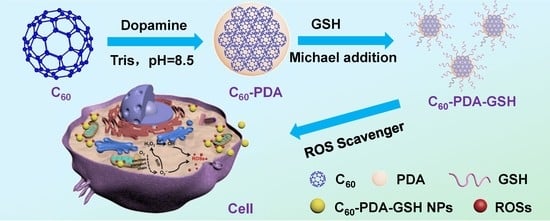
Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants
Abstract
:1. Introduction
2. Experimental Sections
2.1. Materials and Instrumentation
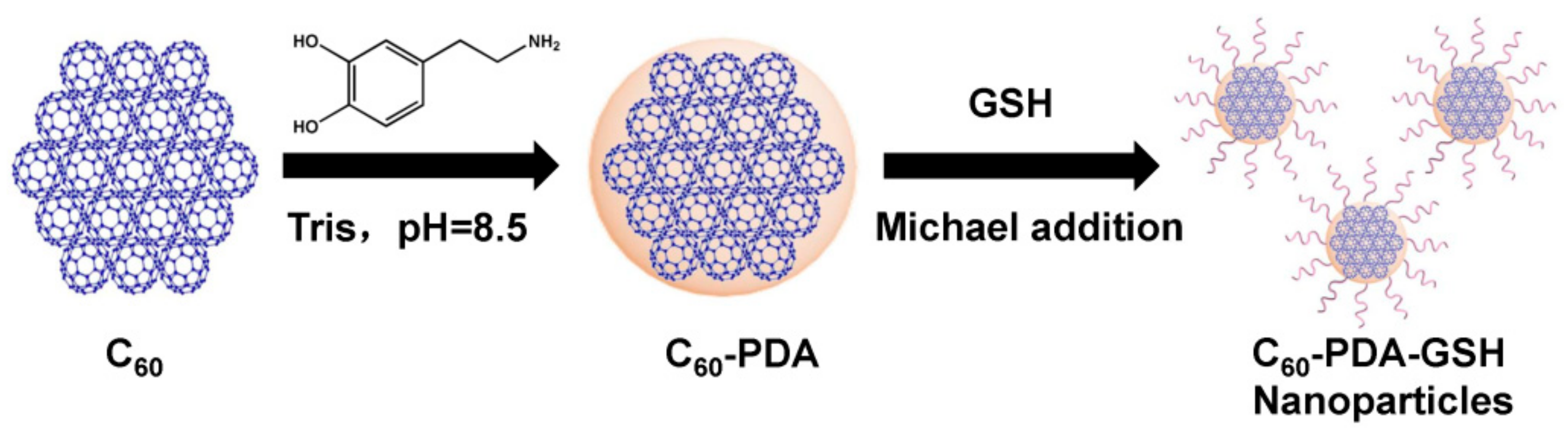
2.2. Preparation of C60-PDA-GSH
2.3. DPPH Scavenging Activity of C60-PDA-GSH
2.4. Scavenging Effects of C60-PDA-GSH on Hydroxyl Radicals
2.5. Cell Culture and Treatment
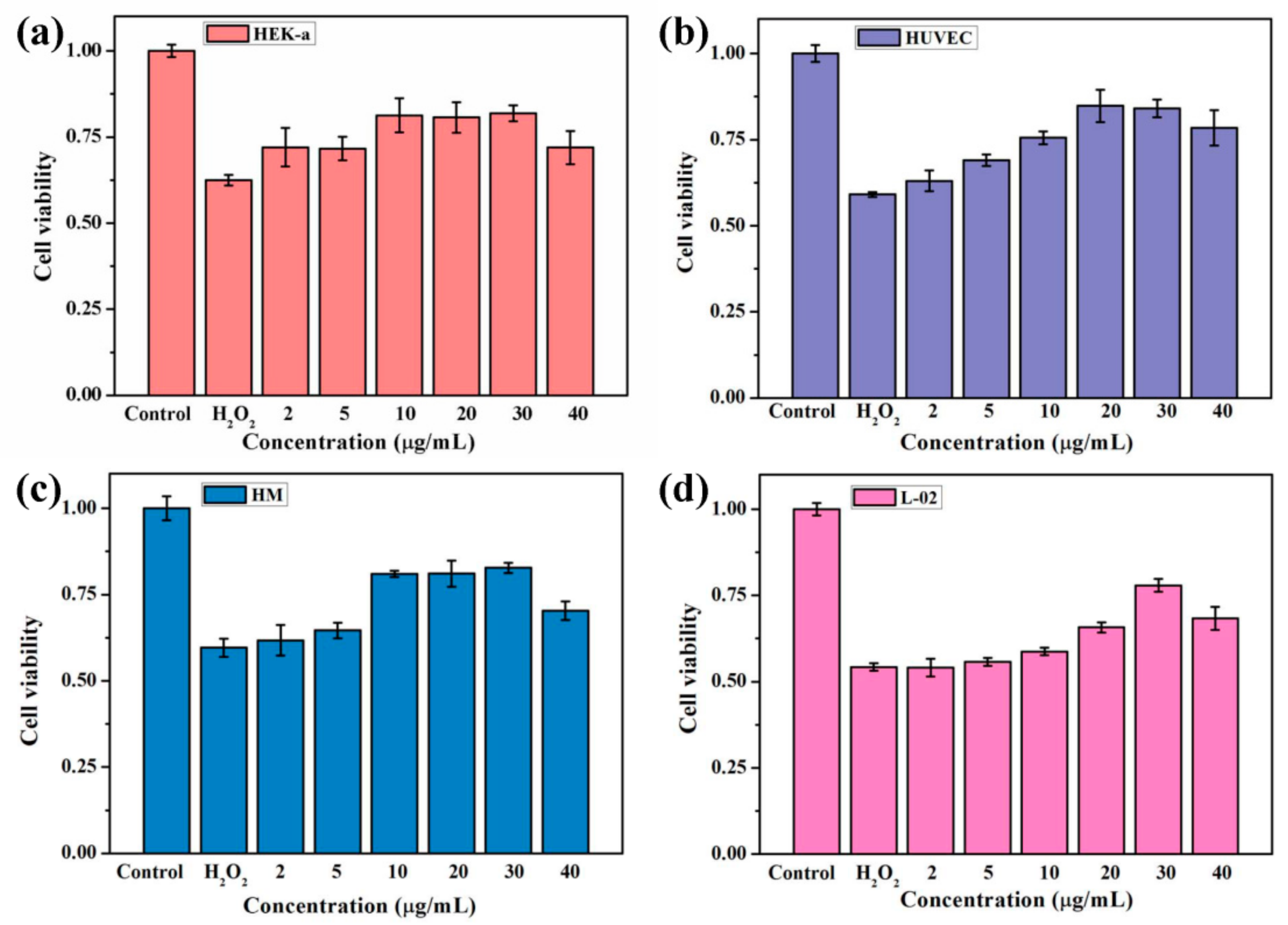
2.6. Cytotoxicity Test Using HEK-a Cells
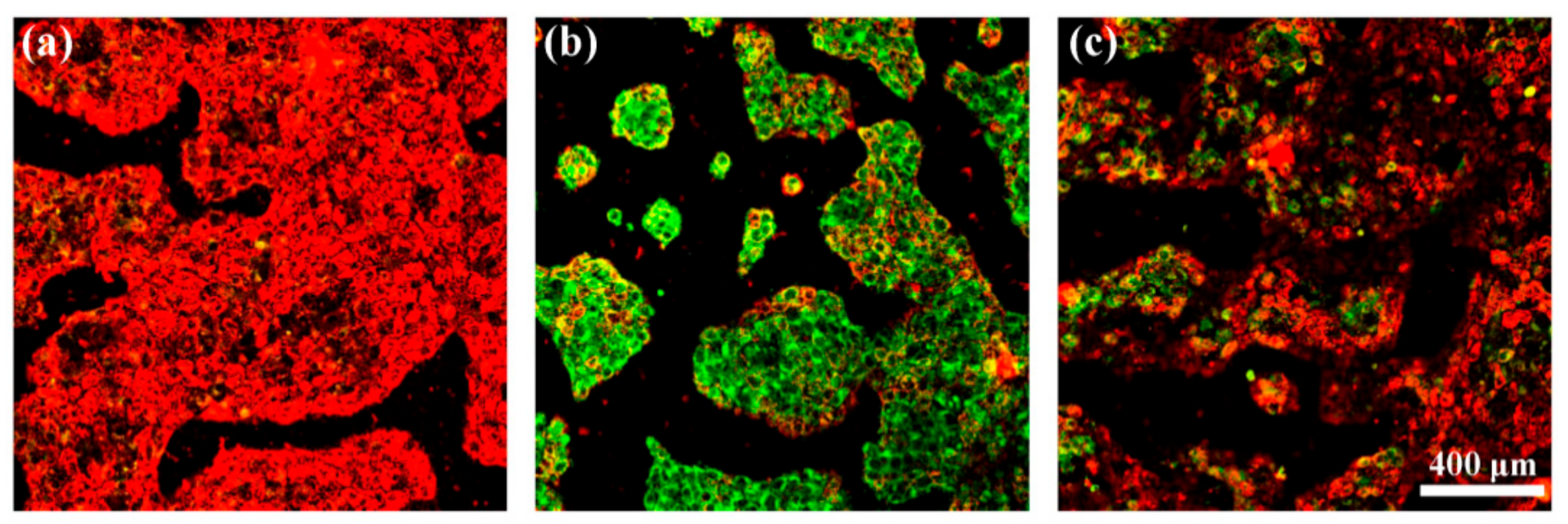
2.7. Protection of Cells against Oxidative Stress
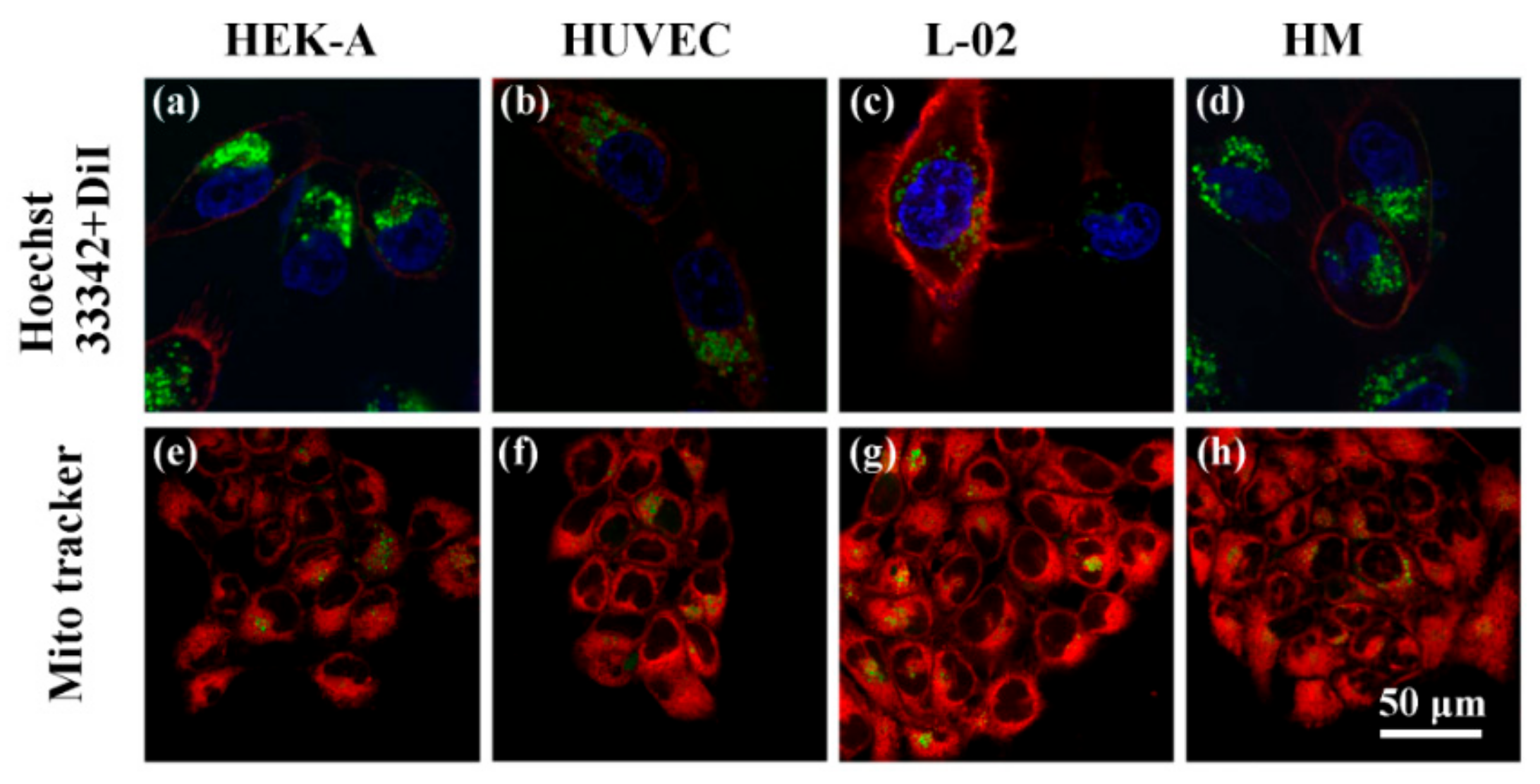
2.8. Fluorescence Costaining and Imaging
3. Results and Discussion
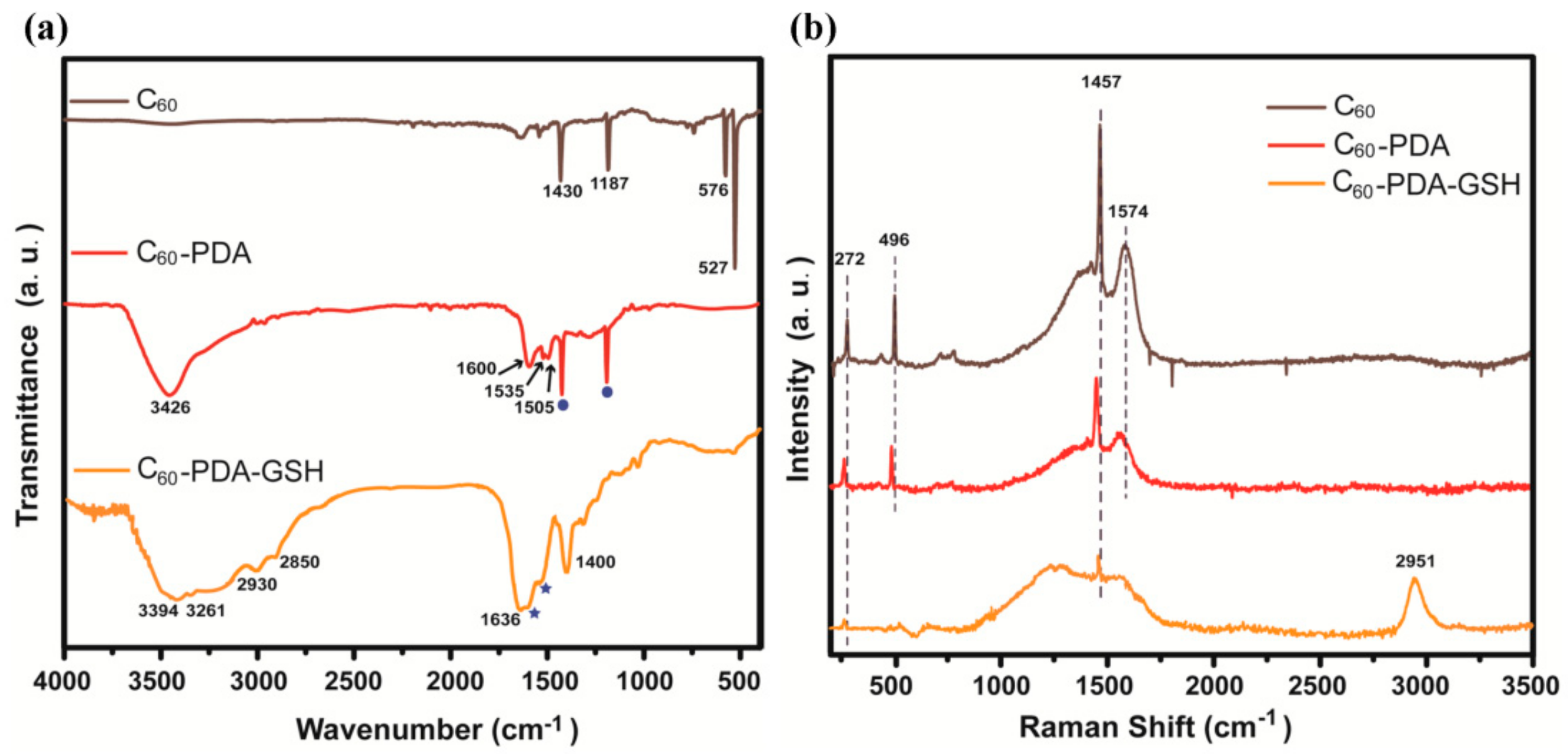
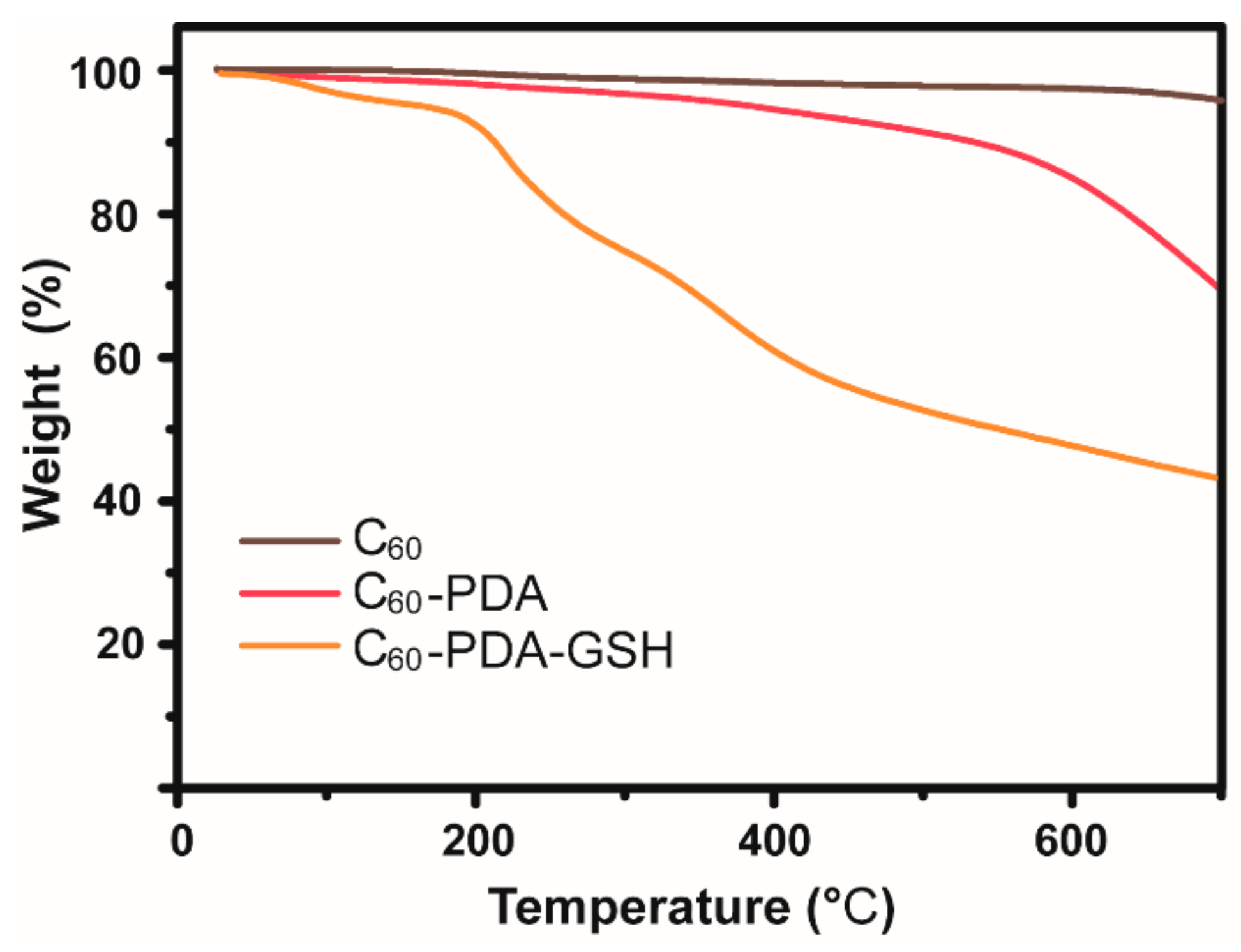
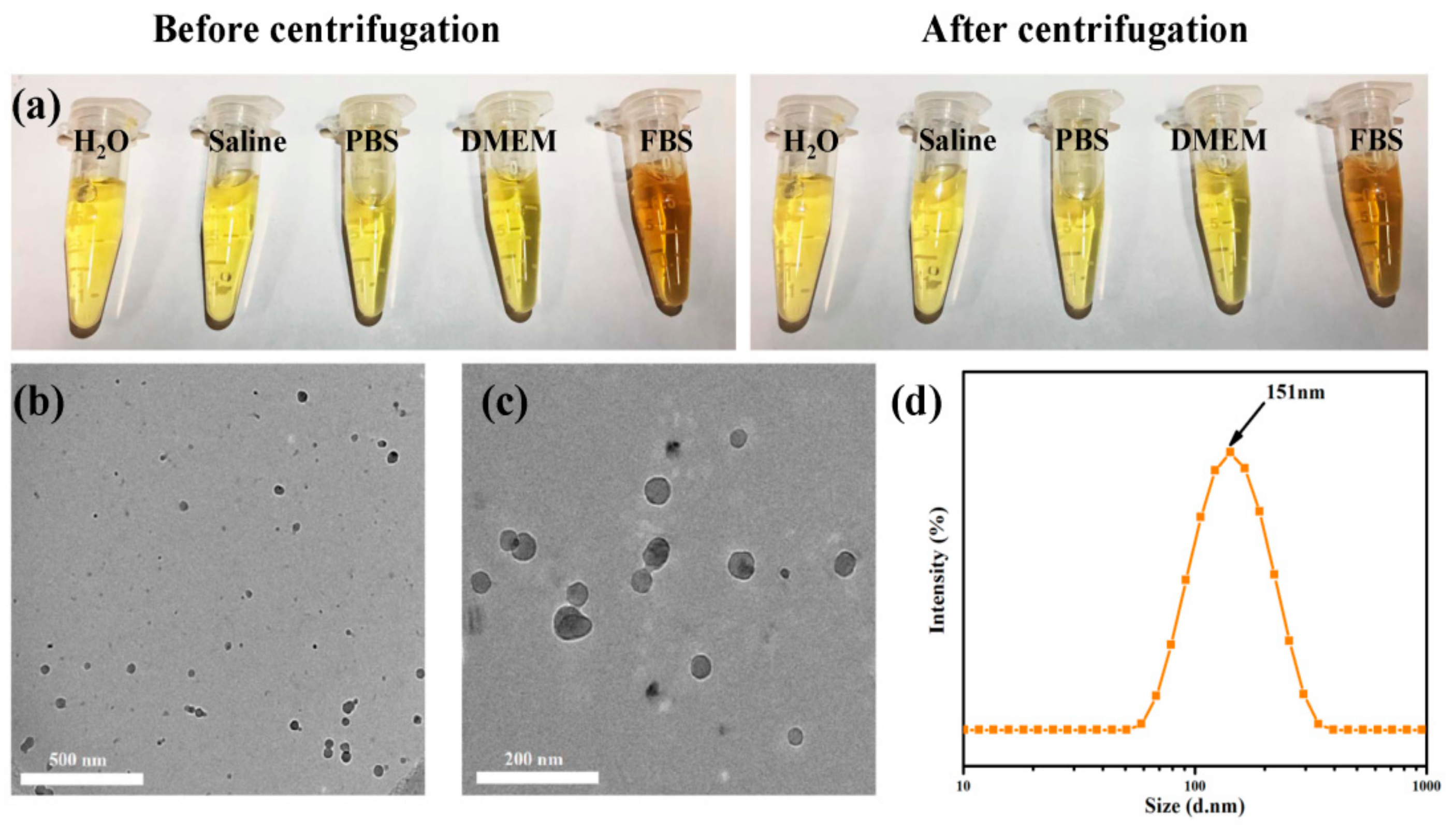
3.1. Characterizations of C60-PDA-GSH Nanoparticles
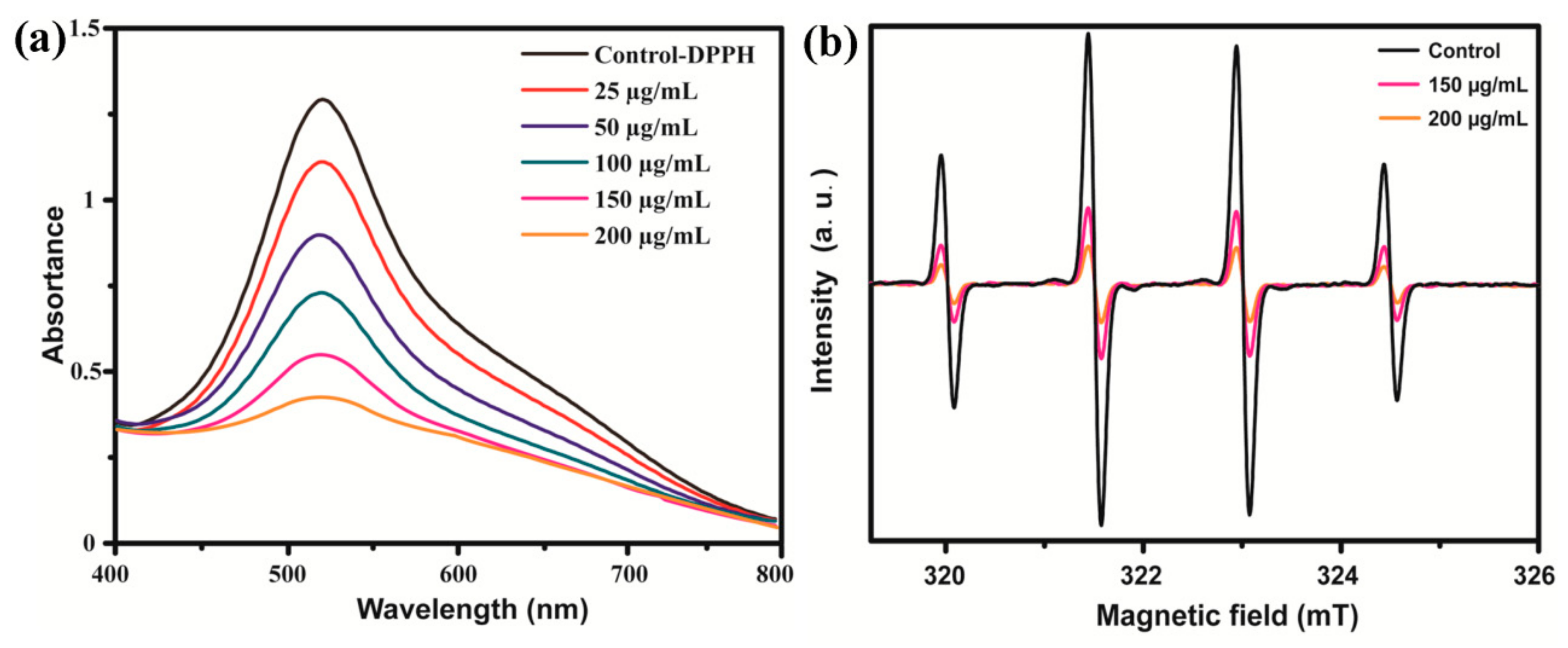
3.2. Scavenging Free Radicals Activity of C60-PDA-GSH Nanoparticles
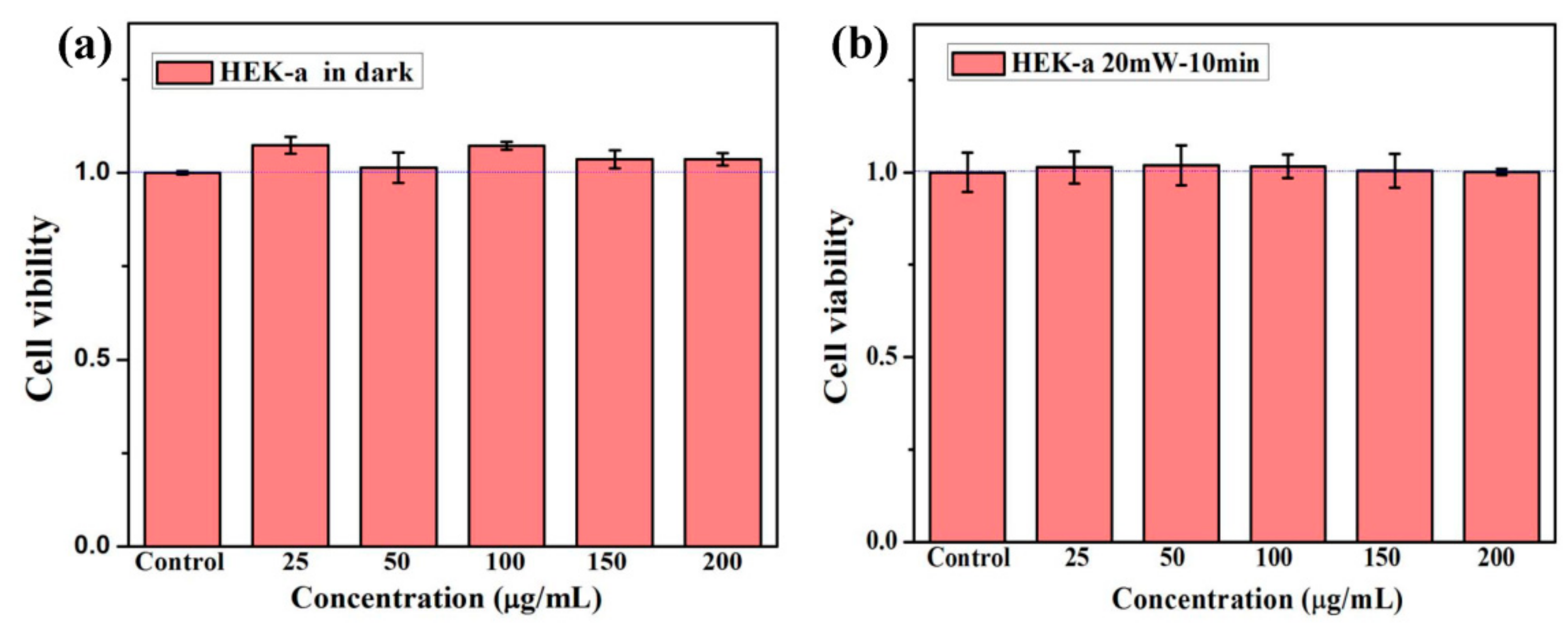
3.3. Celluar Performance of C60-PDA-GSH Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef]
- Dellinger, A.; Zhou, Z.G.; Connor, J.; Madhankumar, A.B.; Pamujula, S.; Sayes, C.M.; Kepley, C.L. Application of fullerenes in nanomedicine: An update. Nanomed. UK 2013, 8, 1191–1208. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Maroto, E.E.; Izquierdo, M.; Reboredo, S.; Marco-Martinez, J.; Filippone, S.; Martin, N. Chiral Fullerenes from Asymmetric Catalysis. Acc. Chem. Res. 2014, 47, 2660–2670. [Google Scholar] [CrossRef] [PubMed]
