Synthesis of Au@Pt Core—Shell Nanoparticles as Efficient Electrocatalyst for Methanol Electro-Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AuNP Seeds
2.3. Synthesis of Au@Pt Bimetallic Core–Shell Nanoparticles
2.4. Structural Characterization
2.5. Preparation of Electrocatalysts
2.6. Electrocatalytic Measurements
3. Results
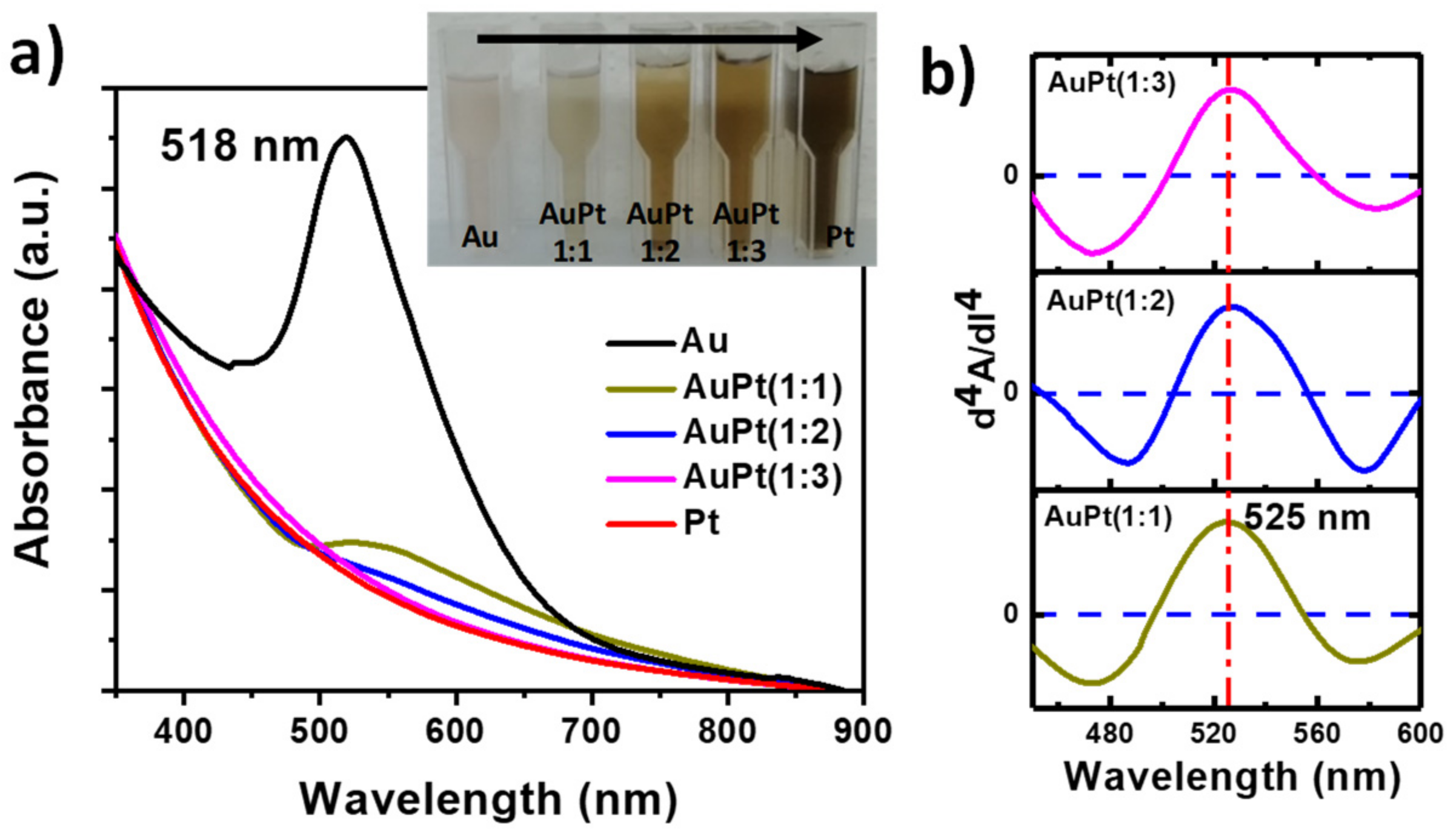
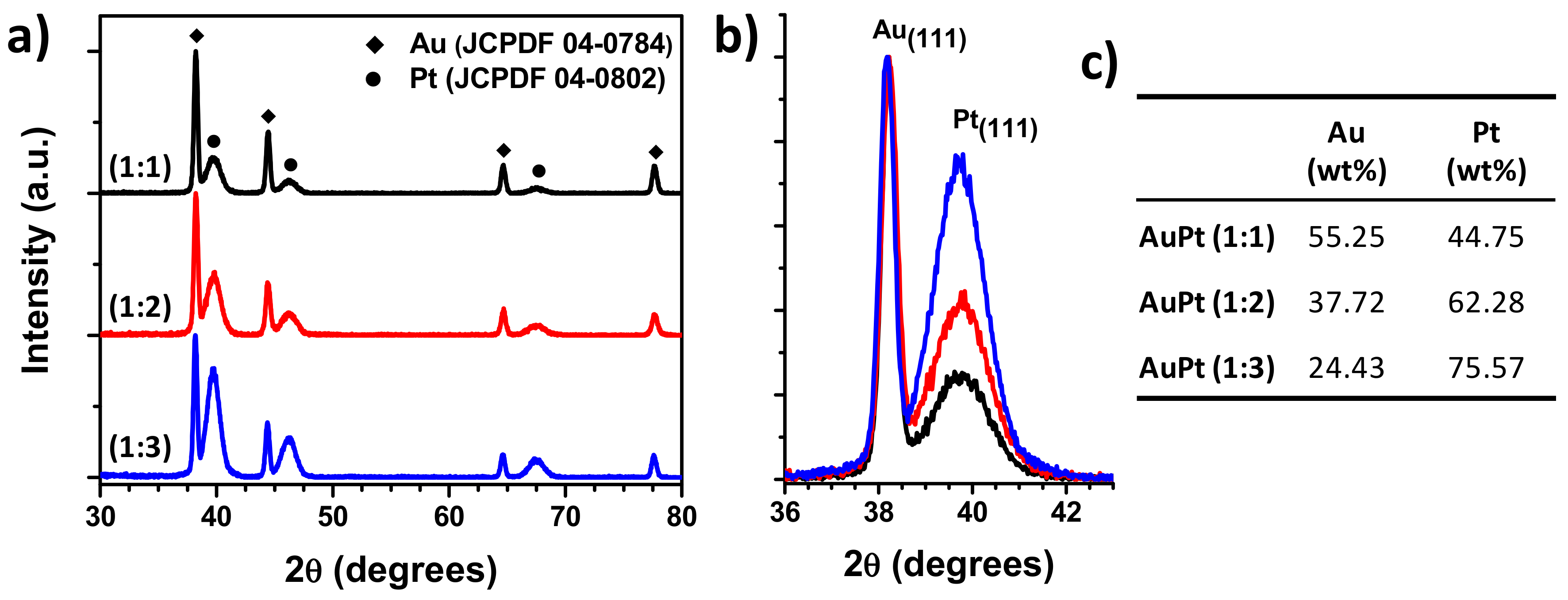
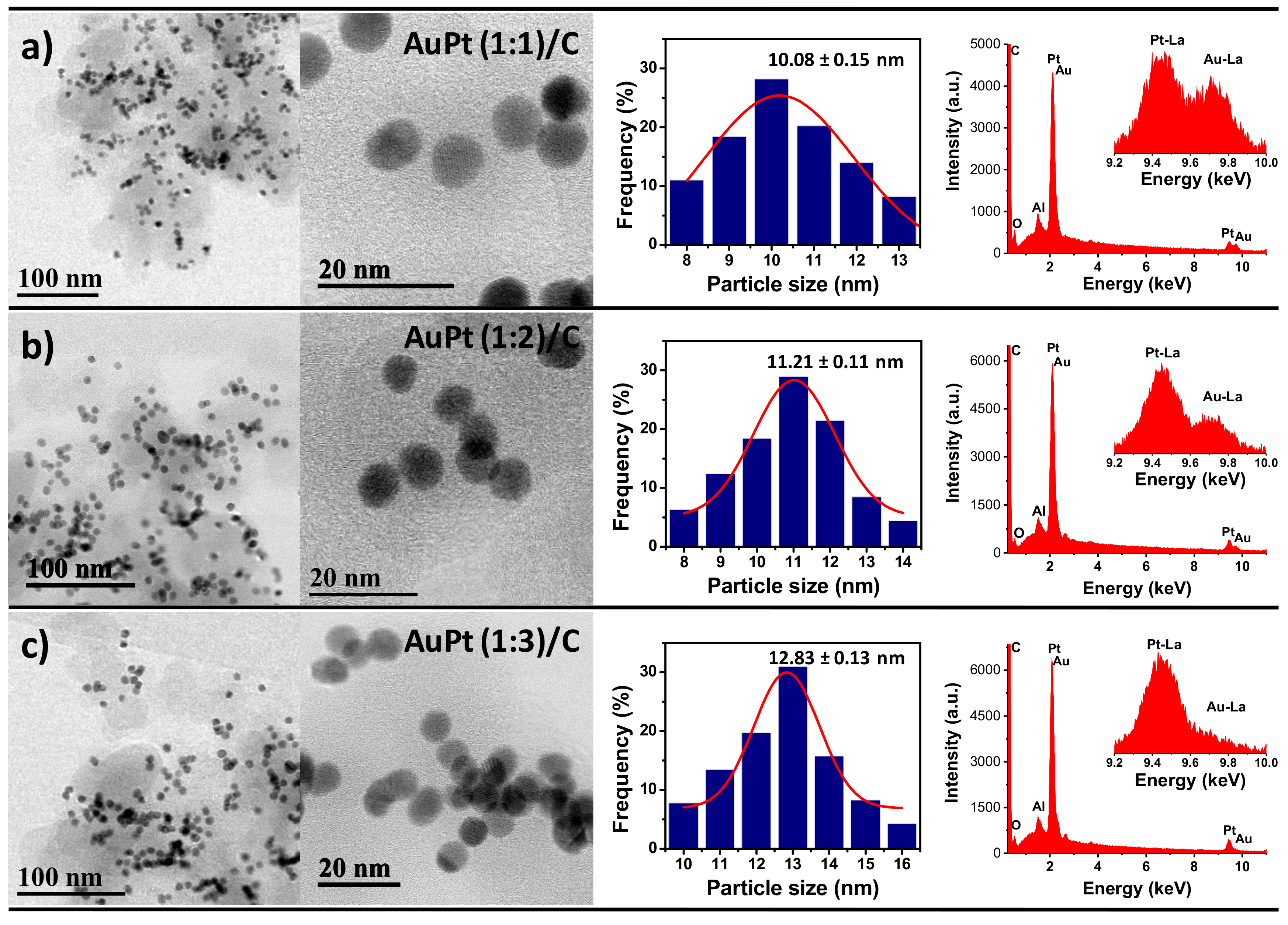
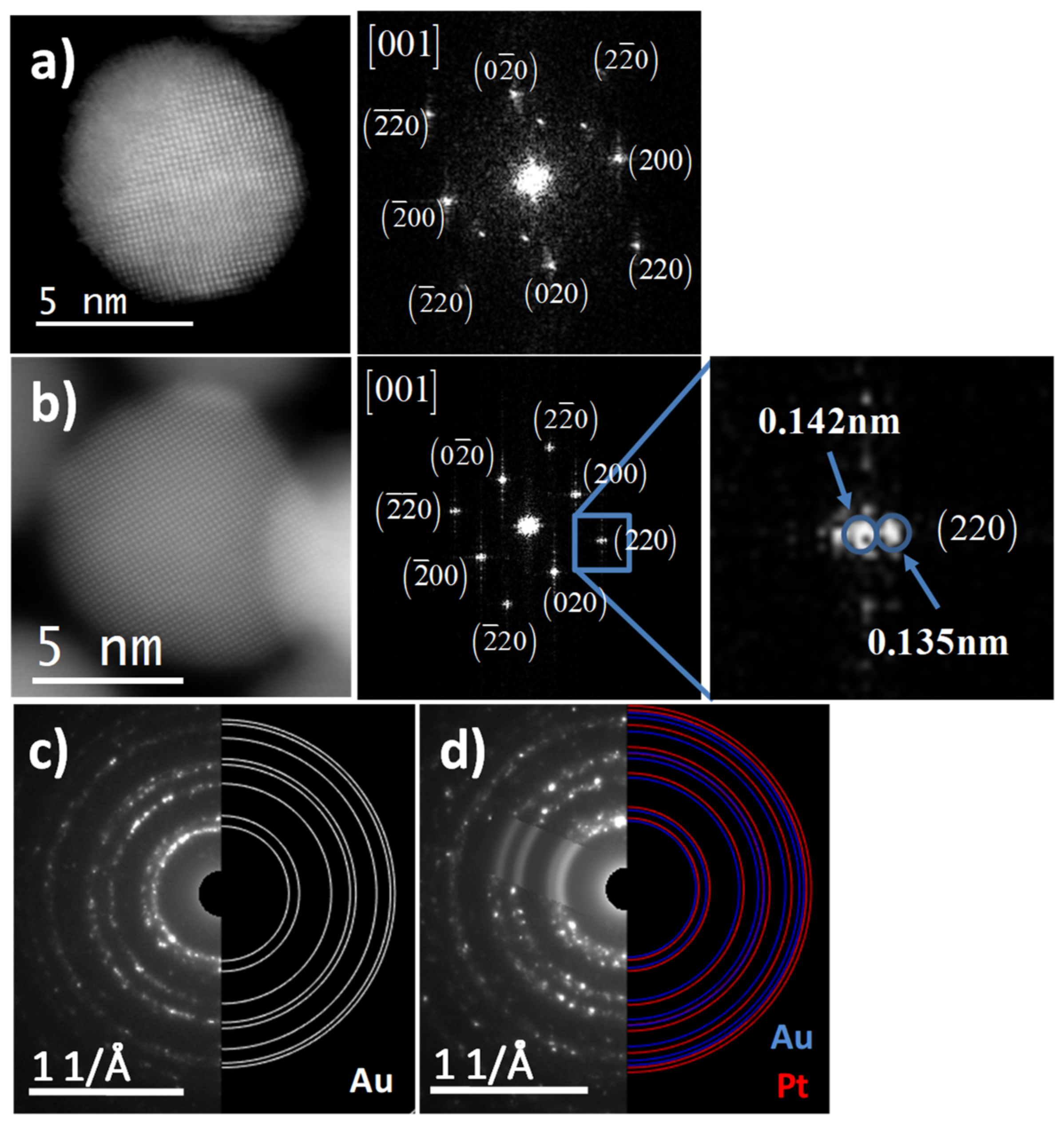
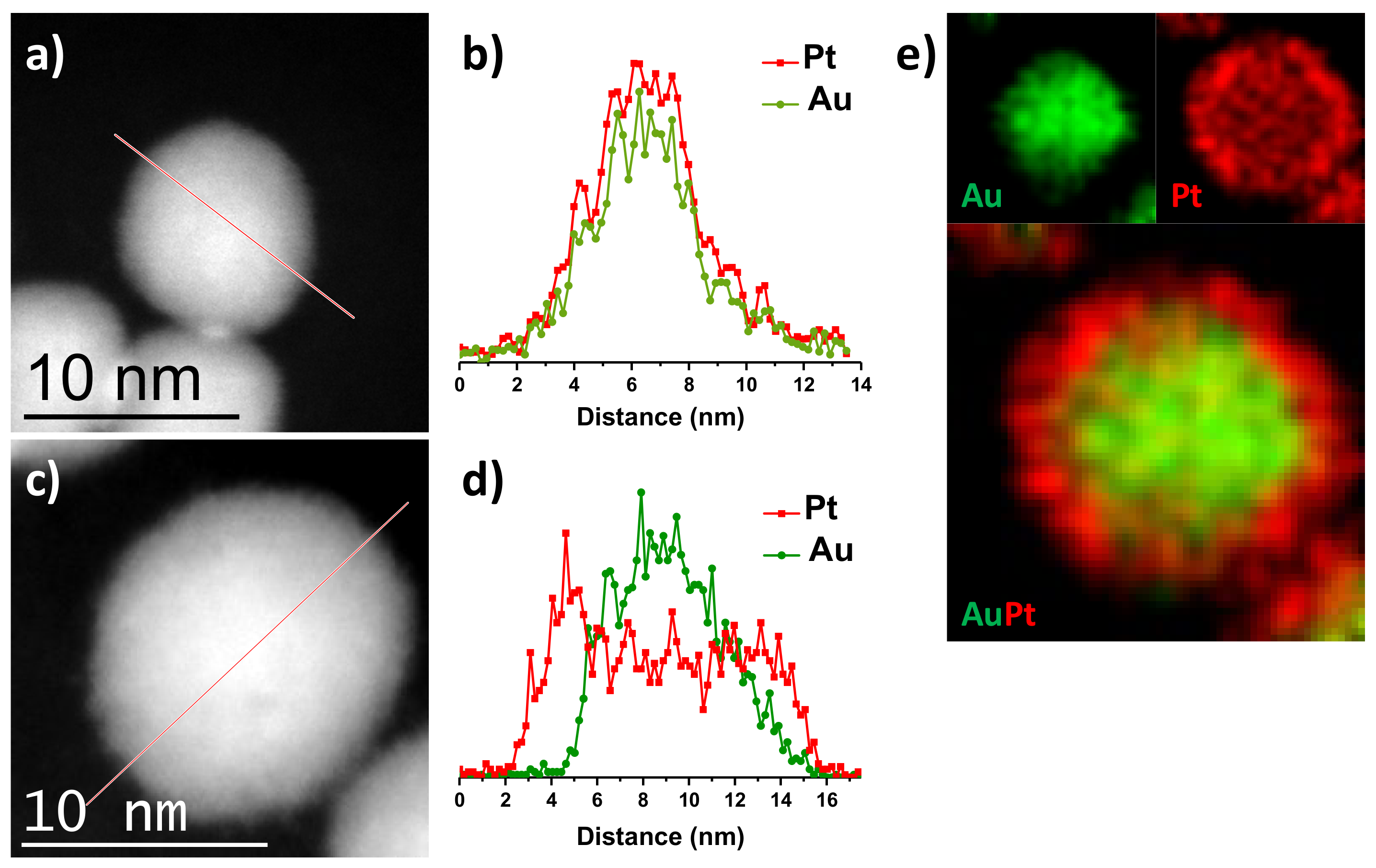
3.1. Structural Characterization
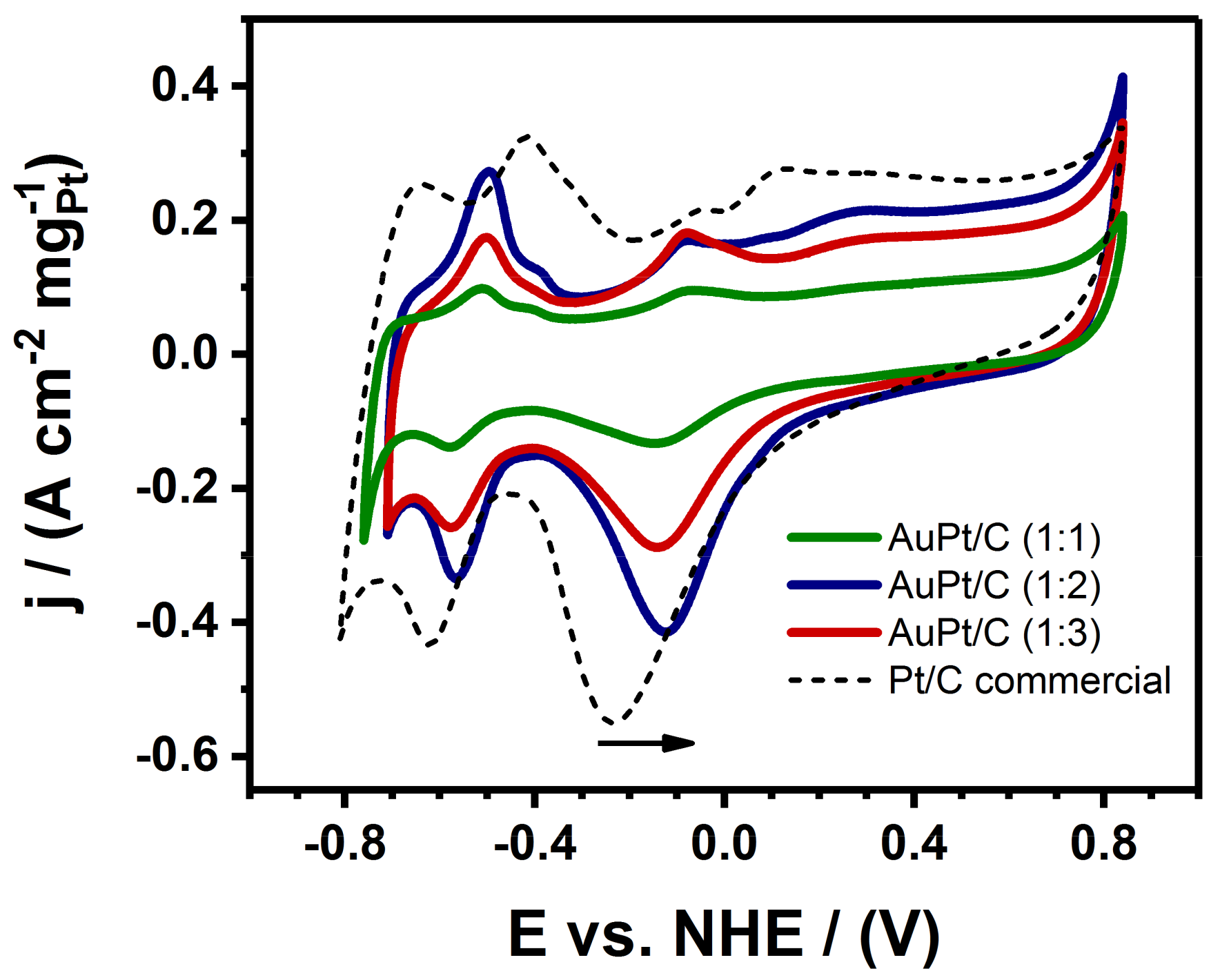
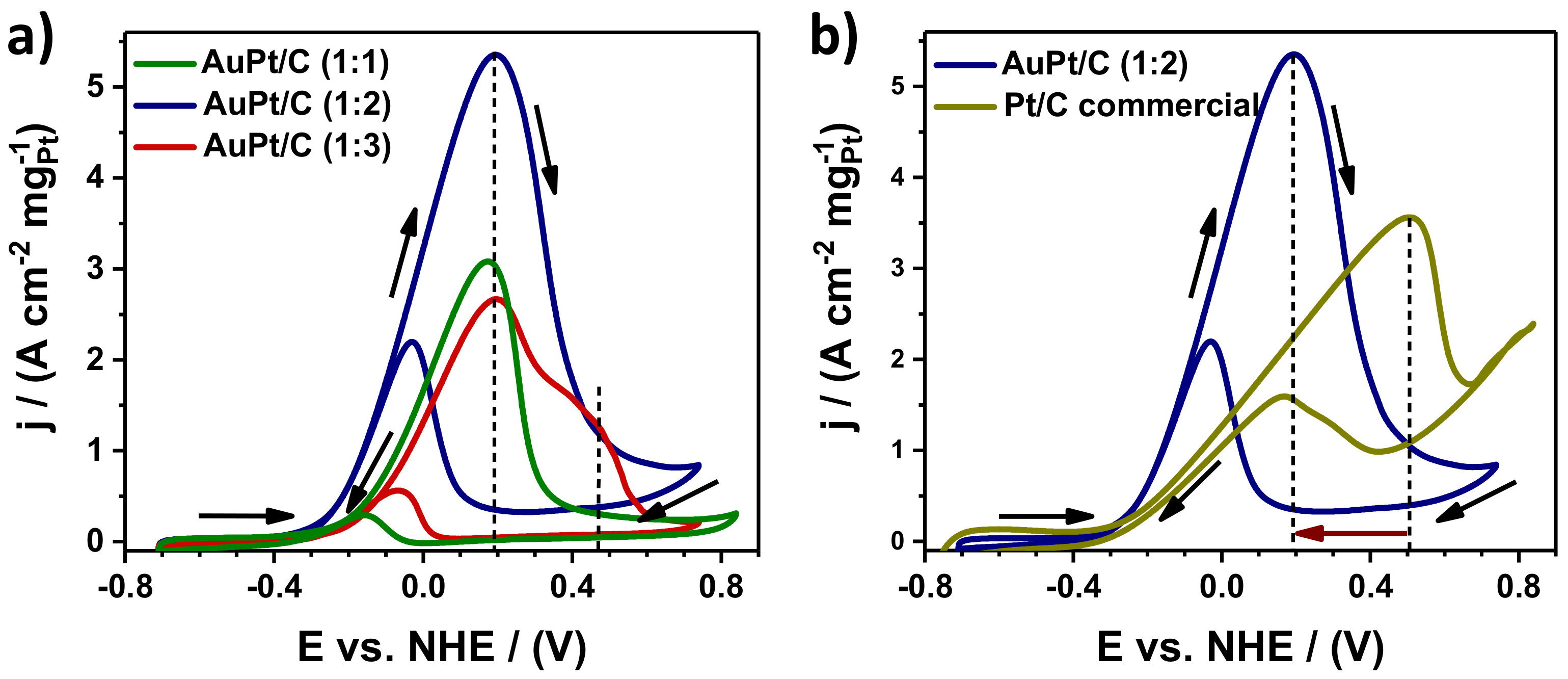
3.2. Electrocatalytic Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Yin, M.; Ma, L.; Liang, L.; Liu, C.; Liao, J.; Lu, T.; Xing, W. Recent Advances in Catalysts for Direct Methanol Fuel Cells. Energy Environ. Sci. 2011, 4, 2736–2753. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, M.; Kwon, H.C.; Cho, S.J.; Yun, S.; Kim, H.T.; Mayrhofer, K.J.J.; Kim, H.; Choi, M. Tuning Selectivity of Electrochemical Reactions by Atomically Dispersed Platinum Catalyst. Nat. Commun. 2016, 7, 10922. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Varela, A.S.; Kuhl, S.; Strasser, P.; Cuenya, B.R. Nanostructured Electrocatalysts with Tunable Activity and Selectivity. Nat. Rev. Mater. 2016, 1, 16009. [Google Scholar] [CrossRef]
- Chen, A.; Holt Hindle, P. Platinum-Based Nanostructured Materials: Synthesis, Properties, and Applications. Chem. Rev. 2010, 110, 3767–3804. [Google Scholar] [CrossRef]
