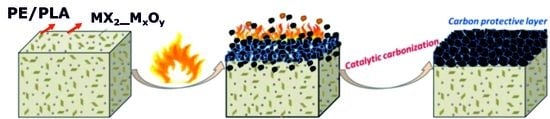
DOPO-Functionalized Molybdenum Disulfide and its Impact on the Thermal Properties of Polyethylene and Poly(Lactic Acid) Composites
Abstract
:1. Introduction
2. Methods
2.1. Materials
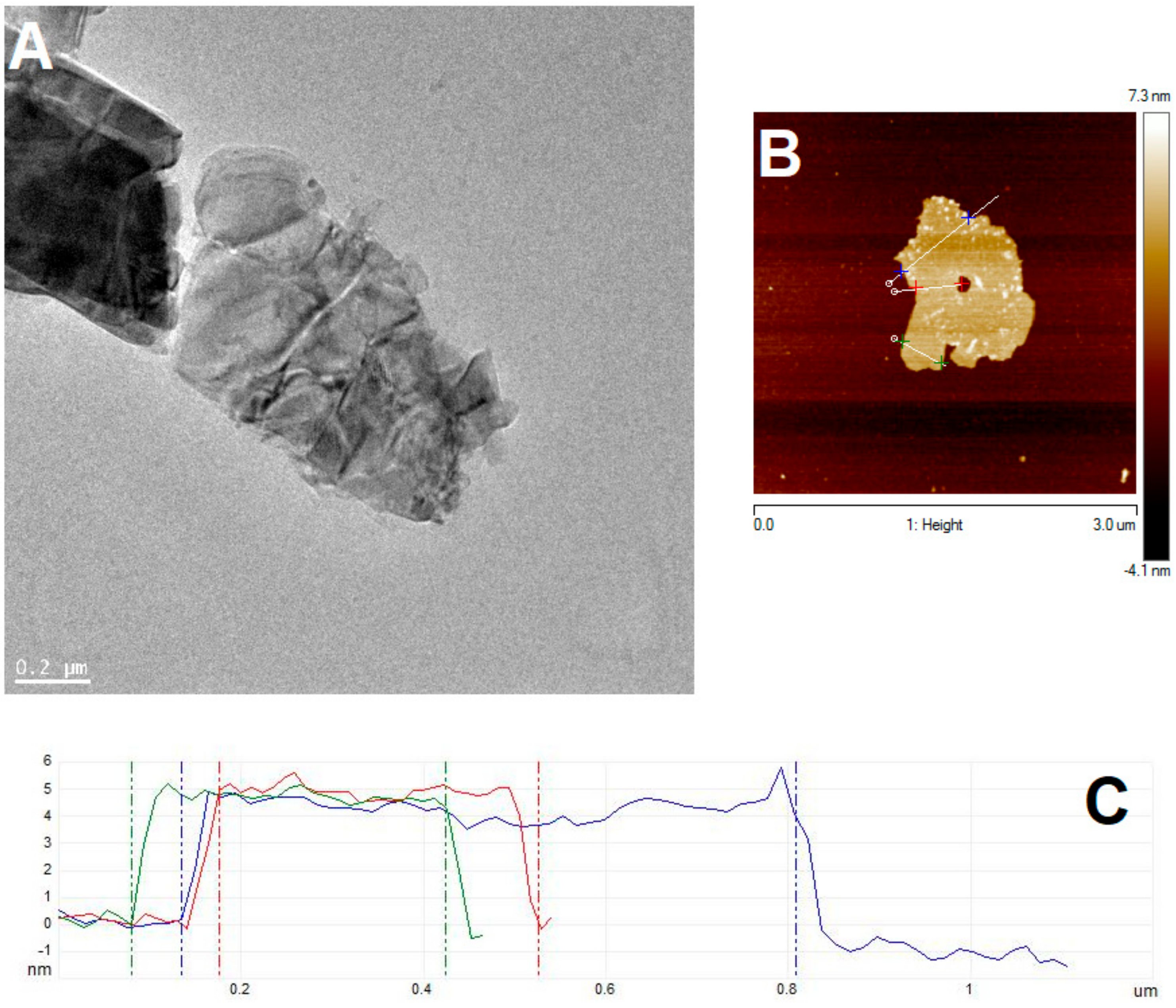
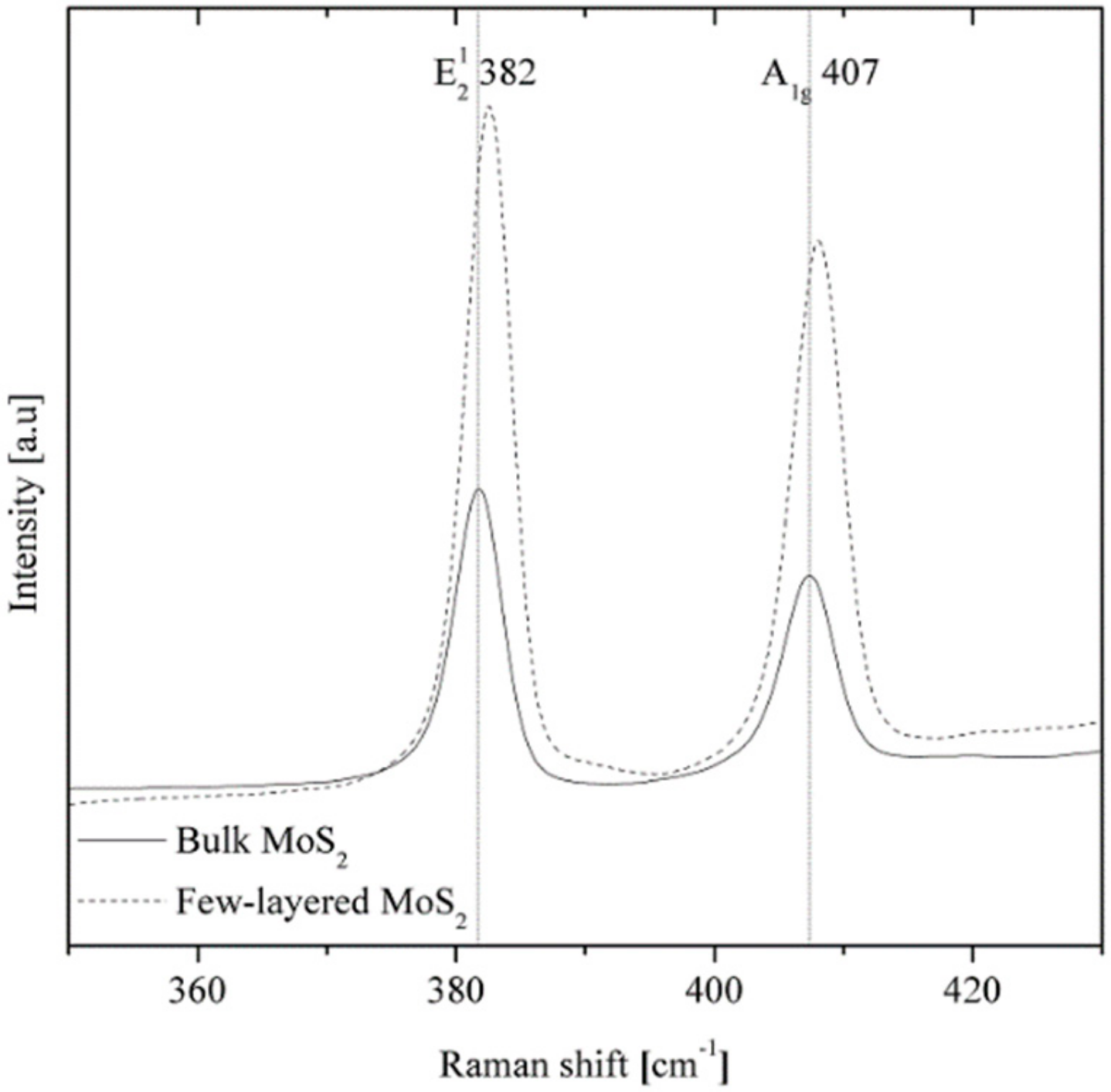
2.2. Preparation of Few-Layered MoS2
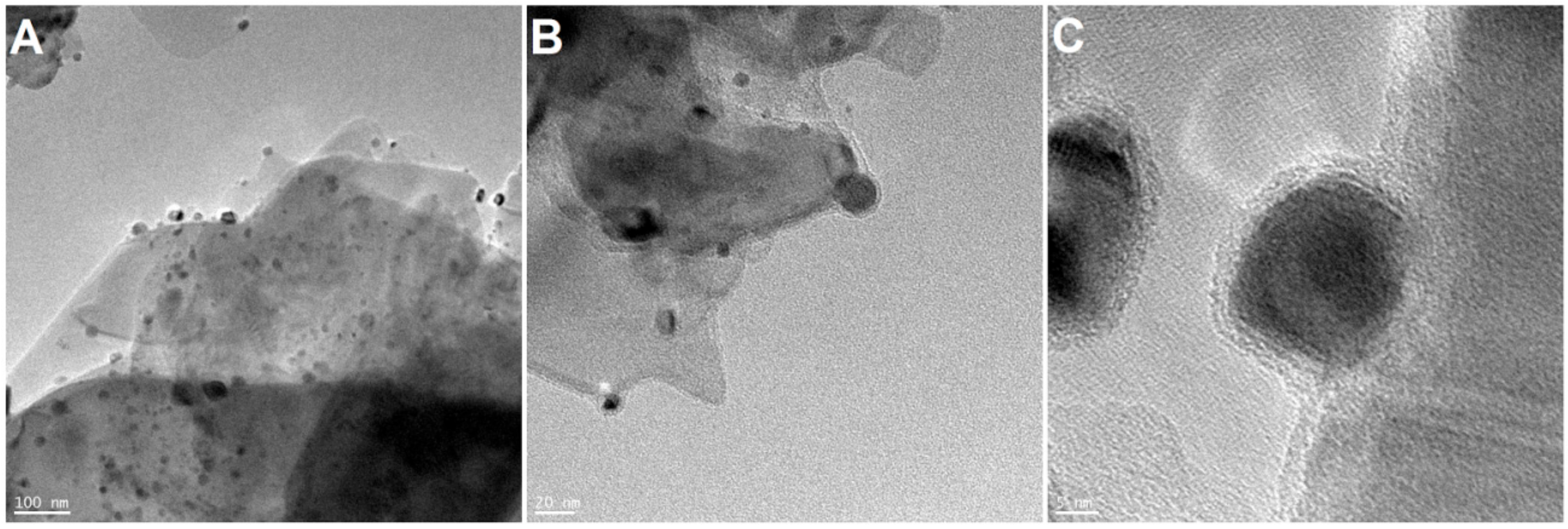
2.3. Modification of MoS2 with Ni2O3 Nanoparticles
2.4. Functionalization of MoS2/Ni2O3 Nanomaterials with DOPO
2.5. Preparation of PE and PLA Nanocomposites
2.6. Characterization
3. Results and Discussion
3.1. Thermal Stability
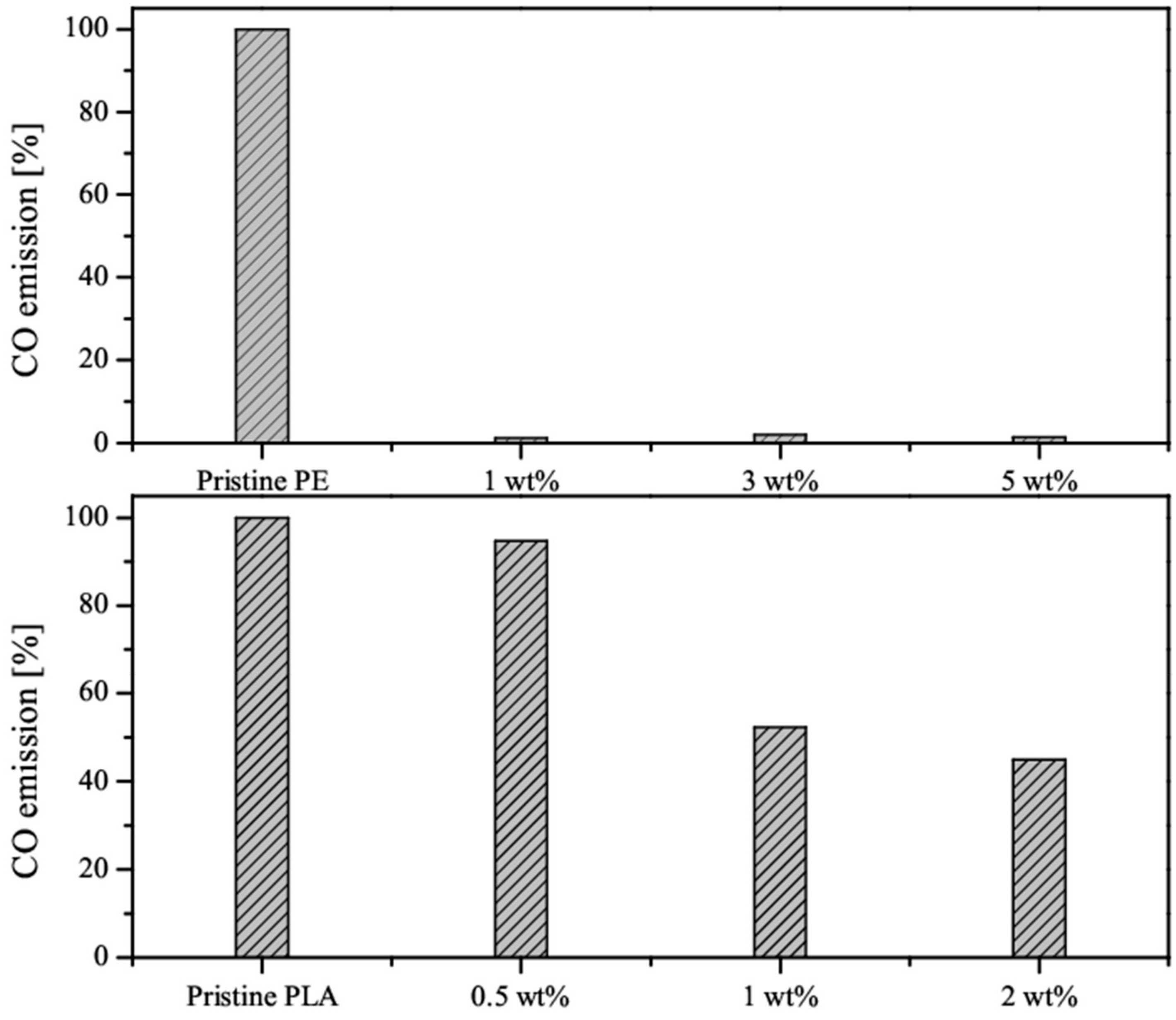
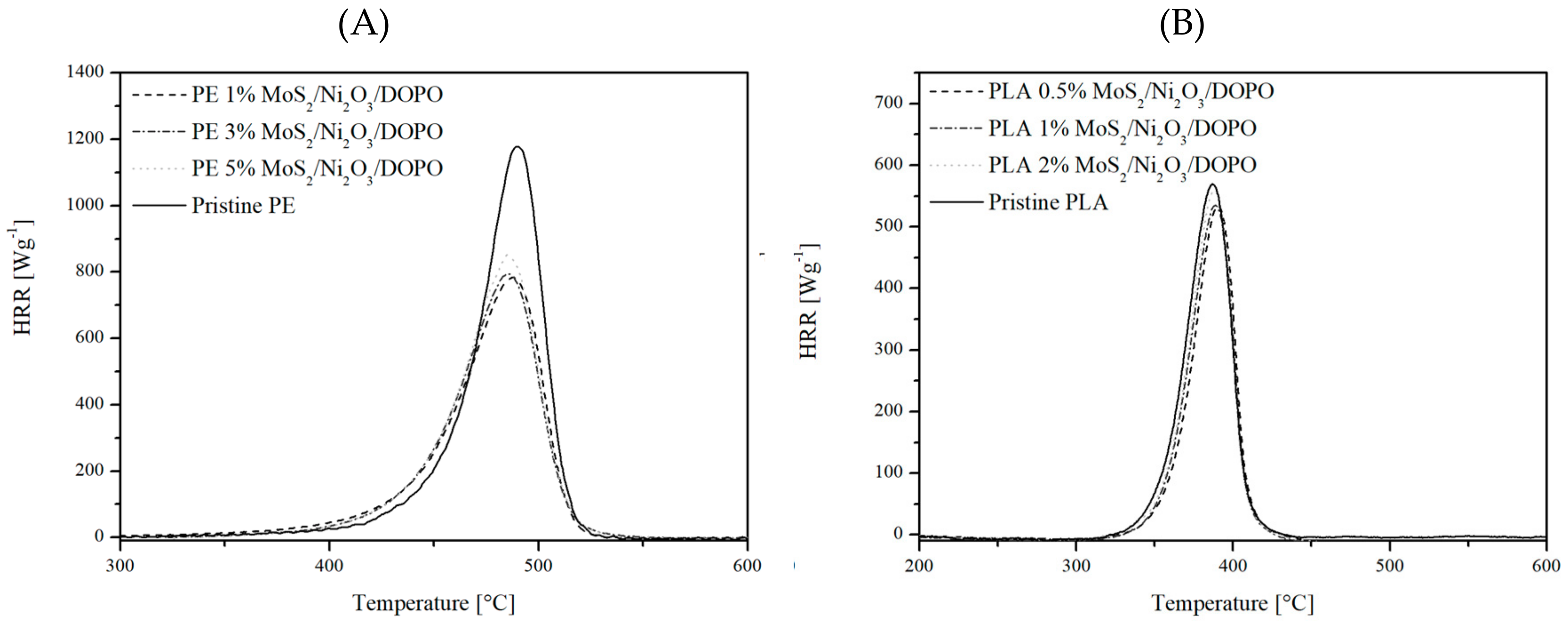
3.2. Flammability Studies
3.3. Thermal Conductivity Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Data Availability
Abbrevations
| AFM | atomic force microscopy |
| CO | carbon oxide |
| DOPO | 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide |
| DOPO-MA | maleic acid functionalized DOPO |
| FR | flame retardant |
| GNS | graphene nanosheets |
| HRC | heat release capacity |
| LOI | limiting oxygen index |
| MCC | microscale combustion calorimetry |
| MoS2 | molybdenum disulfide |
| Ni2O3 | nickel(III) oxide |
| NMP | N-Methyl-2-pyrrolidone |
| PE | polyethylene |
| pHHR | peak heat release rate |
| PLA | poly(lactic acid) |
| PS | polystyrene |
| PVA | poly(vinyl alcohol) |
| TEM | transmission electron microscopy |
| TGA | thermogravimetric analysis |
| THF | tetrahydrofuran |
| THR | total heat release |
References