- Campisciano, V.; Gruttadauria, M.; Giacalone, F. Modified Nanocarbons for Catalysis. Chemcatchem 2019, 11, 90–133. [Google Scholar] [CrossRef]
- Cui, C.H.; Li, Y.W.; Li, Y.F. Fullerene Derivatives for the Applications as Acceptor and Cathode Buffer Layer Materials for Organic and Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1601251. [Google Scholar] [CrossRef]
- Gatti, T.; Menna, E.; Meneghetti, M.; Maggini, M.; Petrozza, A.; Lamberti, F. The Renaissance of fullerenes with perovskite solar cells. Nano Energy 2017, 41, 84–100. [Google Scholar] [CrossRef]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, Electrochemical, and Structural Properties of Endohedral Metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, M.A.; Chamberlain, T.W.; Khobystov, A.N. Harnessing the Synergistic and Complementary Properties of Fullerene and Transition-Metal Compounds for Nanomaterial Applications. Chem. Rev. 2015, 115, 11301–11351. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.T.; Chen, C.H.; Chen, N.; Echegoyen, L. Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity. Acc. Chem. Res. 2019, 52, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, H.; Finkel, T. Unraveling the Truth About Antioxidants: ROS and disease: Finding the right balance. Nat. Med. 2014, 20, 711. [Google Scholar] [CrossRef] [PubMed]
- Pietraforte, D.; Malorni, W. Focusing at the Double-Edged Sword of Redox Imbalance: Signals for Cell Survival or for Cell Death? Antioxid. Redox Signal. 