Creation of Superhydrophobic Coatings Based on MWCNTs Xerogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Xerogel Preparation
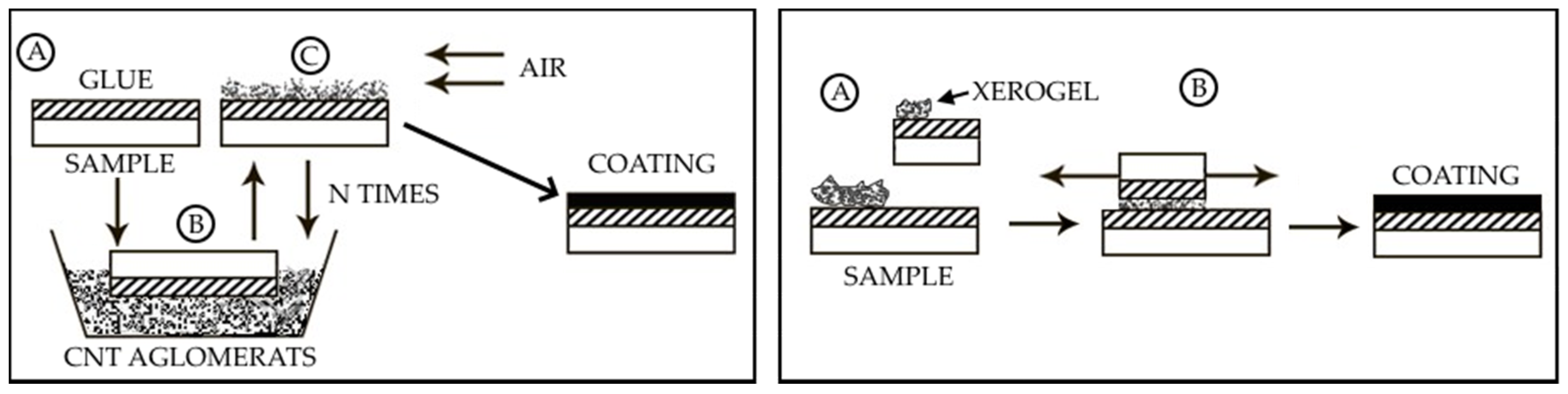
2.2. Obtaining the Coating
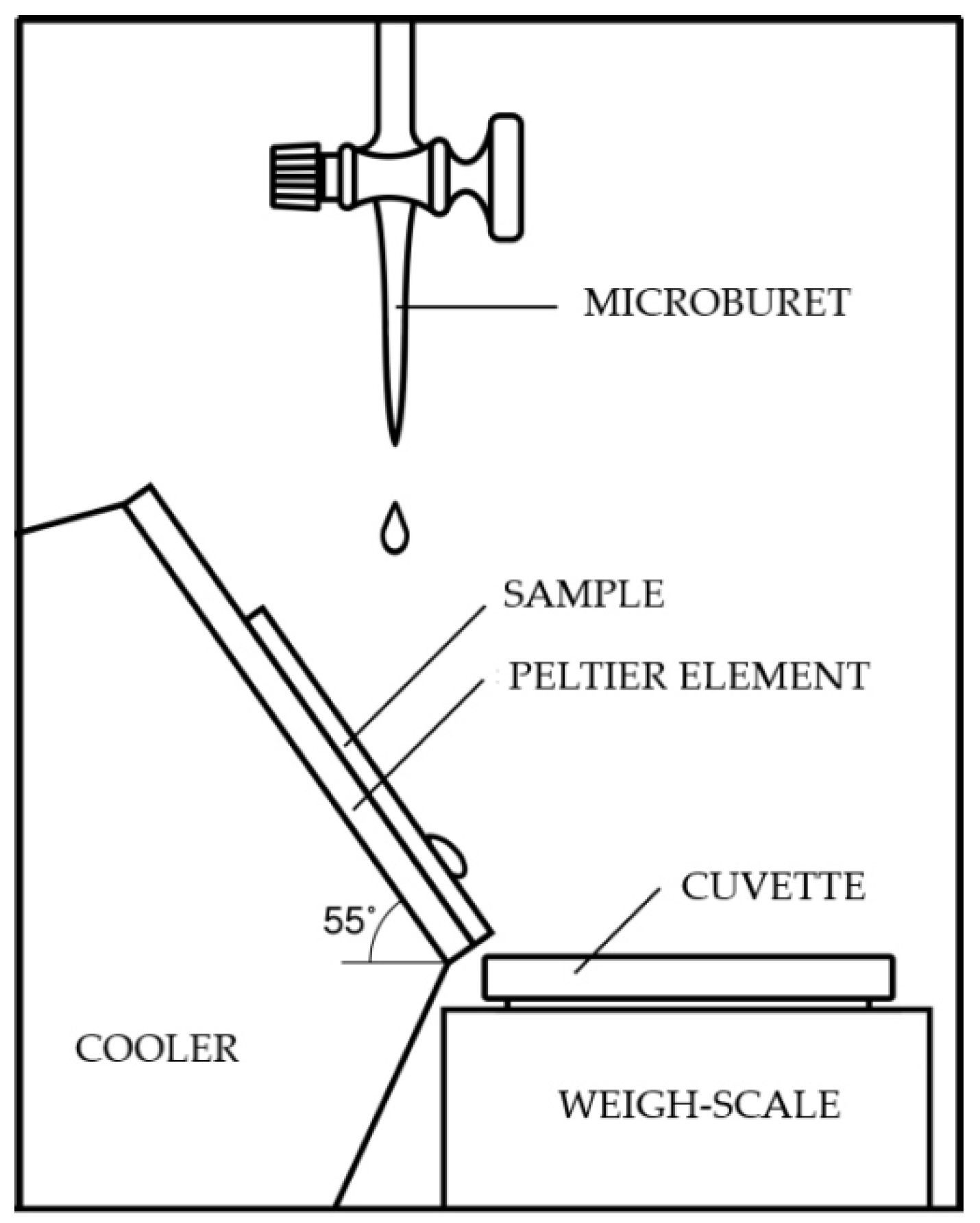
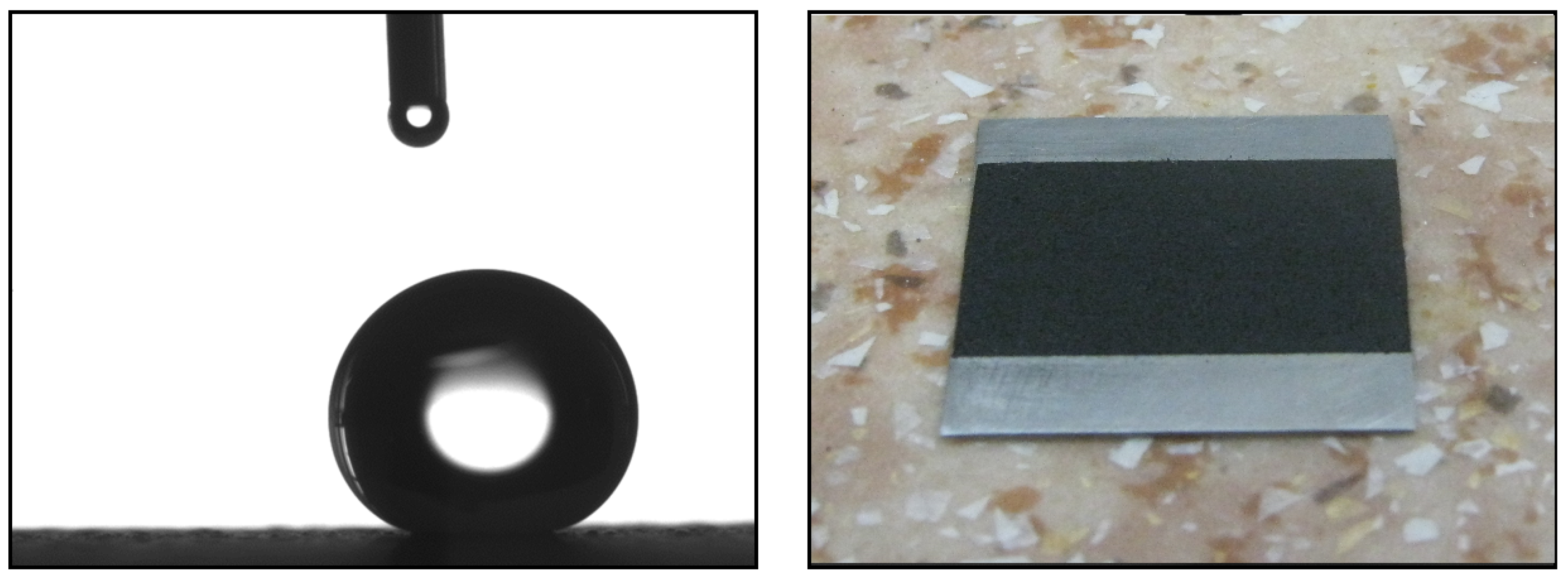
2.3. Wetting Angle Measurements
2.4. Icing
2.5. SLIPS Effect
2.6. Conductivity of the Samples
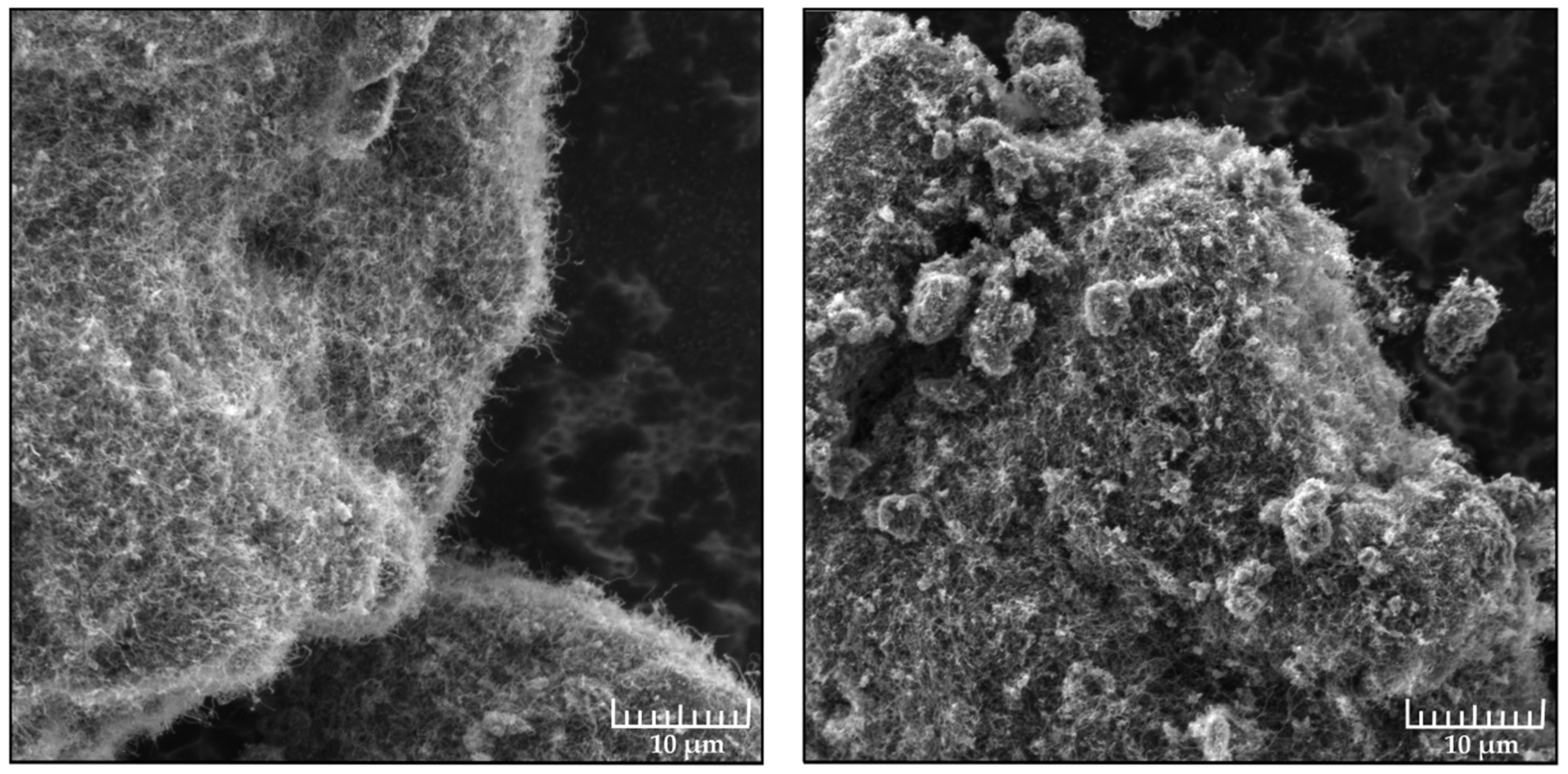
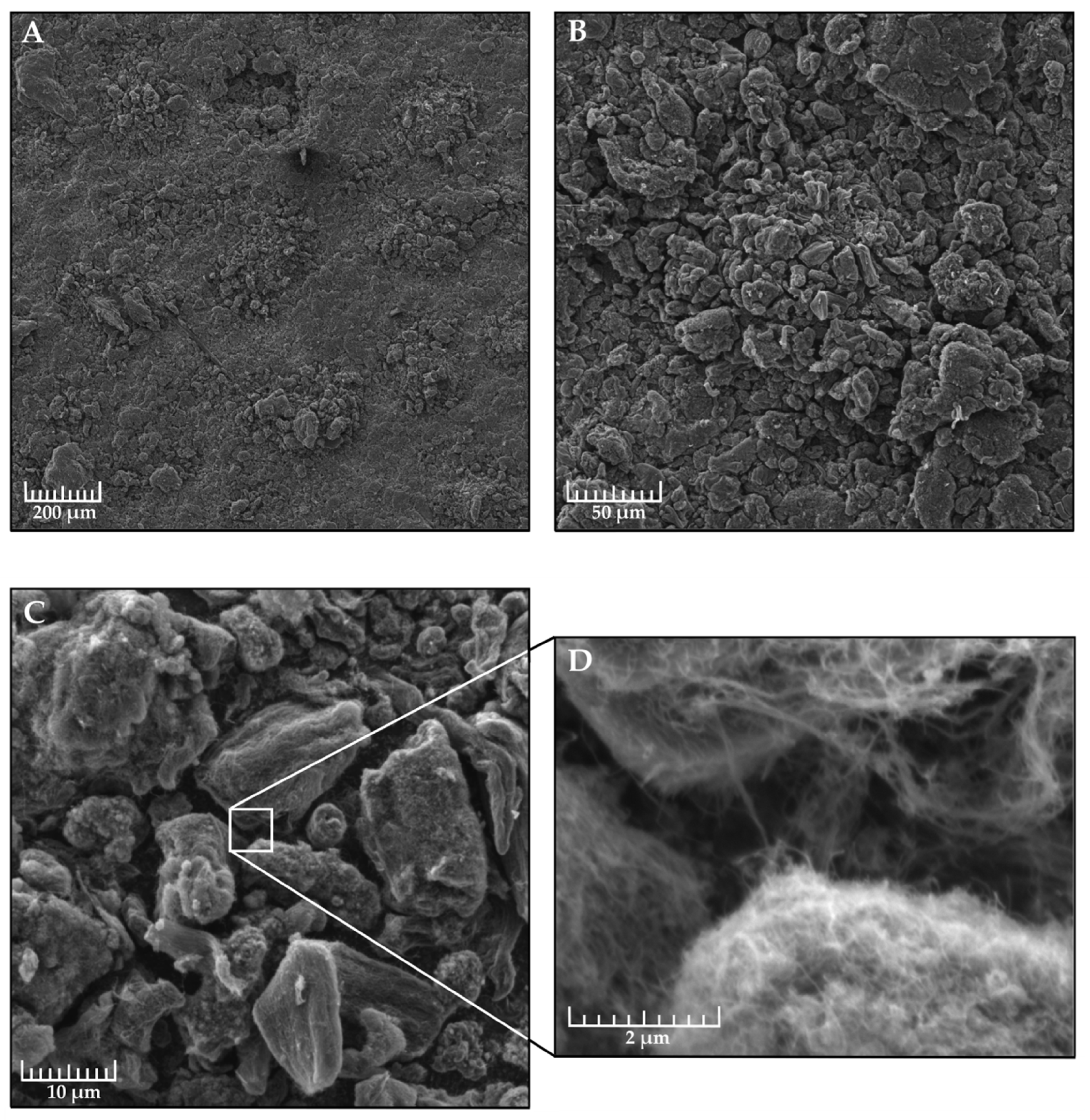
2.7. Scanning Electron Microscopy
3. Results and Discussion
3.1. Hydrophobic Coatings Based on Xerogel and Agglomerates
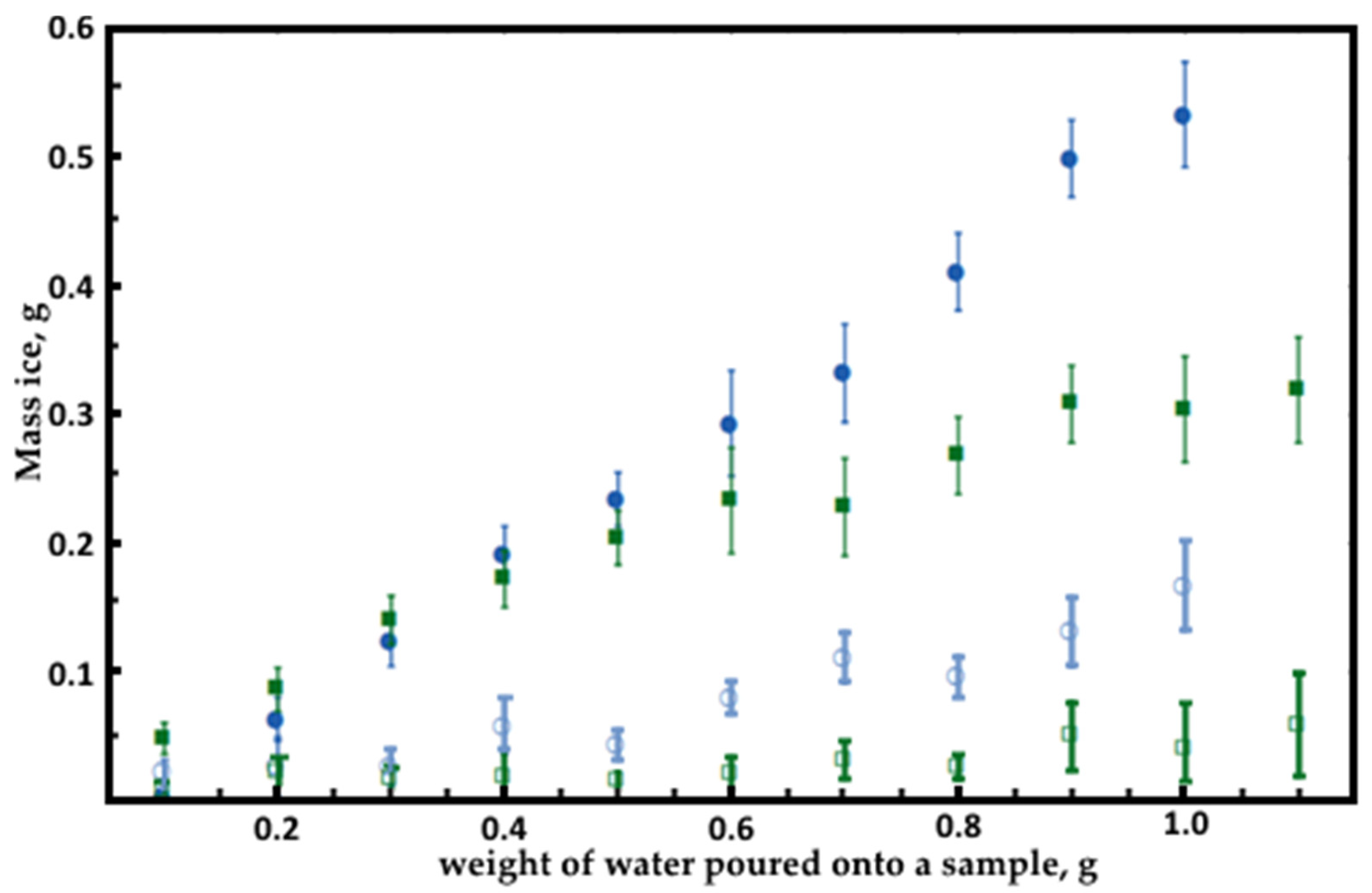
3.2. Icing
3.3. SLIPS Effect
3.4. Conductivity of the Coating
4. Conclusions
- One of the main factors that determine the properties of a coating is the relief of the particle surface made of carbon nanotubes. The morphology of the surface of CNT agglomerates depends heavily on the conditions of synthesis of the CNTs and the conditions of their drying, and can vary in different batches. Furthermore, the surface structure can change under mechanical effect and chemical processing;
- The use of milled xerogel powder allows to obtain a hydrophobic surface with a high homogeneity of properties that is more resistant to mechanical effect. The morphology of xerogel is determined mainly by drying conditions and milling duration;
