3.1. Diffusion Rate of Carbon Atoms on Metal Surface
Ni is known to be more catalytic than Cu in graphene growth [
14]. Previously, we have studied the growth of graphene at 800 °C and found that with the increase of Ni composition in Ni–Cu alloys, the quality of graphene increased gradually [
15]. However, the carbon solid solubility of Ni–Cu alloy also increases with the increase of Ni composition [
16]. We found that when the percentage of Ni reached 50%, graphene showed notable multi-layer areas [
15]. Therefore, at 800 °C, we controlled the proportion of Ni to Cu to be 1:2.
Carbon solid solubility is positively correlated with temperature. At a 600 °C growth temperature, therefore, the carbon solid solubility was to presumably be lower than that at 800 °C. Our initial proposition was, thus, to increase the Ni composition in Ni–Cu alloy at 600 °C, which would improve the quality of graphene, without significantly damaging the uniformity of graphene.
However, we found that the experimental results were not exactly in line with our expectations.
Figure 1 shows the Raman spectra and corresponding optical images of the graphene grown on SiO
2/Si substrates catalyzed by Ni–Cu with different compositions. Plasma was applied to assist the carbon source pyrolysis. At a growth temperature of 600 °C, we did observe that the quality of graphene increased with the increase of Ni composition, which was mainly manifested by the decrease of D peak in Raman spectra of the graphene. The D peak represents inter-valley, defect-induced resonant scattering [
17]. However, the number of graphene layers still increases significantly with the increase of Ni composition. Graphene with different numbers of layers shows different contrasts on 300 nm SiO
2 [
18], so the number of layers can be roughly recognized by observing the color contrast by optical microscopy. We observed that when the Ni–Cu ratios were larger than 1:2 (
Figure 1d,e), the color of graphene appeared to be dark and it was inhomogeneous. In particular, when the ratio reached 2:1 (200 nm Ni + 100 nm Cu), the color of the graphene (see
Figure 1e) was quite dark, indicating that the thickness of graphene was rather great. It shows that with the increase of Ni percentage, the graphene grown at 600 °C becomes thicker and appears with uneven layer distribution. Even though such a phenomenon was observed in our previous work [
15] on the graphene growth at a higher temperature, i.e., 800 °C, it seemed contrary to our expectations, considering the limited carbon solid solubility at low temperatures. We found that this phenomenon could no longer be explained by the known mechanism of carbon atom segregation. According to Laurent Baraton et al. [
19], at 725 °C, the number of carbon atoms dissolved in 200-nm-thick Ni is about 8 × 10
15/cm
2, which corresponds to the number of carbon atoms required to form bilayer graphene. At 600 °C, as in our condition, the number of carbon atoms that can be segregated from the Ni2Cu1 (representing a Ni to Cu ratio of 2:1) alloy should be much less than that of bilayer graphene. However, atomic force microscopy (AFM) measurements show that the maximum thickness of the graphene grown on the alloy was about 88 nm (
Figure 2b). This thickness is equivalent to hundreds of layers of graphene (0.34 nm for a monolayer [
20]), which is much larger than the number of carbon atoms that can be dissolved in Ni–Cu alloy and is even thicker than graphene catalyzed by pure Ni at high temperatures. By contrast, the graphene catalyzed by a Ni1Cu3 (representing a Ni to Cu ratio of 1:3) alloy was only about 1.9 nm thick (
Figure 2a) with an even distribution (
Figure 1b) under the same growth conditions.