- Banham, D.; Ye, S. Current Status and Future Development of Catalyst Materials and Catalyst Layers for Proton Exchange Membrane Fuel Cells: An Industrial Perspective. ACS Energy Lett. 2017, 2, 629–638. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wang, F.F.; Mao, B.W.; Zhan, D.; Tian, Z.Q. Mobility and Reactivity of Oxygen Adspecies on Platinum Surface. J. Am. Chem. Soc. 2016, 138, 9057–9060. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Z.; Ma, C.; Liu, Y.; Giroux, M.; Chi, M.; Jin, J.; Greeley, J.; Wang, C. Plating Precious Metals on Nonprecious Metal Nanoparticles for Sustainable Electrocatalysts. Nano Lett. 2017, 17, 3391–3395. [Google Scholar] [CrossRef]
- Lim, J.; Shin, H.; Kim, M.; Lee, H.; Lee, K.S.; Kwon, Y.; Song, D.; Oh, S.; Kim, H.; Cho, E. Ga–Doped Pt–Ni Octahedral Nanoparticles as a Highly Active and Durable Electrocatalyst for Oxygen Reduction Reaction. Nano Lett. 2018, 18, 2450–2458. [Google Scholar] [CrossRef]
- Wang, C.; Sang, X.; Gamler, J.T.L.; Chen, D.P.; Unocic, R.R.; Skrabalak, S.E. Facet-Dependent Deposition of Highly Strained Alloyed Shells on Intermetallic Nanoparticles for Enhanced Electrocatalysis. Nano Lett. 2017, 17, 5526–5532. [Google Scholar] [CrossRef]
- Bian, T.; Zhang, H.; Jiang, Y.; Jin, C.; Wu, J.; Yang, H.; Yang, D. Epitaxial Growth of Twinned Au–Pt Core–Shell Star-Shaped Decahedra as Highly Durable Electrocatalysts. Nano Lett. 2015, 15, 7808–7815. [Google Scholar] [CrossRef]
- Xie, S.; Choi, S.I.; Lu, N.; Roling, L.T.; Herron, J.A.; Zhang, L.; Park, J.; Wang, J.; Kim, M.J.; Xie, Z.; et al. Atomic Layer-By-Layer Deposition of Pt on Pd Nanocubes for Catalysts with Enhanced Activity and Durability toward Oxygen Reduction. Nano Lett. 2014, 14, 3570–3576. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shan, H.; Li, G.; Xiao, F.; Jiang, Y.; Yan, Y.; Jin, C.; Zhang, H.; Wu, J.; Yang, D. Epitaxial Growth of Multimetallic Pd@PtM (M= Ni, Rh, Ru) Core–Shell Nanoplates Realized by in Situ-Produced CO from Interfacial Catalytic Reactions. Nano Lett. 2016, 16, 7999–8004. [Google Scholar] [CrossRef] [PubMed]