- During the production of xerogel the use of more polar dissolvents allows to obtain particles with a more developed nanorelief of the surface, but with worse mechanical properties;
- It is preferable to use CNTs with a high aspect ratio—they do not only produce better results, but also allow to improve the mechanical properties of xerogel due to a larger number of contact points between the CNTs. It is also possible to improve the mechanical properties of a coating by introducing a binding hydrophobic polymer into the xerogel. The production method of xerogels is widely known; examples can be seen in [26,27,28,29];
- CNTs possess a high sorption capability, which allows to use the suggested coating to reproduce the SLIPS effect. Meanwhile the lubricant can have anti-icing or biocidal properties. The lubricant can also be used for prompt repair—when applied to damaged spots, it reduces the slip start angle of the droplet;
- The high hydrophobicity of the coating allows to decelerate frosting formation. The droplet slips from the coating before it can freeze. As a result, the coating works best at temperatures close to zero, upon further cooling the pace of frosting formation increases.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Bico, J.; Teo, K.B.K.; Chhowalla, M.; Amaratunga, G.A.J.; Milne, W.I.; McKinley, G.H.; Gleason, K.K. Superhydrophobic Carbon Nanotube Forests. Nano Lett. 2003, 3, 1701–1705. [Google Scholar] [CrossRef]
- Lafuma, A.; Quere, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Otten, A.; Herminghaus, S. How plants keep dry: A physicist’s point of view. Langmuir 2004, 20, 2405–2408. [Google Scholar] [CrossRef]
- Puretskiy, N.; Chanda, J.; Stoychev, G.; Synytska, A. Anti–icing superhydrophobic surfaces based on core—Shell fossil particles. Adv. Mater. Interfaces 2015, 2, 1500124–1500131. [Google Scholar] [CrossRef]
- Sohn, S.; Kim, D.; Bae, K.; Choi, M.; Lee, D.U.; Kim, H.M. A stable super-hydrophobic and self-cleaning all surface formed by using roughness combined with hydrophobic coatings. Mol. Cryst. Liq. Cryst. 2014, 6, 9–16. [Google Scholar] [CrossRef]
- Liu, J.; Janjua, Z.A.; Roe, M.; Xu, F.; Turnbull, B.; Choi, K.S.; Hou, X. Super-Hydrophobic/Icephobic Coatings Based on Silica Nanoparticles Modified by Self-Assembled Monolayers. Nanomaterials 2016, 6, 232. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Manik, G. Recent progress in super hydrophobic/hydrophilic self-cleaning surfaces for various industrial applications: A review. Polym. Plast. Technol. Eng. 2018, 57, 1932–1952. [Google Scholar] [CrossRef]
