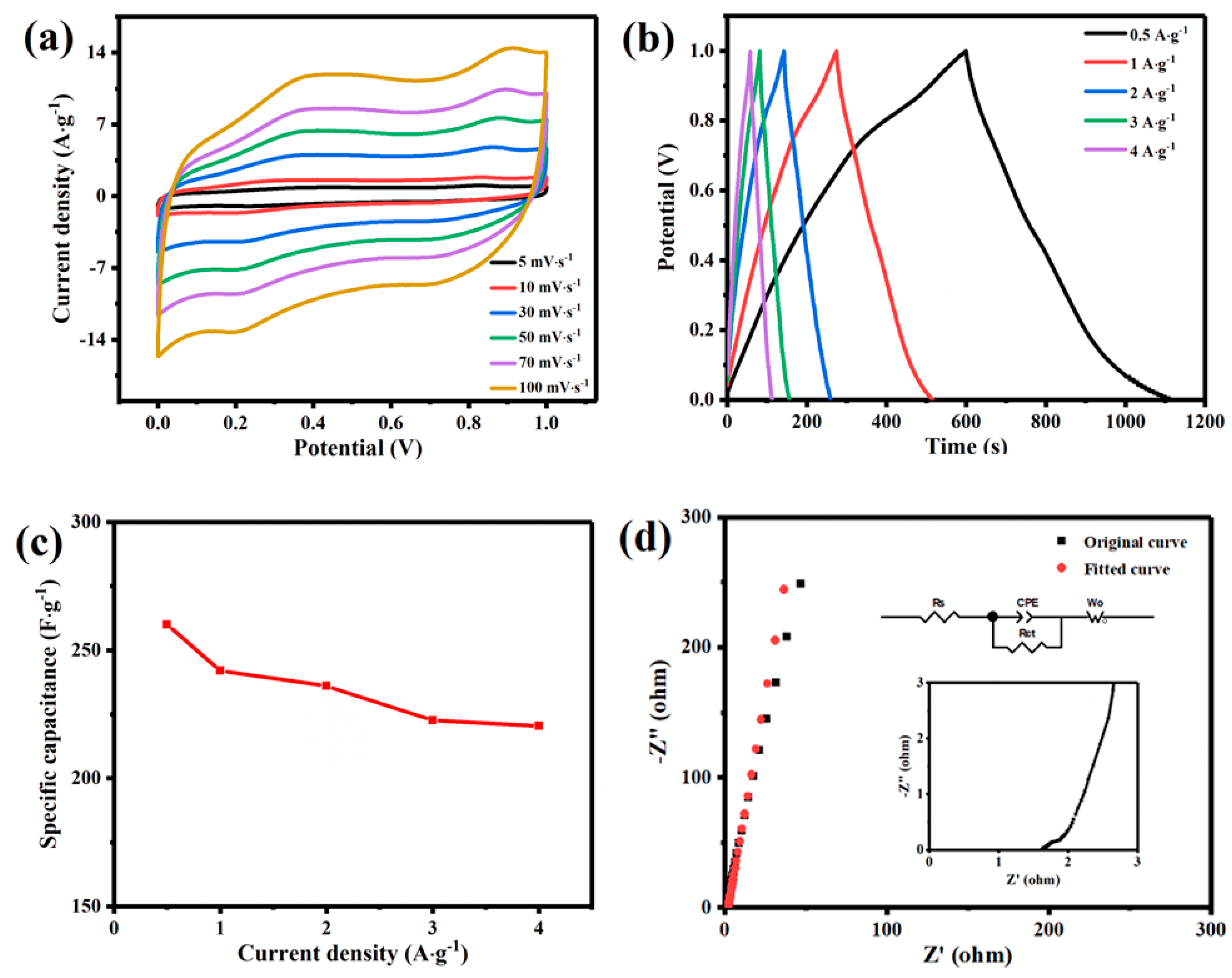
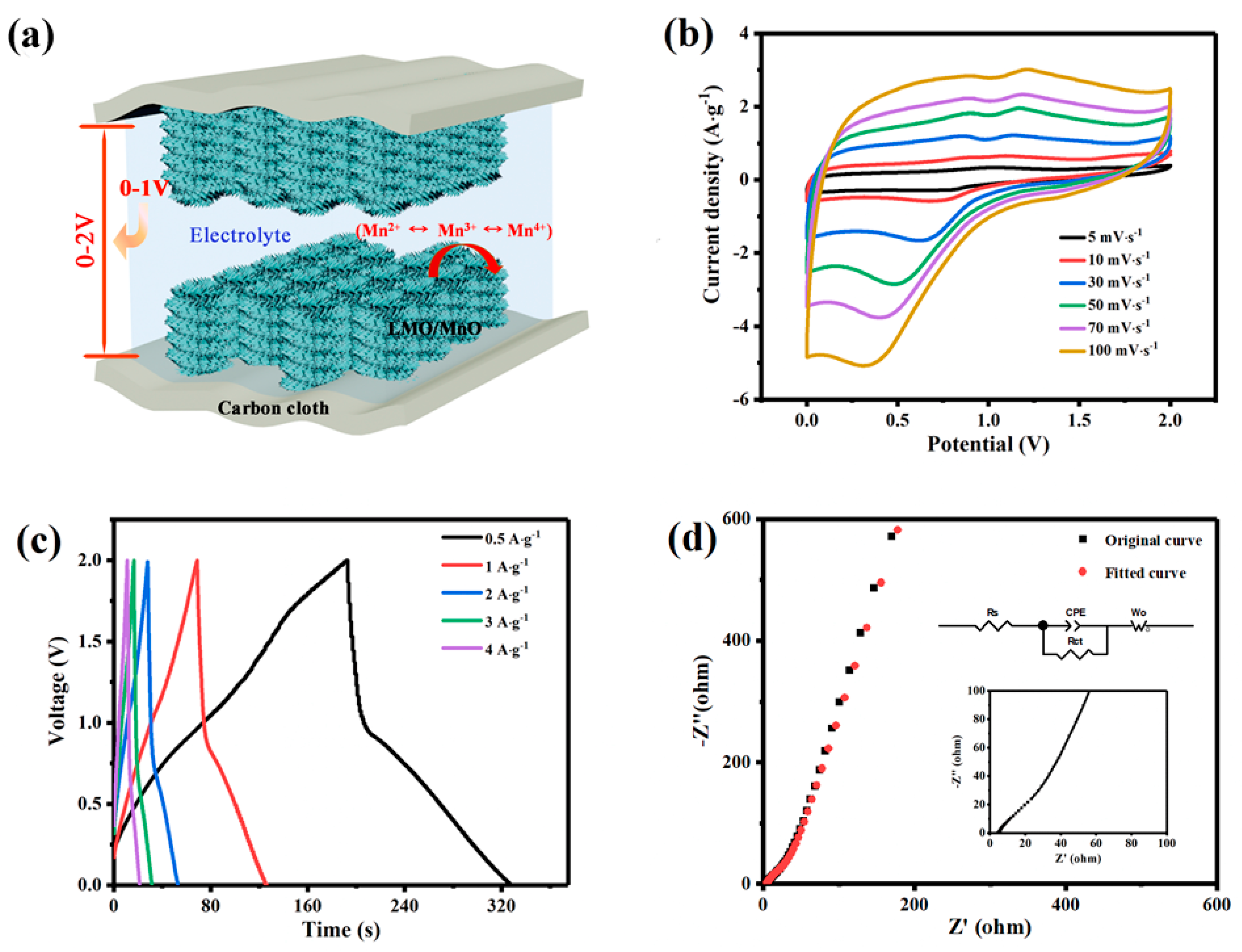
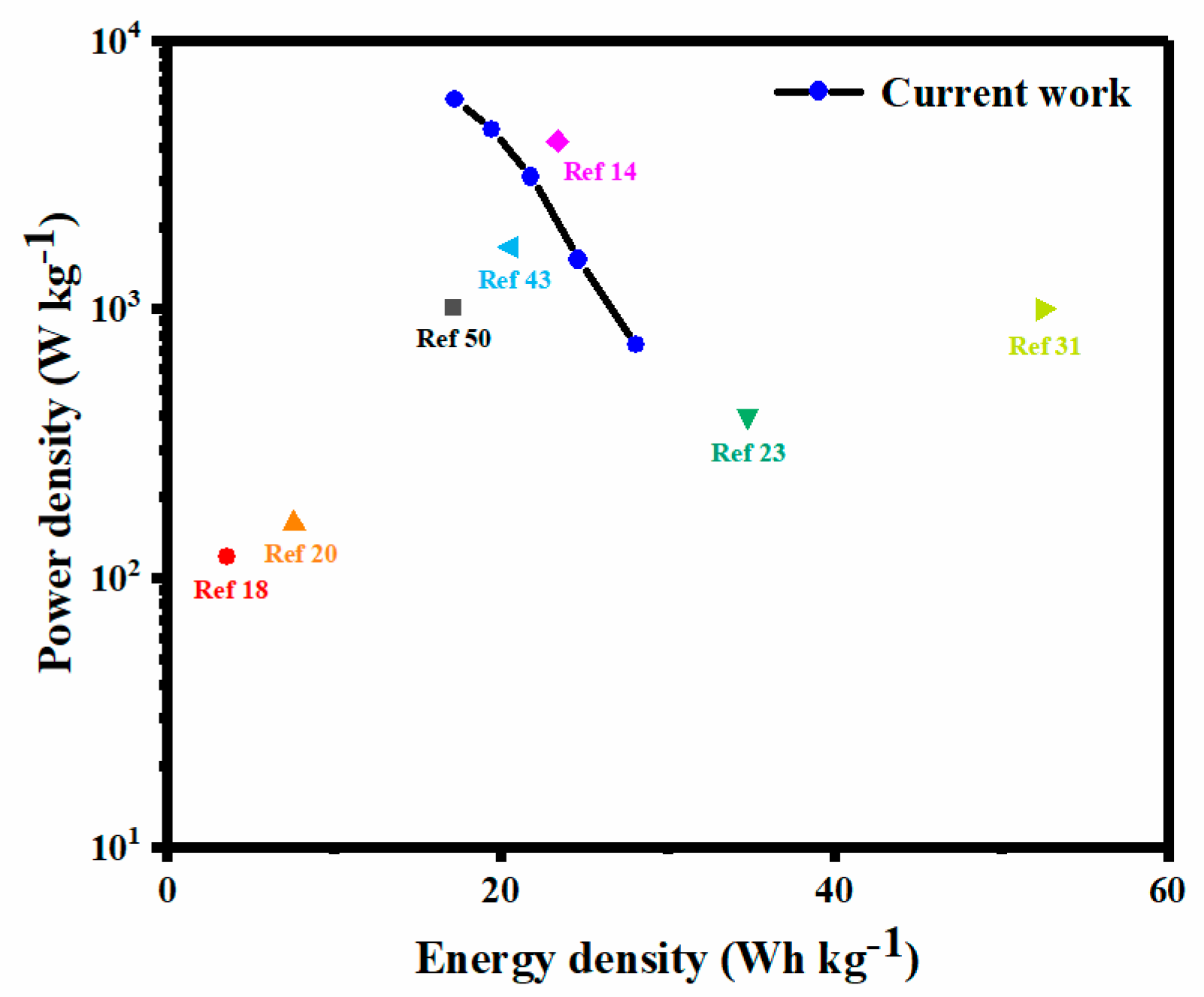
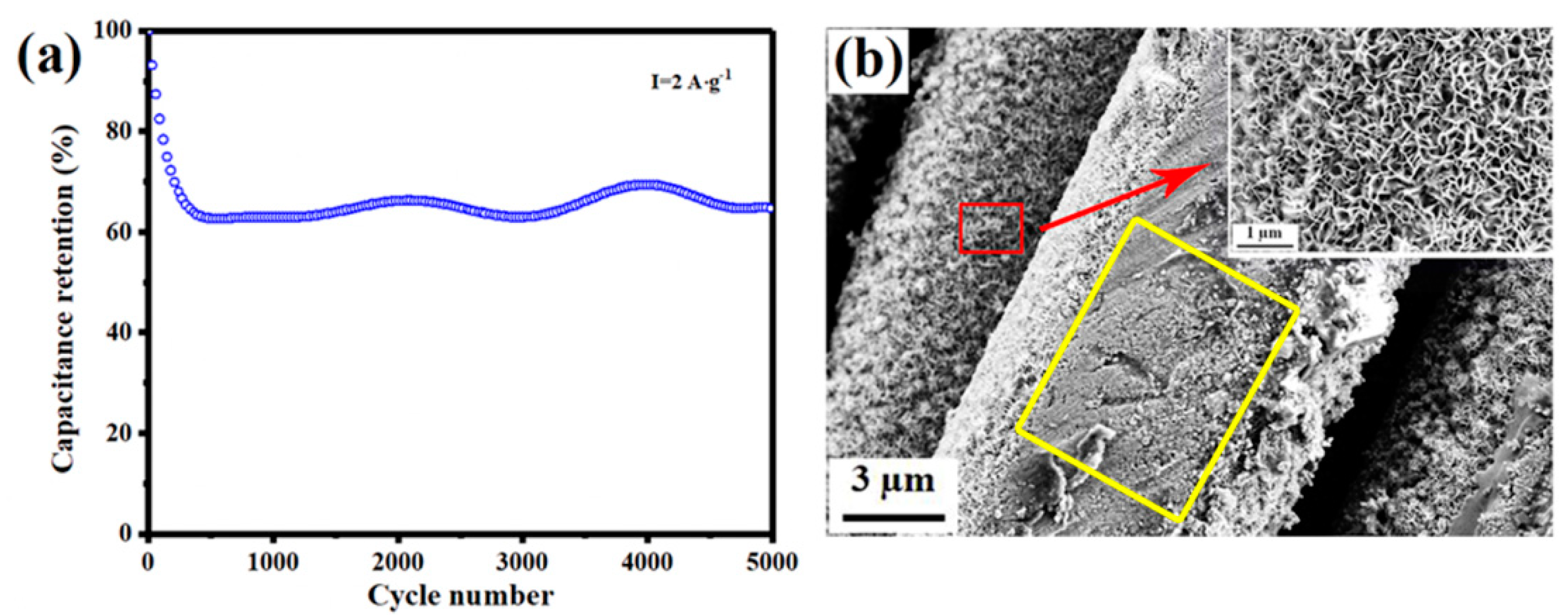
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lukatskaya, M.R.; Dunn, B.; Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 2016, 7, 12647. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.P.; Zhang, L.; Zhang, J.J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.S.; Luo, J.S.; Jiang, J.; Zhou, W.W.; Yang, H.P.; Qi, X.Y.; Zhang, H.; Fan, H.J.; Yu, D.Y.W.; Li, C.M.; et al. Seed-assisted synthesis of highly ordered TiO2@alpha-Fe2O3 core/shell arrays on carbon textiles for lithium-ion battery application. Energy Environ. Sci. 2012, 5, 6559–6566. [Google Scholar] [CrossRef]
- Xie, J.; Yang, P.P.; Wang, Y.; Qi, T.; Lei, Y.; Li, C.M. Puzzles and confusions in supercapacitor and battery: Theory and solutions. J. Power Sources 2018, 401, 213–223. [Google Scholar] [CrossRef]
- Wang, F.X.; Wu, X.W.; Yuan, X.H.; Liu, Z.C.; Zhang, Y.; Fu, L.J.; Zhu, Y.S.; Zhou, Q.M.; Wu, Y.P.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Xie, X.; Pan, L.J.; Bao, Z.N.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Yan, X.; You, H.; Liu, W.; Wang, X.; Wu, D. Free-standing and heteroatoms-doped carbon nanofiber networks as a binder-free flexible electrode for high-performance supercapacitors. Nanomaterials 2019, 9, 1189. [Google Scholar] [CrossRef]
- Yang, P.H.; Ding, Y.; Lin, Z.Y.; Chen, Z.W.; Li, Y.Z.; Qiang, P.F.; Ebrahimi, M.; Mai, W.J.; Wong, C.P.; Wang, Z.L. Low-cost high-performance solid-state asymmetric supercapacitors based on MnO2 nanowires and Fe2O3 nanotubes. Nano Lett. 2014, 14, 731–736. [Google Scholar] [CrossRef]