- Adzic, R.R. Platinum Monolayer Electrocatalysts: Tunable Activity, Stability, and Self-Healing Properties. Electrocatalysis 2012, 3, 163–169. [Google Scholar] [CrossRef]
- Wang, X.; Choi, S.I.; Roling, L.T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M.; Liu, J.; Xie, Z.; Herron, J.A.; et al. Palladium–Platinum Core-Shell Icosahedra with Substantially Enhanced Activity and Durability towards Oxygen Reduction. Nat. Commun. 2015, 6, 7594. [Google Scholar] [CrossRef]
- Ruge, M.; Drnec, J.; Rahn, B.; Reikowski, F.; Harrington, D.A.; Carla, F.; Felici, R.; Stettner, J.; Magnussen, O.M. Structural Reorganization of Pt(111) Electrodes by Electrochemical Oxidation and Reduction. J. Am. Chem. Soc. 2017, 139, 4532–4539. [Google Scholar] [CrossRef]
- Hu, J.; Wu, L.; Kuttiyiel, K.A.; Goodman, K.R.; Zhang, C.; Zhu, Y.; Vukmirovic, M.B.; White, M.G.; Sasaki, K.; Adzic, R.R. Increasing Stability and Activity of Core–Shell Catalysts by Preferential Segregation of Oxide on Edges and Vertexes: Oxygen Reduction on Ti–Au@Pt/C. J. Am. Chem. Soc. 2016, 138, 9294–9300. [Google Scholar] [CrossRef]
- Zhang, G.R.; Zhao, D.; Feng, Y.Y.; Zhang, B.; Su, D.S.; Liu, G.; Xu, B.Q. Catalytic Pt-On-Au Nanostructures: Why Pt Becomes More Active on Smaller Au Particles. ACS Nano 2012, 6, 2226–2236. [Google Scholar] [CrossRef]
- Suntivich, J.; Xu, Z.; Carlton, C.E.; Kim, J.; Han, B.; Lee, S.W.; Bonnet, N.; Marzari, N.; Allard, L.F.; Gasteiger, H.A.; et al. Surface Composition Tuning of Au–Pt Bimetallic Nanoparticles for Enhanced Carbon Monoxide and Methanol Electro-oxidation. J. Am. Chem. Soc. 2013, 135, 7985–7991. [Google Scholar] [CrossRef]