To further understand the growth mechanism at a low temperature, we performed more experiments. We think that the low CDR on the surface of Ni relative to that on Cu plays an important role. We shortened the growth time to 30 s and observed the graphene films grown on Ni1Cu3 (low Ni composition) and Ni2Cu1 (high Ni composition) alloys. As shown in
Figure 2c, the graphene on the Ni1Cu3 alloy grew into a continuous film in the short time and the graphene was uniform. In contrast, the graphene grown on the Ni2Cu1 sample was still discontinuous (
Figure 2d). Meanwhile, the graphene was already very thick. It was found by electrical measurements that the graphene grown on the Ni1Cu3 sample was conductive, while the graphene grown on the Ni2Cu1 sample was not conductive, which further verified that graphene in
Figure 1b was continuous, while the graphene in
Figure 1e was discontinuous. We also observed the samples by scanning electron microscope (SEM). As shown in
Figure 2e,f, the graphene in
Figure 2e is a continuous film, while the graphene in the
Figure 2f is flaky. There is no connection among the flakes, indicating it is discontinuous. The growth rate of graphene on a metal surface is mainly related to the growth conditions, the metal catalyst, nucleation point density and CDR on the metal surface. All the samples in our experiment were grown in the same batch, excluding the influence of different growth conditions. We observed that the average grain size of Ni film is smaller than that of Cu at a same thickness (
Figure S2, Supplementary Materials). In addition, the catalytic activity of Ni is higher than that of Cu [
14]. These observations indicate that, on the surface of the Ni2Cu1 alloy, the graphene nucleation point density should be higher and the cracking rate of methane should be faster. However, within the same growth time, the graphene on the surface of Ni1Cu3 alloy reached full coverage, while the graphene on the surface of Ni2Cu1 did not. This phenomenon indicates that carbon atoms diffuse rapidly on the surface of the low Ni composition alloy (e.g., Ni1Cu3), so they can grow into a continuous graphene film in a very short time. When the graphene fully covers the metal surface, which isolates the contact between methane and the metal, the catalytic action of the metal disappears or at least drastically decreases, and the graphene growth rate is greatly slowed down. For alloys with a high Ni composition, carbon atoms diffuse slowly on their surfaces, and therefore, they accumulate near the graphene nucleation center. Therefore, it takes a longer time to form a continuous graphene film. The catalytic action of the metal will continue until the metal is fully covered, resulting in very thick and also uneven graphene. Analogically, the scenario can be understood as we pour a glass of water on a smooth plane, and the water flows quickly on the plane to form a uniform water film. However, if we pour a glass of sand on the same plane, the sand finds it more difficult to form a uniform layer. As shown in