2014, 21, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.J.; Lao, F.; Fu, P.P.; Wamer, W.G.; Zhao, Y.L.; Wang, P.C.; Qiu, Y.; Sun, B.Y.; Xing, G.M.; Dong, J.Q.; et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials 2009, 30, 611–621. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. BBA-Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Ma, H.J.; Zhen, M.M.; Guo, J.; Wang, L.P.; Jiang, L.; Shu, C.Y.; Wang, C.R. Biocompatible [60]/[70] Fullerenols: Potent Defense against Oxidative Injury Induced by Reduplicative Chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 35539–35547. [Google Scholar] [CrossRef]
- Oberdörster, E. Manufactured Nanomaterials (Fullerenes, C60) Induce Oxidative Stress in the Brain of Juvenile Largemouth Bass. Environ. Health Perspect. 2004, 112, 1058–1062. [Google Scholar] [CrossRef]
- Kovochich, M.; Espinasse, B.; Auffan, M.; Hotze, E.M.; Wessel, L.; Xia, T.; Nel, A.E.; Wiesner, M.R. Comparative Toxicity of C60 Aggregates toward Mammalian Cells: Role of Tetrahydrofuran (THF) Decomposition. Environ. Sci. Technol. 2009, 43, 6378–6384. [Google Scholar] [CrossRef]
- Samal, S.; Geckeler, K.E. Cyclodextrin–fullerenes: A new class of water-soluble fullerenes. Chem. Commun. 2000, 13, 1101–1102. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, D.; Liu, Y. Polysaccharide-porphyrin-fullerene supramolecular conjugates as photo-driven DNA cleavage reagents. Chem. Commun. 2015, 51, 12266–12269. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Doi, Y.; Hashizume, M.; Kikuchi, J.I.; Konishi, T. An Extremely Effective DNA Photocleavage Utilizing Functionalized Liposomes with a Fullerene-Enriched Lipid Bilayer. J. Am. Chem. Soc. 2007, 129, 4140–4141. [Google Scholar] [CrossRef] [PubMed]