- Howarter, J.A.; Genson, K.L.; Youngblood, J.P. Wetting behavior of oleophobic polymer coatings synthesized from fluorosurfactant–macromers. ACS Appl. Mater. Interfaces 2011, 3, 2022–2030. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Self-cleaning and next generation anti-fog surfaces and coatings. Macromol. Rapid Commun. 2008, 29, 455–466. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self–repairing slippery surfaces with pressure–stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Yung, G.S.; Tomlin, N.; Heuerman, K.; Keller, M.V.; White M, G.; Stephens, M.; Lehman, J.H. Plasma modification of vertically aligned carbon nanotubes: Superhydrophobic surfaces with ultra-low reflectance. Carbon 2018, 127, 195–201. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Murugan, P.; Adhigan, M.; Jaisankar, S.N.; Mandal, A.B. “Click” Polymer of Carbon Nanotubes for Superhydrophobic Glass and Leather. Green Mater. 2017, 5, 46–52. [Google Scholar] [CrossRef]
- Zhang, H.F.; TeoChun, M.K.; Yang, C. Superhydrophobic carbon nanotube/polydimethylsiloxane composite coatings. Mater. Sci. Technol. 2015, 31, 1745–1748. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, P.; Xu, X.; Wang, K.; Li, Y.; Zeng, Y. Chemically robust carbon nanotube—PTFE superhydrophobic thin films with enhanced ability of wear resistance. Prog. Nat. Sci. 2017, 27, 396–399. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, J.; Wang, R.; Lu, H.; Li, L.; Kong, Y.; Qi, K.; Xin, J.H. Artificial lotus leaf structures from assembling carbon nanotubes and their applications in hydrophobic textiles. J. Mater. Chem. 2007, 17, 1071–1078. [Google Scholar] [CrossRef]
- Lv, C.; Wang, H.; Liu, Z.; Wang, C.; Zhang, W.; Li, M.; Zhu, Y. Fabrication of durable fluorine-free polyphenylene sulfide/silicone resin composite superhydrophobic coating enhanced by carbon nanotubes/graphene fillers. Prog. Org. Coat. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- He, S.; Wei, J.; Wang, H.; Sun, D.; Yao, Z.; Fu, C.; Xu, R.; Jia, Y.; Zhu, H.; Wang, K.; et al. Stable superhydrophobic surface of hierarchical carbon nanotubes on Si micropillar arrays. Nanoscale Res. Lett. 2013, 8, 412–418. [Google Scholar] [CrossRef]
- Rafiee, J.; Rafiee, M.A.; Yu, Z.; Koratkar, N. Superhydrophobic to superhydrophilic wetting control in graphene films. Adv. Mater. 2010, 22, 2151–2154. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ye, L.; Xie, K.; You, J.; Li, Y. Reversible transition between adhesive and antiadhesive performances by stretching/recovery on superhydrophobic TPU/CNTs composite membrane surface. Appl. Surf. Sci. 2019, 471, 900–903. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Girão, A.V.; Silva, R.F.; Durães, L. Polysilsesquioxane-based silica aerogel monoliths with embedded CNTs. Microporous Mesoporous Mater. 2019, 288, 109575. [Google Scholar] [CrossRef]
- Hong, Y.C.; Uhm, H.S. Superhydrophobicity of a material made from multiwalled carbon nanotubes. Appl. Phys. Lett. 2006, 88, 244101. [Google Scholar] [CrossRef]
- Asthana, A.; Maitra, T.; Büchel, R.; Tiwari, M.K.; Poulikakos, D. Multifunctional superhydrophobic polymer/carbon nanocomposites: Graphene, carbon nanotubes, or carbon black? ACS Appl. Mater. Interfaces 2014, 6, 8859–8867. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. A review of production methods of carbon nanotube and graphen thin films for electrothermal application. Nanoscale 2014, 6, 3037–3045. [Google Scholar] [CrossRef]
- Fath, N.; Lotfy, F.V.; Basta, H.A. Comparative study on the performance of carbon nanotubes prepared from agro- and xerogels as carbon supports. J. Anal. Appl. Pyrolysis 2017, 128, 114–120. [Google Scholar] [CrossRef]
- Rodriguez, N.; Agamez-Pertuz, Y.Y.; Romero, E.; Diaz-Velasquez, J.J.; Odriozola, J.A.; Centeno, M.A. Effect of starch as binder in carbon aerogel and carbon xerogel preparation. J. Non-Cryst. Solids 2019, 522, 119554. [Google Scholar] [CrossRef]