- Demirors, M. The history of polyethylene. ACS Symp. Ser. 2011, 1080, 115–145. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Khanam, P.N.; AlMaadeed, M.A.A. Processing and characterization of polyethylene-based composites. Adv. Manuf. Polym. Compos. Sci. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Karunakaran, S.; Majid, D.L.; Tawil, M.L.M. Flammability of self-extinguishing kenaf/ABS nanoclays composite for aircraft secondary structure. IOP Conf. Ser. Mater. Sci. Eng. 2016, 152, 012068. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; González-Martínez, C.; Chiralt, A. Combination of poly(lactic) acid and starch for biodegradable food packaging. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Gu, X.; Zhang, S.; Sun, J.; Xu, R.; Bourbigot, S.; Duquesne, S.; Casetta, M. Flammability and thermal degradation of poly (lactic acid)/polycarbonate alloys containing a phosphazene derivative and trisilanollsobutyl POSS. Polymer 2015, 79, 221–231. [Google Scholar] [CrossRef]
- Aschberger, K.; Campia, I.; Pesudo, L.Q.; Radovnikovic, A.; Reina, V. Chemical alternatives assessment of different flame retardants—A case study including multi-walled carbon nanotubes as synergist. Pergamon 2017, 101. [Google Scholar] [CrossRef] [PubMed]
- Salmeia, K.A.; Gooneie, A.; Simonetti, P.; Nazir, R.; Kaiser, J.-P.P.; Rippl, A.; Hirsch, C.; Lehner, S.; Rupper, P.; Hufenus, R.; et al. Comprehensive study on flame retardant polyesters from phosphorus additives. Polym. Degrad. Stab. 2018, 155, 22–34. [Google Scholar] [CrossRef]
- Buczko, A.; Stelzig, T.; Bommer, L.; Rentsch, D.; Heneczkowski, M.; Gaan, S. Bridged DOPO derivatives as flame retardants for PA6. Polym. Degrad. Stab. 2014, 107, 158–165. [Google Scholar] [CrossRef]