- Peng, L.; Gan, L.; Wei, Y.; Yang, H.; Li, J.; Du, H.; Kang, F. Pt Submonolayers on Au Nanoparticles: Coverage-Dependent Atomic Structures and Electrocatalytic Stability on Methanol Oxidation. J. Phys. Chem. C 2016, 120, 28664–28671. [Google Scholar] [CrossRef]
- Shao, J.; Liu, M.; Wang, Z.; Li, K.; Bao, B.; Zhao, S.; Zhou, S. Controllable Synthesis of Surface Pt-Rich Bimetallic AuPt Nanocatalysts for Selective Hydrogenation Reactions. ACS Omega 2019, 4, 15621–15627. [Google Scholar] [CrossRef]
- Lu, L.; Peng, L.; Li, L.; Li, J.; Huang, X.; Wei, Z. Improved Hydrogen Oxidation Reaction Under Alkaline Conditions by Au–Pt Alloy Nanoparticles. J. Energy Chem. 2020, 40, 52–56. [Google Scholar] [CrossRef]
- Shim, K.; Lee, W.C.; Heo, Y.U.; Shahabuddin, M.; Park, M.S.; Hossain, M.S.A.; Kim, J.H. Rationally Designed Bimetallic Au@Pt Nanoparticles for Glucose Oxidation. Sci. Rep. 2019, 9, 894. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.; Lee, W.C.; Park, M.S.; Shahabuddin, M.; Yamauchi, Y.; Hossain, M.S.A.; Shim, Y.B.; Kim, J.H. Au Decorated Core-Shell Structured Au@Pt for the Glucose Oxidation Reaction. Sens. Actuators B Chem. 2019, 278, 88–96. [Google Scholar] [CrossRef]
- You, G.; Jiang, J.; Li, M.; Li, L.; Tang, D.; Zhang, J.; Zeng, X.C.; He, R. PtPd(111) Surface Versus PtAu(111) Surface: Which One Is More Active for Methanol Oxidation? ACS Catal. 2017, 8, 132–143. [Google Scholar] [CrossRef]
- Xia, Y.; Gilroy, K.D.; Xia, X.; Peng, H.C.; Peng, H. Seed-Mediated Growth of Colloidal Metal Nanocrystals. Angew. Chem. Int. Ed. 2016, 56, 60–95. [Google Scholar] [CrossRef]