- Antoku, D.; Sugikawa, K.; Ikeda, A. Photodynamic Activity of Fullerene Derivatives Solubilized in Water by Natural-Product-Based Solubilizing Agents. Chem. A Eur. J. 2019, 25, 1854–1865. [Google Scholar] [CrossRef]
- Grebowski, J.; Kazmierska, P.; Krokosz, A. Fullerenols as a New Therapeutic Approach in Nanomedicine. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Postnov, V.N.; Sharoyko, V.V.; Vorotyntsev, I.V.; Galagudza, M.M.; Murin, I.V. Fullerenols: Physicochemical properties and applications. Prog. Solid State Chem. 2016, 44, 59–74. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Suzuki, T.; Ishii, H.; Nakae, D.; Ogata, A. Cytotoxic effects of hydroxylated fullerenes on isolated rat hepatocytes via mitochondrial dysfunction. Arch. Toxicol. 2011, 85, 1429–1440. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Liu, M.Y.; Zeng, G.J.; Wang, K.; Wan, Q.; Tao, L.; Zhang, X.Y.; Wei, Y. Recent developments in polydopamine: An emerging soft matter for surface modification and biomedical applications. Nanoscale 2016, 8, 16819–16840. [Google Scholar] [CrossRef]
- Qu, K.G.; Wang, Y.H.; Vasileff, A.; Jiao, Y.; Chen, H.Y.; Zheng, Y. Polydopamine-inspired nanomaterials for energy conversion and storage. J. Mater. Chem. A 2018, 6, 21827–21846. [Google Scholar] [CrossRef]
- Mrowczynski, R.; Markiewicz, R.; Liebscher, J. Chemistry of polydopamine analogues. Polym. Int. 2016, 65, 1288–1299. [Google Scholar] [CrossRef]