- Patel, M.N.; Wang, X.; Slanac, D.A.; Ferrer, D.A.; Dai, S.; Johnston, K.P.; Stevenson, K.J. High pseudocapacitance of MnO2 nanoparticles in graphitic disordered mesoporous carbon at high scan rates. J. Mater. Chem. 2012, 22, 3160–3169. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xia, X.F.; Ye, H.T.; Wang, L.; Jiao, X.Y.; Lei, W.; Hao, Q.L. Three-dimensional hierarchical structure ZnO@C@NiO on carbon cloth for asymmetric supercapacitor with enhanced cycle stability. ACS Appl. Mater. Interfaces 2018, 10, 3549–3561. [Google Scholar] [CrossRef]
- Xiao, J.W.; Wan, L.; Yang, S.H.; Xiao, F.; Wang, S. Design hierarchical electrodes with highly conductive NiCo2S4 nanotube arrays grown on carbon fiber paper for high-performance pseudocapacitors. Nano Lett. 2014, 14, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Song, R.H.; Lee, S.B.; Lim, T.H.; Park, S.J.; Park, C.O.; Lee, J.W. Facile synthesis of Ca-doped LaCoO3 perovskite via chemically assisted electrodeposition as a protective film on solid oxide fuel cell interconnects. J. Electrochem. Soc. 2016, 163, F1066–F1071. [Google Scholar] [CrossRef]
- Wu, W.; Guan, W.B.; Wang, G.L.; Liu, W.; Zhang, Q.S.; Chen, T.; Wang, W.G. Evaluation of Ni80Cr20/(La0.75Sr0.25)0.95MnO3 dual layer coating on SUS 430 stainless steel used as metallic interconnect for solid oxide fuel cells. Int. J. Hydrog. Energy 2014, 39, 996–1004. [Google Scholar] [CrossRef]
- Mefford, J.T.; Hardin, W.G.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 2014, 13, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Elsiddig, Z.A.; Xu, H.; Wang, D.; Zhang, W.; Guo, X.L.; Zhang, Y.; Sun, Z.M. Modulating Mn4+ ions and oxygen vacancies in nonstoichiometric LaMnO3 perovskite by a facile sol-gel method as high-performance supercapacitor electrodes. Electrochim. Acta 2017, 253, 422–429. [Google Scholar] [CrossRef]