- Toldy, A.; Szabó, A.; Novák, C.; Madarász, J.; Tóth, A.; Marosi, G. Intrinsically flame retardant epoxy resin —Fire performance and background—Part II. Polym. Degrad. Stab. 2008, 93, 2007–2013. [Google Scholar] [CrossRef]
- Price, D.; Bullett, K.J.; Cunliffe, L.K.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P. Cone calorimetry studies of polymer systems flame retarded by chemically bonded phosphorus. Polym. Degrad. Stab. 2005, 88, 74–79. [Google Scholar] [CrossRef]
- Bindra, S.K.; Narang, R.S. Combustion of flame retardants II. Chemosphere 1996, 33, 1981–1996. [Google Scholar] [CrossRef]
- Sato, H.; Kondo, K.; Tsuge, S.; Ohtani, H.; Sato., N. Mechanisms of thermal degradation of a polyester flame-retarded with antimony oxide/brominated polycarbonate studied by temperature-programmed analytical pyrolysis. Polym. Degrad. Stab. 1998, 62, 41–48. [Google Scholar] [CrossRef]
- Legler, J.; Brouwer, A. Are brominated flame retardants endocrine disruptors? Environ. Int. 2003, 29, 879–885. [Google Scholar] [CrossRef]
- Poma, G.; Malysheva, S.V.; Goscinny, S.; Malarvannan, G.; Voorspoels, S.; Covaci, A.; Van Loco, J. Occurrence of selected halogenated flame retardants in Belgian foodstuff. Chemosphere 2018, 194, 256–265. [Google Scholar] [CrossRef]
- Brits, M.; de Vos, J.; Weiss, J.M.; Rohwer, E.R.; de Boer, J. Critical review of the analysis of brominated flame retardants and their environmental levels in Africa. Chemosphere 2016, 164, 174–189. [Google Scholar] [CrossRef]
- Sun, Z.; Hou, Y.; Hu, Y.; Hu, W. Effect of additive phosphorus-nitrogen containing flame retardant on char formation and flame retardancy of epoxy resin. Mater. Chem. Phys. 2018, 214, 154–164. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.; Li, Z.; Yan, Y.; Ji, K. Aluminated mesoporous silica as novel high-effective flame retardant in polylactide. Compos. Sci. Technol. 