Figure 2g,h, the diffusion of carbon atoms on Cu surface is equivalent to “pouring water on a smooth plane”, while the diffusion of carbon on Ni is equivalent to “pouring sand”.
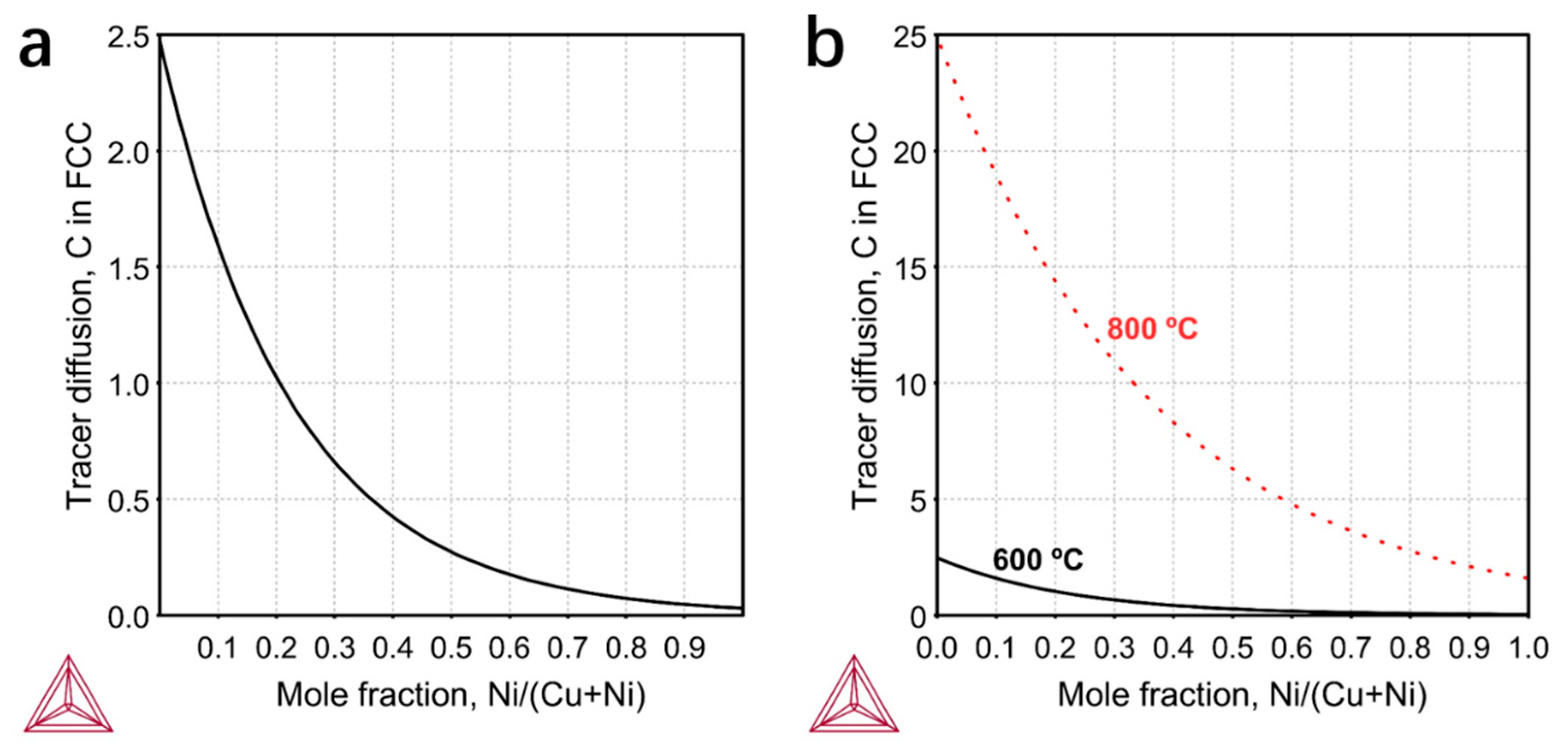
In order to verify our assumptions, we tried to estimate the relative surface CDR theoretically. In similar external environments, the relative CDR on the surface of Ni–Cu alloys is mainly determined by the alloy composition. There may be different mechanisms and factors for the diffusions inside a bulk and along a surface. The absolute quantity differs a lot between the bulk/surface-diffusivity even for the same material. Nevertheless, the surface CDR is in principle correlated to the bulk CDR. Hancke and Haugsrud [
21] confirmed the positive correlation between surface kinetic and bulk diffusion. Therefore, we may assume the composition-dependence of surface CDR is positively correlated to the bulk CDR. In the present case, the bulk CDRs we calculated for various Ni–Cu alloy compositions at 600 °C may have served as a semi-quantitative factor. The calculation was made based on the Thermo-Calc Software
®, <
www.thermocalc.se>, version 2019a, using the MOBCU2 atomic mobility database. As shown in
Figure 3a, with the increase of Ni composition, the CDR in the alloy decreases rapidly. The CDRs in pure Cu and Ni are 2.5 × 10
−12 m
2/s and 3 × 10
−14 m
2/s respectively, which are about two orders of magnitude different. In
Figure 1, we observe that when the ratio of Ni to Cu reaches 1:1, the graphene layers are obviously thick and begin to become inhomogeneous. Thus, from
Figure 3a we can roughly get that a critical diffusion rate of 2.5 × 10
−13 m
2/s is needed to ensure the uniformity of graphene layers. We also calculated the bulk CDR at 800 °C (
Figure 3b). The temperature and diffusion rate are positively correlated. It can be seen that when the temperature increases by 200 °C, the CDR in pure Ni has already exceeded the critical value of 2.5 × 10
−13 m
2/s, so the CDR will not affect the uniformity of graphene at 800 °C. In this sense, it is understandable that little attention has been paid to CDR in previous reports where the growth temperatures are high. To our knowledge, this is the first work to study the effect of CDR on the thickness and uniformity of graphene in low temperature growth.