- Barclay, T.G.; Hegab, H.M.; Clarke, S.R.; Ginic-Markovic, M. Versatile Surface Modification Using Polydopamine and Related Polycatecholamines: Chemistry, Structure, and Applications. Adv. Mater. Interfaces 2017, 4, 1601192. [Google Scholar] [CrossRef]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busque, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Huang, Q.; Deng, F.J.; Huang, H.Y.; Wan, Q.; Liu, M.Y.; Wei, Y. Mussel-inspired fabrication of functional materials and their environmental applications: Progress and prospects. Appl. Mater. Today 2017, 7, 222–238. [Google Scholar] [CrossRef]
- Cheng, W.; Zeng, X.W.; Chen, H.Z.; Li, Z.M.; Zeng, W.F.; Mei, L.; Zhao, Y.L. Versatile Polydopamine Platforms: Synthesis and Promising Applications for Surface Modification and Advanced Nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, Y.; Zhang, C.; Liu, L.; Li, J.; Wang, Y. Graphene–polydopamine–C60 nanohybrid: An efficient protective agent for NO-induced cytotoxicity in rat pheochromocytoma cells. J. Mater. Chem. B 2014, 2, 8587–8597. [Google Scholar] [CrossRef]
- Qu, K.G.; Zheng, Y.; Dai, S.; Qiao, S.Z. Graphene oxide-polydopamine derived N,S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Q.; Liu, M.; Tian, J.; Zeng, G.; Li, Z.; Wang, K.; Zhang, Q.; Wan, Q.; Deng, F.; et al. Preparation of amine functionalized carbon nanotubes via a bioinspired strategy and their application in Cu2+ removal. Appl. Surf. Sci. 2015, 343, 19–27. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Xu, D.; Mao, L.; Huang, Q.; Deng, F.; Tian, J.; Wen, Y.; Zhang, X.; Wei, Y. Facile fabrication of glycosylated and PEGylated carbon nanotubes through the combination of mussel inspired chemistry and surface-initiated ATRP. Mater. Sci. Eng. C 2020, 106, 110157. [Google Scholar] [CrossRef]