2013, 82, 1–7. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; El Saeed, A.M.; Dardir, M.M.; El-Sockary, M.A. Studying the effect of organo-modified nanoclay loading on the thermal stability, flame retardant, anti-corrosive and mechanical properties of polyurethane nanocomposite for surface coating. Prog. Org. Coat. 2015, 89, 212–219. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Hawkins, S.; Mannan, M.S.; Cheng, Z. Study of thermal and mechanical behaviors of flame retardant polystyrene-based nanocomposites prepared via in-situ polymerization method. J. Loss Prev. Process Ind. 2017. [Google Scholar] [CrossRef]
- Hapuarachchi, T.D.; Peijs, T. Multiwalled carbon nanotubes and sepiolite nanoclays as flame retardants for polylactide and its natural fibre reinforced composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 954–963. [Google Scholar] [CrossRef]
- Zhang, T.; Du, Z.; Zou, W.; Li, H.; Zhang, C. The flame retardancy of blob-like multi-walled carbon nanotubes/silica nanospheres hybrids in poly (methyl methacrylate). Polym. Degrad. Stab. 2012, 97, 1716–1723. [Google Scholar] [CrossRef]
- Feng, X.; Wen, P.; Cheng, Y.; Liu, L.; Tai, Q.; Hu, Y.; Liew, K.M. Defect-free MoS2 nanosheets: Advanced nanofillers for polymer nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 81, 61–68. [Google Scholar] [CrossRef]
- Feng, X.; Xing, W.; Yang, H.; Yuan, B.; Song, L.; Hu, Y.; Liew, K.M. High-performance poly(ethylene oxide)/molybdenum disulfide nanocomposite films: Reinforcement of properties based on the gradient interface effect. ACS Appl. Mater. Interfaces 2015, 7, 13164–13173. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Y.; Zuo, X.; Zhang, L.; Yang, Z.; Zhou, Y.; Marmorat, C.; He, S.; Rafailovich, M. Capitalizing on the molybdenum disulfide/graphene synergy to produce mechanical enhanced flame retardant ethylene-vinyl acetate composites with low aluminum hydroxide loading. Polym. Degrad. Stab. 2017, 144, 155–166. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, R.; Qian, X. Self-assembly of exfoliated molybdenum disulfide (MoS2) nanosheets and layered double hydroxide (LDH): Towards reducing fire hazards of epoxy. J. Hazard. Mater. 2017, 338, 343–355. [Google Scholar] [CrossRef]
- Thakur, S.; Bandyopadhyay, P.; Kim, S.H.; Kim, N.H.; Lee, J.H. Enhanced physical properties of two dimensional MoS2/poly(vinyl alcohol) nanocomposites. Compos. Part A Appl. Sci. Manuf. 2018, 110, 284–293. [Google Scholar] [CrossRef]
- Eksik, O.; Gao, J.; Shojaee, S.A.; Thomas, A.; Chow, P.; Bartolucci, S.F.; Lucca, D.A.; Koratkar, N. Epoxy nanocomposites with two-dimensional transition metal dichalcogenide additives. ACS Nano 2014, 8, 5282–5289. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y. The influence of graphene based smoke suppression agents on reduced fire hazards of polystyrene composites. Compos. Part. A Appl. Sci. Manuf. 2016, 80, 217–227. [Google Scholar] [CrossRef]
- Zhou, K.; Yang, W.; Tang, G.; Wang, B.; Jiang, S.; Hu, Y.; Gui, Z. Comparative study on the thermal stability, flame retardancy and smoke suppression properties of polystyrene composites containing molybdenum disulfide and graphene. RSC Adv. 2013, 3, 25030. [Google Scholar] [CrossRef]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. Part B Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Doring, M.; Kramer, J.; Altstädt, V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur. Polym. J. 2011, 47, 1081–1089. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Döring, M.; Krämer, J.; Altstädt, V. A new halogen-free flame retardant based on 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide for epoxy resins and their carbon fiber composites for the automotive and aviation industries. Macromol. Mater. Eng. 2011, 296, 14–30. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Yu, T.; Tuerhongjiang, T.; Sheng, C.; Li, Y. Phosphorus-containing diacid and its application in jute/poly(lactic acid) composites: Mechanical, thermal and flammability properties. Compos. Part A Appl. Sci. Manuf. 2017, 97, 60–66. [Google Scholar] [CrossRef]
- Long, L.; Chang, Q.; He, W.; Xiang, Y.; Qin, S.; Yin, J.; Yu, J. Effects of bridged DOPO derivatives on the thermal stability and flame retardant properties of poly(lactic acid). Polym. Degrad. Stab. 2017, 139, 55–66. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Wu, Y.; Jiao, L. Controlled synthesis of highly crystalline MoS2 flakes by chemical vapor deposition. J. Am. Chem. Soc. 2013, 135, 5304–5307. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of Raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Shi, X.; Peng, X.; Zhu, J.; Lin, G.; Kuang, T. Synthesis of DOPO-HQ-functionalized graphene oxide as a novel and efficient flame retardant and its application on polylactic acid: Thermal property, flame retardancy, and mechanical performance. J. Colloid Interface Sci. 2018, 524, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Gui, Z.; Hu, Y.; Jiang, S.; Tang, G. The influence of cobalt oxide-graphene hybrids on thermal degradation, fire hazards and mechanical properties of thermoplastic polyurethane composites. Compos. Part A Appl. Sci. Manuf. 