- Weiner, R.G.; Kunz, M.R.; Skrabalak, S.E. Seeding a New Kind of Garden: Synthesis of Architecturally Defined Multimetallic Nanostructures by Seed-Mediated Co-Reduction. Accounts Chem. Res. 2015, 48, 2688–2695. [Google Scholar] [CrossRef]
- Duan, T.; Zhang, R.; Ling, L.; Wang, B. Insights into the Effect of Pt Atomic Ensemble on HCOOH Oxidation OverPt-Decorated Au Bimetallic Catalyst To Maximize Pt Utilization. J. Phys. Chem. C 2016, 120, 2234–2246. [Google Scholar] [CrossRef]
- Sugioka, D.; Kameyama, T.; Kuwabata, S.; Yamamoto, T.; Torimoto, T. Formation of a Pt-Decorated Au Nanoparticle Monolayer Floating on an Ionic Liquid by the Ionic Liquid/Metal Sputtering Method and Tunable Electrocatalytic Activities of the Resulting Monolayer. ACS Appl. Mater. Interfaces 2016, 8, 10874–10883. [Google Scholar] [CrossRef]
- Banerjee, I.; Kumaran, V.; Santhanam, V. Synthesis and Characterization of Au@Pt Nanoparticles with Ultrathin Platinum Overlayers. J. Phys. Chem. C 2015, 119, 5982–5987. [Google Scholar] [CrossRef]
- Tan, L.; Li, L.; Peng, Y.; Guo, L. Synthesis of Au@Pt Bimetallic Nanoparticles with Concave Au Nanocuboids as Seeds and Their Enhanced Electrocatalytic Properties in the Ethanol Oxidation Reaction. Nanotechnology 2015, 26, 505401. [Google Scholar] [CrossRef]
- Wang, J.X.; Inada, H.; Wu, L.; Zhu, Y.; Choi, Y.M.; Liu, P.; Zhou, W.P.; Adzic, R.R. Oxygen Reduction on Well-Defined Core−Shell Nanocatalysts: Particle Size, Facet, and Pt Shell Thickness Effects. J. Am. Chem. Soc. 2009, 131, 17298–17302. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gokcen, D.; Bertocci, U.; Moffat, T.P. Self-Terminating Growth of Platinum Films by Electrochemical Deposition. Science. 