The high CDR in/on Cu relative to that of Ni is attributed to the following factors. The diffusion coefficient is given as D = D
0exp (-Q/k
BT), in which D
0 (the frequency factor) is approximately temperature-independent. Q is the diffusion activation energy, k
B is the Boltzmann constant and T is the absolute temperature in Kelvin. We see that D
0 depends on the crystal lattice. In the present case, Cu has a larger D
0 value, mainly due to a larger lattice constant than Ni. The activation energy Q sitting in the exponent function should have a stronger impact on D, in comparison with D
0 which is proportional to D. The high diffusivity in Cu can, therefore, be attributed to two factors: (1) a larger lattice constant, and (2) a lower diffusion barrier which can be further interpreted based on the filling status of the d electron shell. Hu et al. [
22] studied the solubility and mobility of C interstitials in transition metals used for the growth of 2D materials. They found the interstitial formation energies of C in Cu are much higher than those in Ni, which indicates Cu is less likely to accommodate C. Consistently, they confirmed that the migration barriers of C interstitials in Cu are lower than those in Ni, indicating an easier mobility for C in Cu.
Thus, the performances of Ni–Cu alloys at low-temperature growth are dramatically different from the previous reports at high temperatures [
23,
24,
25,
26]. Ni is mainly used to provide high catalytic activity to improve the quality of graphene grown at low temperatures, while Cu is mainly used to improve the CDR on the surface of the alloy to ensure a thin and uniform graphene film. By compromising the quality and uniformity of graphene, we found that the Ni–Cu ratio of 1:2 was the best ratio. This ratio guarantees both the uniformity and relatively high quality of graphene.
This work also gave us inspiration for our follow-up work. We can further explore alloys with higher catalytic activity and higher CDR on its surface simultaneously, in order to obtain even more uniform and higher quality graphene at low temperatures. The selection of new alloys must meet the following requirements: (1) high catalytic activity, (2) fast CDR in the metal and (3) forming a homogenous single-phase solid solution alloy. We screened CDR in various metals [
22] and the catalytic activities of these metals [
14], and inspected the phase diagrams of various binary alloys. Finally, three potential alloy catalysts for low temperature growth were identified; namely, Cu–Pd, Au–Ni and Co–Ru. The detailed analysis process is shown in the
Supplementary Materials. In the future, we will try to use these alloys for graphene growth.
3.2. Plasma Enhancement
We also studied the influence of plasma on the quality of graphene. The purpose of applying plasma during the growth is mainly to accelerate the low temperature cracking of methane. Interestingly, we found that plasma is also very important to improving the quality of graphene. In fact, graphene can also be grown on the surface of Cu–Ni alloys at 600 °C even without plasma. It was found that the lowest growth temperature catalyzed by pure Ni without plasma is around 450 °C (
Figure S3, Supplementary Materials). However, without plasma participation, the quality of graphene decreases significantly (
Figure 4a). This is mainly because without plasma, it is difficult for methane to achieve complete pyrolysis. Therefore, during growth, the starting time of plasma is best synchronized with the time of methane inflow. If we turn on the plasma after methane has been introduced for some time, the graphene has already grown on the metal surface, resulting in poor quality of the graphene. The plasma power also affects the quality of graphene. As shown in
Figure 4b, we experimented with four levels of power—25 W, 50 W, 100 W and 150 W, with a frequency of 100 kHz. Raman results show that 50 W is the best because the D peak of the graphene is the smallest. For higher power, the growth reaction will become very violent, leading to the increase of defects in graphene. The plasma electrodes we used are located below the heater (
Figure 4d). Therefore, for lower power, it is difficult for the plasma electric field to cover the surface of the heater, resulting in incomplete pyrolysis of methane and a decrease of the graphene’s quality. The distance between the plasma electrode and the heater will also directly affect the electric field strength on the heater’s surface. When the plasma electrode is closer to the heater, the optimal power should be reduced appropriately. The thickness of graphene is also affected by the plasma power. It was found that with a higher plasma power, the graphene was thicker.