2016, 88, 10–18. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, X.; Huang, S.; Tian, X.; Song, L.; Yu, Q.; Wang, Z. Performance comparison of flame retardant epoxy resins modified by DPO–PHE and DOPO–PHE. Polym. Degrad. Stab. 2018, 156, 89–99. [Google Scholar] [CrossRef]
- Chen, X.; Zhuo, J.; Jiao, C. Thermal degradation characteristics of flame retardant polylactide using TG-IR. Polym. Degrad. Stab. 2012, 97, 2143–2147. [Google Scholar] [CrossRef]
- Gu, L.; Qiu, J.; Sakai, E. Effect of DOPO-containing flame retardants on poly(lactic acid): Non-flammability, mechanical properties and thermal behaviors. Chem. Res. Chin. Univ. 2017, 33, 143–149. [Google Scholar] [CrossRef]
- Yan, R.; Simpson, J.R.; Bertolazzi, S.; Brivio, J.; Watson, M.; Wu, X.; Kis, A.; Luo, T.; Walker, A.R.H.; Xing, H.G. Thermal conductivity of monolayer molybdenum disulfide obtained from temperature-dependent raman spectroscopy. ACS Nano 2014, 8, 986–993. [Google Scholar] [CrossRef]
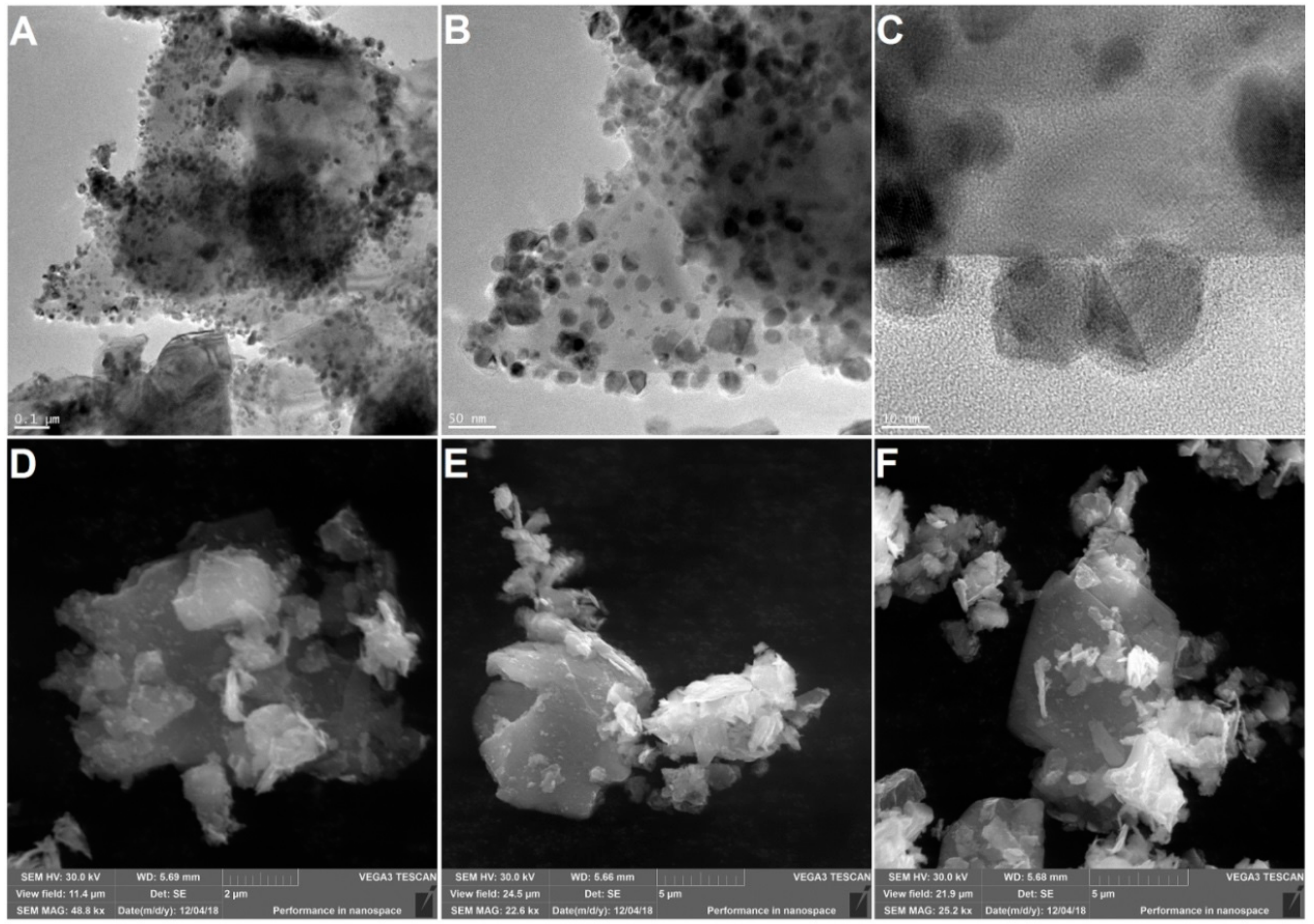
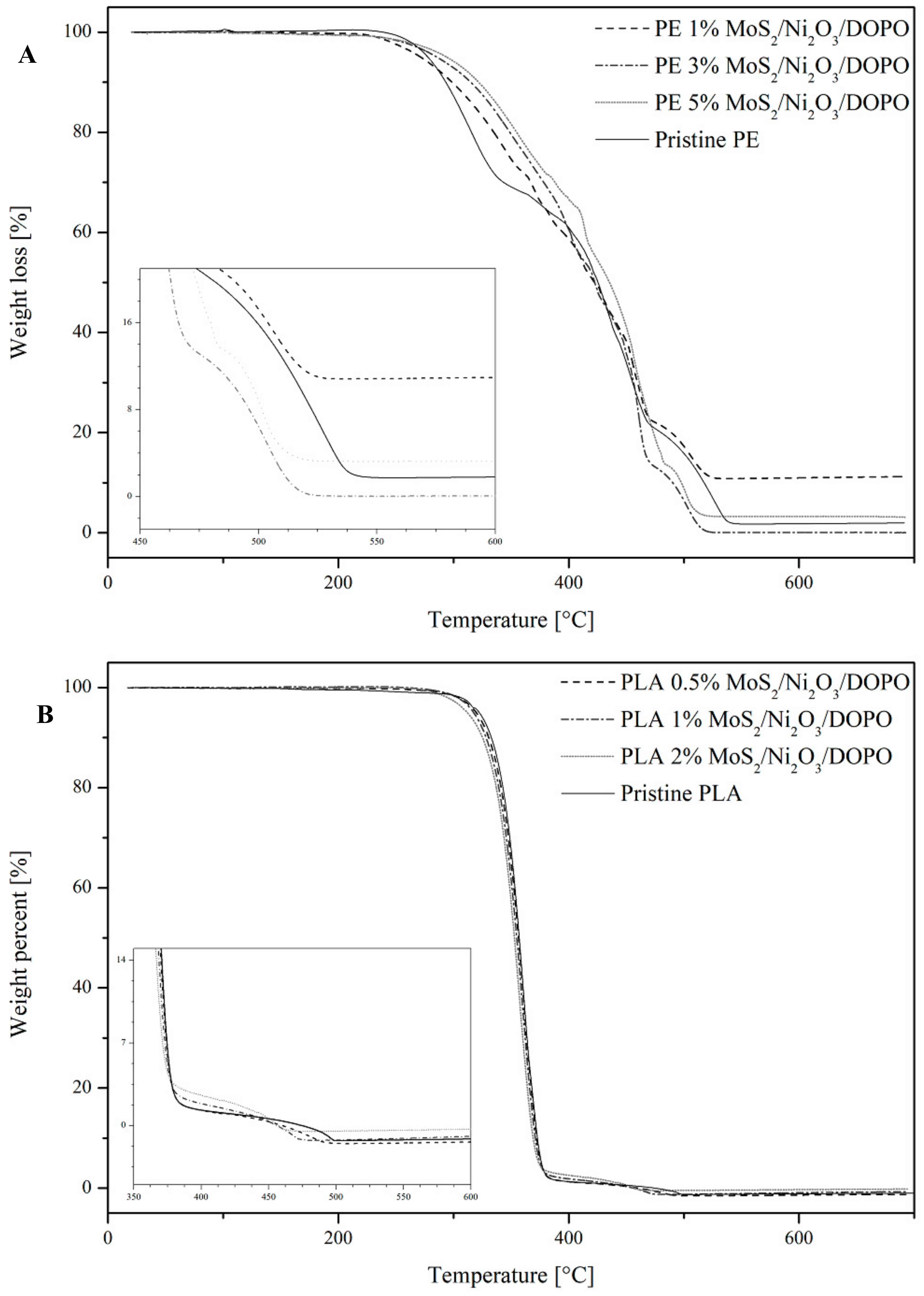
| FR Load (wt%) | T10wt% (°C) | T50wt% (°C) | Tmax (°C) | |
|---|---|---|---|---|
| PE | - | 292 | 424 | 552 |
| PE MoS2/Ni2O3/DOPO | 1 | 299 | 422 | 530 |
| 3 | 315 | 422 | 524 | |
| 5 | 322 | 435 | 526 | |
| PLA | - | 333 | 357 | 499 |
| PLA MoS2/Ni2O3/DOPO | 0.5 | 330 | 356 | 495 |
| 1 | 328 | 354 | 475 | |
| 2 | 328 | 353 | 462 |
| FR Load (wt%) | HRC (J g−1K−1) | pHRR (W g−1) | THR (kJ g−1) | |
|---|---|---|---|---|
| PE | - | 1222 | 1175 | 47.0 |