- Ho, K.H.; Wang, J. Hydrazine reduction of LaNiO3 for active materials insupercaacitors. J. Am. Ceram. Soc. 2017, 100, 4629–4637. [Google Scholar] [CrossRef]
- Guo, Y.Z.; Shao, T.Y.; You, H.H.; Li, S.; Li, C.; Zhang, L. Polyvinylpyrrolidone-assisted solvothermal synthesis of porous LaCoO3 nanospheres as supercapacitor electrode. Int. J. Electrochem. Sci. 2017, 12, 7121–7127. [Google Scholar] [CrossRef]
- Lang, X.; Mo, H.; Hu, X.; Tian, H. Supercapacitor performance of perovskite La1-xSrxMnO3. Dalton Trans. 2017, 46, 13720–13730. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhu, Q.Q.; Wang, X.E.; Zhang, H.C.; Zhang, J.J.; Wang, L.F. Structural andelectrochemical properties of La0.85Sr0.15MnO3 powder as an electrode material for supercapacitor. J. Alloy. Compd. 2016, 675, 195–200. [Google Scholar] [CrossRef]
- Mo, H.; Nan, H.; Lang, X.; Liu, S.; Qiao, L.; Hu, X.; Tian, H. Influence of calcium doping on performance of LaMnO3 supercapacitors. Ceram. Int. 2018, 44, 9733–9741. [Google Scholar] [CrossRef]
- Jin, C.; Cao, X.C.; Zhang, L.Y.; Zhang, C.; Yang, R.Z. Preparation and electrochemical properties of urchin-like La0.8Sr0.2MnO3 perovskite oxide as a bifunctional catalyst for oxygen reduction and oxygen evolution reaction. J. Power Sources 2013, 241, 225–230. [Google Scholar] [CrossRef]
- Kumar, S.R.; Abinaya, C.V.; Amirthapandian, S.; Ponpandian, N. Enhanced visible light photocatalytic activity of porous LaMnO3 sub-micron particles in the degradation of rose Bengal. Mater. Res. Bull. 2017, 93, 270–281. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.P.; Sun, Y.; Yang, H.; Zhang, X.Q. Symmetric/asymmetric supercapacitor based on the perovskite-type lanthanum cobaltate nanofibers with Sr-substitution. Electrochim. Acta 2015, 178, 398–406. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, B.P.; Sun, Y.; Yang, H.; Zhang, X.Q. Synthesis, structure and electrochemical properties of lanthanum manganese nanofibers doped with Sr and Cu. J. Alloy. Compd. 2015, 638, 204–213. [Google Scholar] [CrossRef]