- Shi, Y.G.; Liu, M.Y.; Wang, K.; Deng, F.J.; Wan, Q.; Huang, Q.; Fu, L.H.; Zhang, X.Y.; Wei, Y. Bioinspired preparation of thermo-responsive graphene oxide nanocomposites in an aqueous solution. Polym. Chem. 2015, 6, 5876–5883. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Huang, Q.; Jiang, R.; Huang, H.; Deng, F.; Wen, Y.; Tian, J.; Zhang, X.; Wei, Y. A novel light-induced ATRP for the preparation of water dispersible fluorescent nanodiamonds and their biological imaging applications. Ceram. Int. 2018, 44, 9907–9914. [Google Scholar] [CrossRef]
- Li, S.; Yang, P.; Liu, X.; Zhang, J.; Xie, W.; Wang, C.; Liu, C.; Guo, Z. Graphene oxide based dopamine mussel-like cross-linked polyethylene imine nanocomposite coating with enhanced hexavalent uranium adsorption. J. Mater. Chem. A 2019, 7, 16902–16911. [Google Scholar] [CrossRef]
- Yu, L.; Lin, F.; Xiao, W.; Luo, D.; Xi, J. CNT@polydopamine embedded mixed matrix membranes for high-rate and long-life vanadium flow batteries. J. Membr. Sci. 2018, 549, 411–419. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Cheng, Y.; Chen, X.; Li, Y.; Zhang, A. Mussel-inspired preparation of C60 nanoparticles as photo-driven DNA cleavage reagents. New J. Chem. 2018, 42, 18102–18108. [Google Scholar] [CrossRef]