| PE MoS2/Ni2O3/DOPO | 1 | 1087 | 783 | 41.3 |
| 3 | 978 | 794 | 40.5 | |
| 5 | 1004 | 851 | 41.8 | |
| PLA | - | 715 | 573 | 21.8 |
| PLA MoS2/Ni2O3/DOPO | 0.5 | 719 | 562 | 20.6 |
| 1 | 685 | 522 | 19.4 | |
| 2 | 730 | 564 | 20.6 |
| FR Load (wt %) | Thermal Conductivity (W m−1K−1) | Increase (%) | |
|---|---|---|---|
| PLA | - | 0.326 | - |
| PLA MoS2/Ni2O3/DOPO | 0.5 | 0.325 | 0 |
| 1 | 0.325 | 0 | |
| 2 | 0.326 | 0 | |
| PE | - | 0.186 | - |
| PE MoS2/Ni2O3/DOPO | 1 | 0.363 | 95 |
| 3 | 0.436 | 134 | |
| 5 | 0.394 | 112 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenelska, K.; Homa, P.; Popovic, S.; Maslana, K.; Mijowska, E. DOPO-Functionalized Molybdenum Disulfide and its Impact on the Thermal Properties of Polyethylene and Poly(Lactic Acid) Composites. Nanomaterials 2019, 9, 1637. https://doi.org/10.3390/nano9111637
Wenelska K, Homa P, Popovic S, Maslana K, Mijowska E. DOPO-Functionalized Molybdenum Disulfide and its Impact on the Thermal Properties of Polyethylene and Poly(Lactic Acid) Composites. Nanomaterials. 2019; 9(11):1637. https://doi.org/10.3390/nano9111637
Chicago/Turabian StyleWenelska, Karolina, Piotr Homa, Stefan Popovic, Klaudia Maslana, and Ewa Mijowska. 2019. "DOPO-Functionalized Molybdenum Disulfide and its Impact on the Thermal Properties of Polyethylene and Poly(Lactic Acid) Composites" Nanomaterials 9, no. 11: 1637. https://doi.org/10.3390/nano9111637
APA StyleWenelska, K., Homa, P., Popovic, S., Maslana, K., & Mijowska, E. (2019). DOPO-Functionalized Molybdenum Disulfide and its Impact on the Thermal Properties of Polyethylene and Poly(Lactic Acid) Composites. Nanomaterials, 9(11), 1637. https://doi.org/10.3390/nano9111637