- Horng, Y.Y.; Lu, Y.C.; Hsu, Y.K.; Chen, C.C.; Chen, L.C.; Chen, K.H. Flexible supercapacitor based on polyaniline nanowires/carbon cloth with both high gravimetric and area-normalized capacitance. J. Power Sources 2010, 195, 4418–4422. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Wang, X.F.; Chen, G.; Chen, D.; Zhou, C.W.; Shen, G.Z. Hierarchical three-dimensional ZnCo2O4 nanowire arrays/carbon cloth anodes for a novel class of high-performance flexible lithium-ion batteries. Nano Lett. 2012, 12, 3005–3011. [Google Scholar] [CrossRef]
- Chen, W.; Xia, C.; Alshareef, H.N. One-step electrodeposited nickel cobalt sulfide nanosheet arrays for high-performance asymmetric supercapacitors. ACS Nano 2014, 8, 9531–9541. [Google Scholar] [CrossRef]
- Park, B.K.; Song, R.H.; Lee, S.B.; Lim, T.H.; Park, S.J.; Park, C.O.; Lee, J.W. A perovskite-type lanthanum cobaltite thin film synthesized via an electrochemical route and its application in SOFC interconnects. J. Electrochem. Soc. 2015, 162, F1549–F1554. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Dinamani, M.; Vishnu Kamath, P. Electrochemical synthesis of perovskite oxides. J. Appl. Electrochem. 2005, 35, 459–465. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, G.; Sun, S.; Xu, B.; Zhou, J.; Zhang, Y.; Yao, J. Growth of NiCo2S4 nanotubes on carbon nanofibers for high performance flexible supercapacitors. J. Electroanal. Chem. 2017, 804, 212–219. [Google Scholar] [CrossRef]