- Sabounchei, S.J.; Hashemi, A.; Sayadi, M.; Bayat, M.; Sedghi, A.; Karamian, R.; Moazzami Farida, S.H.; Gable, R.W. New highly soluble [6,6]-methanofullerene derivatives incorporating both α-keto and α, β-ester stabilized phosphorus ylides; synthesis, characterization, theoretical and biological studies. J. Mol. Struct. 2018, 1165, 142–152. [Google Scholar] [CrossRef]
- Li, J.; Guan, M.R.; Wang, T.S.; Zhen, M.M.; Zhao, F.W.; Shu, C.Y.; Wang, C.R. Gd@C-82-(ethylenediamine)(8) Nanoparticle: A New High-Efficiency Water-Soluble ROS Scavenger. ACS Appl. Mater. Interfaces 2016, 8, 25770–25776. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, M.Y.; Zhang, Y.L.; Yang, B.; Ji, Y.; Feng, L.; Tao, L.; Li, S.X.; Wei, Y. Combining mussel-inspired chemistry and the Michael addition reaction to disperse carbon nanotubes. RSC Adv. 2012, 2, 12153–12155. [Google Scholar] [CrossRef]
- Guan, J.; Zhong, X.W.; Chen, X.; Zhu, X.J.; Li, P.L.; Wu, J.H.; Lu, Y.L.; Yu, Y.; Yang, S.F. Expanding pore sizes of ZIF-8-derived nitrogen-doped microporous carbon via C-60 embedding: Toward improved anode performance for the lithium-ion battery. Nanoscale 2018, 10, 2473–2480. [Google Scholar] [CrossRef]
- Wei, T.; Martin, O.; Yang, S.F.; Hauke, F.; Hirsch, A. Modular Covalent Graphene Functionalization with C-60 and the Endohedral Fullerene Sc3N@C-80: A Facile Entry to Synthetic-Carbon-Allotrope Hybrids. Angew. Chem. Int. Ed. 2019, 58, 816–820. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K. Efficient Synthesis of Heteroatom (N or S)-Doped Graphene Based on Ultrathin Graphene Oxide-Porous Silica Sheets for Oxygen Reduction Reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Dou, J.; Huang, Q.; Huang, H.; Gan, D.; Chen, J.; Deng, F.; Wen, Y.; Zhu, X.; Zhang, X.; Wei, Y. Mussel-inspired preparation of layered double hydroxides based polymer composites for removal of copper ions. J. Colloid Interface Sci. 2019, 533, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhen, M.; Li, J.; Zhou, Y.; Ma, H.; Jia, W.; Wang, C. Anti-apoptosis effect of amino acid modified gadofullerene via a mitochondria mediated pathway. Dalton Trans. 2019, 48, 7884–7890. [Google Scholar] [CrossRef] [PubMed]
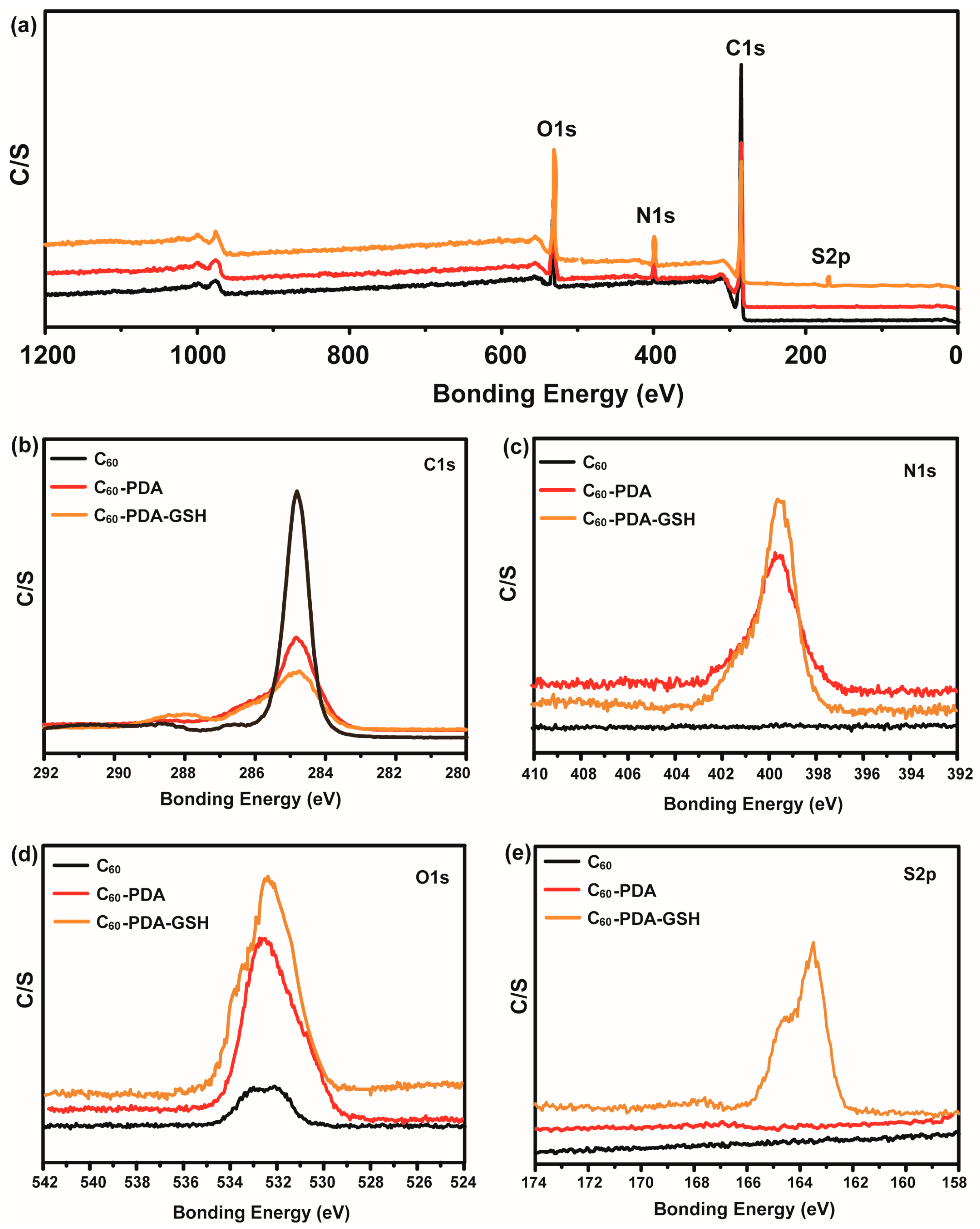
| Samples | C1s (At%) | N1s (At%) | O1s (At%) | S2p (At%) |
|---|---|---|---|---|
| C60 | 97.50 | 0 | 2.50 | 0 |
| C60-PDA | 77.41 | 6.48 | 16.11 | 0 |
| C60-PDA-GSH | 66.51 | 9.08 | 22.14 | 2.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ma, Y.; Fu, S.; Zhang, A. Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants. Nanomaterials 2019, 9, 1647. https://doi.org/10.3390/nano9121647
Zhang X, Ma Y, Fu S, Zhang A. Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants. Nanomaterials. 2019; 9(12):1647. https://doi.org/10.3390/nano9121647
Chicago/Turabian StyleZhang, Xiaoyan, Yihan Ma, Sheng Fu, and Aiqing Zhang. 2019. "Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants" Nanomaterials 9, no. 12: 1647. https://doi.org/10.3390/nano9121647
APA StyleZhang, X., Ma, Y., Fu, S., & Zhang, A. (2019). Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants. Nanomaterials, 9(12), 1647. https://doi.org/10.3390/nano9121647