2012, 338, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Liu, Y.; Moffat, T.P. Ultrathin Platinum Films for Methanol and Formic Acid Oxidation: Activity as a Function of Film Thickness and Coverage. ACS Catal. 2015, 5, 2124–2136. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Antonov, L.; Nedeltcheva, D. Resolution of Overlapping UV–Vis Absorption Bands and Quantitative Analysis. Chem. Soc. Rev. 2000, 29, 217–227. [Google Scholar] [CrossRef]
- Mohan, P.; Takahashi, M.; Higashimine, K.; Mott, D.; Maenosono, S. AuFePt Ternary Homogeneous Alloy Nanoparticles with Magnetic and Plasmonic Properties. Langmuir 2017, 33, 1687–1694. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, C.; Liu, W.; Chen, R.; Jiang, X. Tuning the Composition of AuPt Bimetallic Nanoparticles for Antibacterial Application. Angew. Chem. 2014, 126, 8265–8269. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Glaspell, G.; Nguyen, M.; Howe, J.M.; El-Shall, M.S. Laser Synthesis of Bimetallic Nanoalloys in the Vapor and Liquid Phases and the Magnetic Properties of PdM and PtM Nanoparticles (M=Fe, Co and Ni). Faraday Discuss. 2008, 138, 163–180. [Google Scholar] [CrossRef]
- Wang, C.L.; Hsao, B.J.; Lai, S.F.; Chen, W.C.; Chen, H.H.; Chen, Y.Y.; Chien, C.C.; Cai, X.; Kempson, I.M.; Hwu, Y.; et al. One-Pot Synthesis of AuPt Alloyed Nanoparticles by Intense x-Ray Irradiation. Nanotechnology 2011, 22, 065605. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-Decorated Gold Nanoparticles with Dual Functionalities for Ultrasensitive Colorimetric in Vitro Diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Klinger, M. More Features, More Tools, More CrystBox. J. Appl. Crystallogr. 2017, 50, 1226–1234. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Y.; Han, D.; Zhang, Y.; Shen, Y.; Wang, Z.; Niu, L. Synthesis of Highly Faceted Multiply Twinned Gold Nanocrystals Stabilized by Polyoxometalates. Nanotechnology 2006, 17, 4689–4694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, G.; Chen, D.; Lv, P.; Kong, X.; Sun, Y.; Wang, Z.; Yuan, Z.; Liu, H.; Yang, J. Core-Shell Au-Pd Nanoparticles as Cathode Catalysts for Microbial Fuel Cell Applications. Sci. Rep. 2016, 6, 35252. [Google Scholar] [CrossRef]
- Gaw, S.L.; Wang, J.; Sun, S.; Dong, Z.; Reches, M.; Lee, P.S.; Xu, Z.J. Electrochemical Cycling Induced Surface Segregation of AuPt Nanoparticles in HClO4and H2SO4. J. Electrochem. Soc. 2016, 163, F752–F760. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lee, K.J.; Sung, Y.E. Methanol Electro-Oxidation on the Pt Surface: Revisiting the Cyclic Voltammetry Interpretation. J. Phys. Chem. C 2016, 120, 9028–9035. [Google Scholar] [CrossRef]
- Dorjgotov, A.; Jeon, Y.; Hwang, J.; Ulziidelger, B.; Kim, H.S.; Han, B.; Shul, Y.G. Synthesis of Durable Small-sized Bilayer Au@Pt Nanoparticles for High Performance PEMFC Catalysts. Electrochim. Acta 2017, 228, 389–397. [Google Scholar] [CrossRef]
- Zheng, J.N.; Li, S.S.; Ma, X.; Chen, F.Y.; Wang, A.J.; Chen, J.R.; Feng, J.J. Popcorn-Like PtAu Nanoparticles Supported on Reduced Graphene Oxide: Facile Synthesis and Catalytic Applications. J. Mater. Chem. A 2014, 2, 8386–8395. [Google Scholar] [CrossRef]
- Feng, J.J.; Chen, L.X.; Ma, X.; Yuan, J.; Chen, J.R.; Wang, A.J.; Xu, Q.Q. Bimetallic AuPt Alloy Nanodendrites/Reduced Graphene Oxide: One-Pot Ionic Liquid-Assisted Synthesis and Excellent Electrocatalysis Towards Hydrogen Evolution and Methanol Oxidation Reactions. Int. J. Hydrog. Energy 2017, 42, 1120–1129. [Google Scholar] [CrossRef]