- Shafi, P.M.; Joseph, N.; Thirumurugan, A.; Bose, A.C. Enhanced electrochemical performances of agglomeration-free LaMnO3 perovskite nanoparticles and achieving high energy and power densities with symmetric supercapacitor design. Chem. Eng. J. 2018, 338, 147–156. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Jiang, G.H.; Xu, W.C.; Cao, C.; Liu, Y.K.; Lei, N.; Evariste, U.; Ma, P.P. Construction -of NiMoO4/CoMoO4 nanorod arrays wrapped by Ni-Co-S nanosheets on carbon cloth as high performance electrode for suppercapacitor. J. Alloy. Compd. 2019, 799, 415–424. [Google Scholar] [CrossRef]
- Zhao, W.; Zheng, Y.W.; Cui, L.; Jia, D.D.; Zheng, R.K.; Barrow, C.; Yang, W.R.; Liu, J.Q. M-OF derived Ni-Co-S nanosheets on electrochemically activated carbon cloth via an etching/ion exc-hange method for wearable hybrid supercapacitors. Chem. Eng. J. 2019, 371, 461–469. [Google Scholar] [CrossRef]
- Najjar, H.; Batis, H. Development of Mn-based perovskite materials: Chemical structure and applications. Catal. Rev.-Sci. Eng. 2016, 58, 371–438. [Google Scholar] [CrossRef]
- Louca, D.; Egami, T.; Brosha, E.L.; Roder, H.; Bishop, A.R. Local Jahn-Teller distortion in La1-xSrxMnO3 observed by pulsed neutron diffraction. Phys. Rev. B Condens. Matter. 1997, 56, R8475–R8478. [Google Scholar] [CrossRef]
- Ma, P.P.; Zhu, B.; Lei, N.; Liu, Y.K.; Yu, B.; Lu, Q.L.; Dai, J.M.; Li, S.H.; Jiang, G.H. Effect of Sr substitution on structure and electrochemical properties of perovskite-type LaMn0.9Ni0.1O3 nanofibers. Mater. Lett. 2019, 252, 23–26. [Google Scholar] [CrossRef]
- Lin, H.; Liu, P.; Wang, S.; Zhang, Z.; Dai, Z.; Tan, S.; Chen, D. A highly efficient electrocatalyst for oxygen reduction reaction: Three-dimensionally ordered macroporous perovskite LaMnO3. J. Power Sources 2019, 412, 701–709. [Google Scholar] [CrossRef]