The growth equipment we used included a vertical, cold wall graphene growth system (Black magic, Aixtron). As shown in
Figure 4c, the plasma electrode was made of Cu upon our reconstruction and the heater was made of graphite, which was heated by Joule heat. When growing, a heavily doped silicon wafer was placed on the heater and the sample was placed on the silicon.
Figure 4d was taken during the growth process. The methane/hydrogen ratio was 5 sccm/20 sccm. Methane was used to grow graphene, and hydrogen was used to etch the defects in graphene to improve its quality [
27,
28]. These two effects are both dynamic processes, and the reaction speeds of the two were greatly accelerated with the enhancement of plasma. The methane/hydrogen ratio of 5 sccm/20 sccm was chosen to ensure the stability of the chamber environment after multiple growths (
Figure S4, Supplementary Materials).
3.3. Wafer-Scale Patterned Graphene Direct Growth
One of the applications of low temperature growth is the combination with direct growth technology, which makes it possible to directly grow graphene on a variety of substrates that cannot withstand very high temperatures. We combined this 600 °C graphene growth method with our previously-reported in situ growth technique [
15,
29] and obtained wafer-scale patterned graphene directly on the SiO
2/Si substrate.
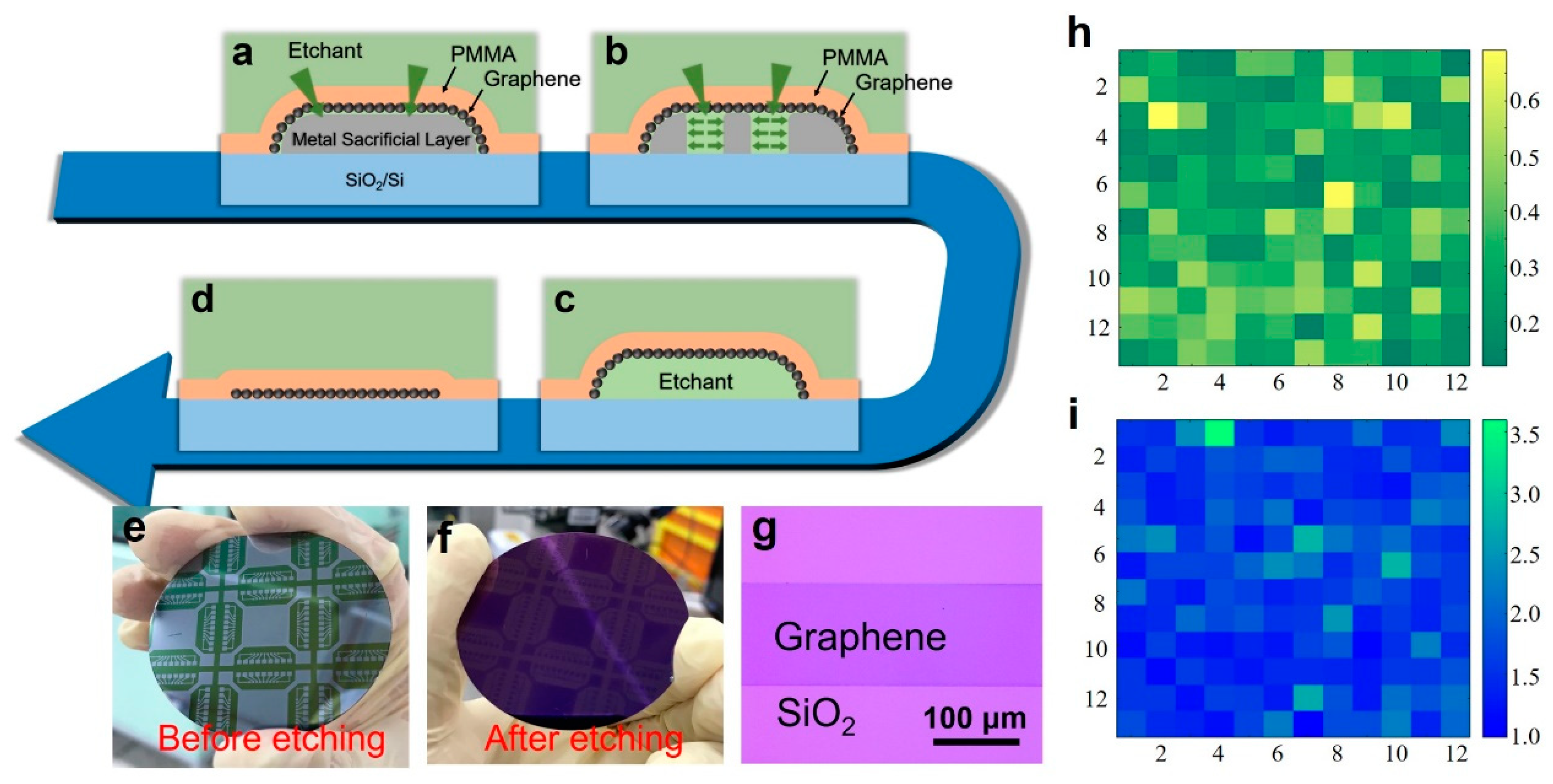
First, we need to give a brief introduction of our in situ growth technology. This method uses a sacrificial metal layer to assist growth. The essence of the growth method is the etching mechanism of the metal sacrificial layer. As shown in
Figure 5a, we deposit patterned metal film on insulating substrates, such as quartz, SiO
2/Si and sapphire. The graphene is grown on the metal surface. After that, a PMMA supporting layer is spun on the sample. Therefore, the metal sacrificial layer is completely covered by the PMMA/graphene films. Interestingly, we find that when the sample is immersed in the metal etchant, the etchant can efficiently penetrate the PMMA and graphene to accomplish the metal etching (