- Long, X.Y.; Gao, N.; Yueying, G.; Huo, S.; Mohideen, M.M.; Hong, S.; Liy, Y. Controllable preparation of methyltriethoxysilane xerogel nanofibers. J. Mater. Sci. 2019, 54, 10130–10140. [Google Scholar] [CrossRef]
- Mahato, N.R.; Hansda, K.M.; Das, A.; Banerjee, J.; Mondal, S.; Mahata, N. Synthesis of Mesoporous Carbon Xerogel and Activation by Oxidative Treatment. Asian J. Chem. 2019, 31, 2139–2142. [Google Scholar] [CrossRef]
| Characteristic | Taunit | Taunit–M | Taunit–MD |
|---|---|---|---|
| Outer diameter, nm | 20–50 | 10–30 | 8–30 |
| Internal diameter, nm | 10–20 | 5–15 | 5–15 |
| Length, μm | ≥2 | ≥2 | ≥20 |
| The total amount of impurities, % | ≤10 | ≤5 | ≤5 |
| Specific surface, m2/g | ≥160 | ≥270 | ≥270 |
| Bulk density, g/cm3 | 0.3–0.6 | 0.025–0.06 | 0.025–0.06 |
| Cost, dollar per gram | 0.2 | 1.5 | 1.5 |
| Model | Application through Sticking | Application through Grinding |
|---|---|---|
| Taunit | 110.7° ± 10.8° | 135.1° ± 4.8° |
| Taunit-М | 121.1° ± 8.3° | 143.7° ± 4.1° |
| Taunit-МD | 127.0° ± 6.4° | 146.0° ± 3.4° |
| Xerogel Material | Taunit-M and Isopropanol | Taunit-MD and Isopropanol | Taunit-M and Ethanol | |||
|---|---|---|---|---|---|---|