- Tenney, S.A.; Ratliff, J.S.; Roberts, C.C.; He, W.; Ammal, S.C.; Heyden, A.; Chen, D.A. Adsorbate-Induced Changes in the Surface Composition of Bimetallic Clusters: Pt−Au on TiO2(110). J. Phys. Chem. C 2010, 114, 21652–21663. [Google Scholar] [CrossRef]
- Hong, W.; Li, C.W. Microstructural Evolution of Au@Pt Core-Shell Nanoparticles under Electrochemical Polarization. ACS Appl. Mater. Interfaces 2019, 11, 30977–30986. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higareda, A.; Kumar-Krishnan, S.; García-Ruiz, A.F.; Maya-Cornejo, J.; Lopez-Miranda, J.L.; Bahena, D.; Rosas, G.; Pérez, R.; Esparza, R. Synthesis of Au@Pt Core—Shell Nanoparticles as Efficient Electrocatalyst for Methanol Electro-Oxidation. Nanomaterials 2019, 9, 1644. https://doi.org/10.3390/nano9111644
Higareda A, Kumar-Krishnan S, García-Ruiz AF, Maya-Cornejo J, Lopez-Miranda JL, Bahena D, Rosas G, Pérez R, Esparza R. Synthesis of Au@Pt Core—Shell Nanoparticles as Efficient Electrocatalyst for Methanol Electro-Oxidation. Nanomaterials. 2019; 9(11):1644. https://doi.org/10.3390/nano9111644
Chicago/Turabian StyleHigareda, América, Siva Kumar-Krishnan, Amado F. García-Ruiz, José Maya-Cornejo, José L. Lopez-Miranda, Daniel Bahena, Gerardo Rosas, Ramiro Pérez, and Rodrigo Esparza. 2019. "Synthesis of Au@Pt Core—Shell Nanoparticles as Efficient Electrocatalyst for Methanol Electro-Oxidation" Nanomaterials 9, no. 11: 1644. https://doi.org/10.3390/nano9111644
APA StyleHigareda, A., Kumar-Krishnan, S., García-Ruiz, A. F., Maya-Cornejo, J., Lopez-Miranda, J. L., Bahena, D., Rosas, G., Pérez, R., & Esparza, R. (2019). Synthesis of Au@Pt Core—Shell Nanoparticles as Efficient Electrocatalyst for Methanol Electro-Oxidation. Nanomaterials, 9(11), 1644. https://doi.org/10.3390/nano9111644