Figure 5b). As shown in
Figure 5c,d, after the etching of the metal, the graphene film will fall on the substrate because it is firmly fixed on the substrate surface by PMMA. Then, the sample is immersed in deionized water for cleaning. After the sample is dried, the PMMA/graphene films are baked at 150 °C for 15 min to enhance the adhesion to the substrate. Finally, the PMMA is removed by acetone. In that manner, in-situ, transfer-free growth of graphene is realized. We call this method “in-situ” and “transfer-free” because the graphene is grown and finally used on the same one substrate and there is not any transfer during the whole process. Besides, the graphene patterns and the positions on the substrate are identical to those of the sacrificial metal layer. By this method, we can grow graphene films of any pattern at any position on the substrate [
15,
29].
Using this method, a patterned graphene film was grown directly on a 2-inch SiO
2/Si substrate. The plasma power was selected to be 50 W; the ratio of methane to hydrogen was 5 sccm/20 sccm; and the growth time was 5 min.
Figure 5e is a photograph of the sample after the spinning of PMMA and before etching of the alloy.
Figure 5f is a photograph of the final, wafer-scale patterned graphene sample.
Figure 5g is an optical image showing the graphene in a local area of the sample. The graphene we grew exhibited a relatively uniform color, indicating that the graphene was macroscopically uniform. The graphene had patterns. Limited by the size of the heater (
Figure 5c), 2 inches is the largest area we can grow. In principle, our method can be scaled up.
Figure 5h,i show the Raman mapping data measured in a 50 × 50 µm area (12 × 12 points).
Figure 5h is the mapping of D/G peak ratio, where I
D/I
G lies in the range of 0.12–0.69.
Figure 5i is the mapping of G/2D peak ratio with I
G/I
2D of typically 0.97–3.6.