- Zeng, S.; Zhao, R.; Li, A.; Xue, S.; Lv, D.; Luo, Q.; Shu, D.; Chen, H. MnO/carbon fibers prepared by an electrospinning method and their properties used as anodes for lithium ion batteries. Appl. Surf. Sci. 2019, 463, 211–216. [Google Scholar] [CrossRef]
- Li, Y.; Guan, B.; Maclennan, A.; Hu, Y.; Li, D.; Zhao, J.; Wang, Y.; Zhang, H. Porous waxberry-like MnO2/La2O3 microspheres for high performance asymmetric supercapacitor. Electrochim. Acta 2017, 241, 395–405. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Sun, Y.; Pang, C.; Zhuang, J.; Ouyang, P.; Li, Z.; Liu, S. Perovskite-type oxide LaMnO3: An efficient and recyclable heterogeneous catalyst for the wet aerobic oxidation of lignin to aromatic aldehydes. Catal. Lett. 2008, 126, 106–111. [Google Scholar] [CrossRef]
- Zhang, C.; Hua, W.; Wang, C.; Guo, Y.; Guo, Y.; Lu, G.; Baylet, A.; Giroir-Fendler, A. The effect of A-site substitution by Sr, Mg and Ce on the catalytic performance of LaMnO3 catalysts for the oxidation of vinyl chloride emission. Appl. Catal. B Environ. 2013, 134–135, 310–315. [Google Scholar] [CrossRef]
- Lang, X.Q.; Sun, X.C.; Liu, Z.T.; Nan, H.S.; Li, C.I.; Hu, X.Y.; Tian, H.W. Ag nanoparticles decorated perovskite La0.85Sr0.15MnO3 as electrodematerials for supercapacitors. Mater. Lett. 2019, 243, 34–37. [Google Scholar] [CrossRef]
- Nan, H.S.; Hu, X.Y.; Tian, H.W. Recent advances in perovskite oxides for anion-intercalation supercapacitor: A review. Mater. Sci. Semicond. Process. 2019, 94, 35–50. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, L.; Lin, H.; Ma, L.; Zhao, P.; Hu, Y.; Chen, T.; Chen, R.; Wang, Y.; Tie, Z.; et al. Walnut-like multicore-shell MnO encapsulated nitrogen-rich carbon nanocapsules as anode material for long-cycling and soft-packed lithium-ion batteries. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Li, L.; Zhu, J.; Niu, Y.; Chen, Z.; Liu, Y.; Liu, S.; Xu, M.; Li, C.M.; Jiang, J. Efficient production of coaxial core–shell MnO@carbon nanopipes for sustainable electrochemical energy storage applications. ACS Sustain. Chem. Eng. 2017, 5, 6288–6296. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Z.; Qi, X.; Ren, X.; Xu, G.; Wan, P.; Sun, X.; Zhang, H. Wall-like hierarchical metal oxide nanosheet arrays grown on carbon cloth for excellent supercapacitor electrodes. Nanoscale 2016, 8, 13273–13279. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Cao, P.; Cai, C.; Fu, Y.; Ma, X. Mesoporous composite nickel cobalt oxide/graphene oxide synthesized via a template-assistant co-precipitation route as electrode material for supercapacitors. J. Power Sources 2016, 306, 742–752. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Vijayakumar, S.; Ryu, K.S. Cerium oxide mixed LaMnO3 nanoparticles as the negative electrode for aqueous asymmetric supercapacitor devices. Mater. Chem. Phys. 2017, 199, 543–551. [Google Scholar] [CrossRef]
- Lang, X.; Zhang, H.; Xue, X.; Li, C.; Sun, X.; Liu, Z.; Nan, H.; Hu, X.; Tian, H. Rational design of La0.85Sr0.15MnO3@NiCo2O4core–shell architecture supported on Ni foam for high performance supercapacitors. J. Power Sources 2018, 402, 213–220. [Google Scholar] [CrossRef]
- Sun, S.; Huang, M.; Wang, P.; Lu, M. Controllable hydrothermal synthesis of Ni/Co MOF as hybrid advanced electrode materials for supercapacitor. J. Electrochem. Soc. 2019, 166, A1799–A1805. [Google Scholar] [CrossRef]
- Liu, Y.; Dinh, J.; Tade, M.O.; Shao, Z.P. Design of perovskite oxides as anion-intercalation-type electrodes for supercapacitors: Cation leaching effect. ACS Appl. Mater. Interfaces 2016, 8, 23774–23783. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Xu, J.C.; Hui, K.N.; Yang, Y.; Su, S.C.; Li, L.; Zhang, X.T.; Ng, K.W.; Wang, S.P.; Tang, Z.K. Homogeneous core/shell NiMoO4@NiMoO4 and activated carbon for high performance asymmetric supercapacitor. Nanomaterials 2019, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, X.; Xue, H.; Feng, L. A nitrogen-doped CoP nanoarray over 3D porous Co foam as an efficient bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2019, 7, 13242–13248. [Google Scholar] [CrossRef]
- Xue, W.D.; Yin, H.; Wang, W.J.; Zhao, R. Design and fabrication of petal-like NiCo2O4@NiMoO4 core/shell nanosheet arrys electrode for asymmetric supercapacitors batteries and energy storage. J. Electrochem. Soc. 2017, 164, A482–A489. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, P.; Lei, N.; Yu, B.; Liu, Y.; Jiang, G.; Dai, J.; Li, S.; Lu, Q. Flexible Supercapacitor Electrodes Based on Carbon Cloth-Supported LaMnO3/MnO Nano-Arrays by One-Step Electrodeposition. Nanomaterials 2019, 9, 1676. https://doi.org/10.3390/nano9121676
Ma P, Lei N, Yu B, Liu Y, Jiang G, Dai J, Li S, Lu Q. Flexible Supercapacitor Electrodes Based on Carbon Cloth-Supported LaMnO3/MnO Nano-Arrays by One-Step Electrodeposition. Nanomaterials. 2019; 9(12):1676. https://doi.org/10.3390/nano9121676
Chicago/Turabian StyleMa, Pianpian, Na Lei, Bo Yu, Yongkun Liu, Guohua Jiang, Jianming Dai, Shuhong Li, and Qiuling Lu. 2019. "Flexible Supercapacitor Electrodes Based on Carbon Cloth-Supported LaMnO3/MnO Nano-Arrays by One-Step Electrodeposition" Nanomaterials 9, no. 12: 1676. https://doi.org/10.3390/nano9121676
APA StyleMa, P., Lei, N., Yu, B., Liu, Y., Jiang, G., Dai, J., Li, S., & Lu, Q. (2019). Flexible Supercapacitor Electrodes Based on Carbon Cloth-Supported LaMnO3/MnO Nano-Arrays by One-Step Electrodeposition. Nanomaterials, 9(12), 1676. https://doi.org/10.3390/nano9121676