| Fraction Size, mm | Water Contact Angle, ° | Slip Start Angle, ° | Water Contact Angle, ° | Slip Start Angle, ° | Water Contact Angle, ° | Slip Start Angle, ° |
| 0.350–0.250 | 143.5 ± 3.3 | 15.0 ± 8.5 | 144.8 ± 2.1 | 10.5 ± 5.0 | 151.5 ± 2.2 | 12.6 ± 5.6 |
| 0.250–0.140 | 145.9 ± 1.9 | 12.7 ± 6.2 | 147.2 ± 1.9 | 9.1 ± 5.2 | 153.8 ± 2.8 | 5.1 ± 1.5 |
| 0.140–0.125 | 146.3 ± 1.2 | 9.3 ± 4.4 | 148.3 ± 1.7 | 8.0 ± 1.0 | 155.9 ± 1.5 | 3.7 ± 1.8 |
| 0.125–0.100 | 147.2 ± 0.5 | 5.1 ± 2.2 | 150.5 ± 0.6 | 4.4 ± 0.4 | 155.6 ± 1.1 | 3.6 ± 1.2 |
| 0.100–0.071 | 146.3 ± 1.1 | 5.3 ± 2.8 | 148.8 ± 0.9 | 4.7 ± 1.5 | 155.6 ± 1.0 | 3.7 ± 1.1 |
| Slip Start angle, ° | Before Applying the Lubricant | The droplet doesn’t slide | 88.2 ± 12.5 | 41.9 ± 15.7 | 36.2 ± 13.2 | 25.2 ± 15.1 | 19.9 ± 5.7 | 14.3 ± 4.5 |
| After Applying the Lubricant | 6.4 ± 2.5 | 6.3 ± 1.2 | 4.5 ± 1.4 | 2.5 ± 1.1 | 6.3 ± 2.1 | 7.0 ± 1.8 | 3.4 ± 1.7 |
| Sample | Water Contact Angle | Slip Start Angle |
|---|---|---|
| Before applying perfluorodecalin | 157.7 ± 1.4 | 4.7 ± 1.8 |
| With perfluorodecalin | 102.0 ± 0.9 | 2.5 ± 0.6 |
| After drying perfluorodecalin | 154.5 ± 2.7 | 5.6 ± 1.9 |
| Fraction size, mm | 0.100–0.071 | 0.125–0.100 | 0.140–0.125 | 0.250–0.140 | 0.350–0.250 |
| Resistance, kOhm/sm2 | 0.47 ± 0.03 | 0.44 ± 0.05 | 0.35 ± 0.03 | 0.34 ± 0.01 | 0.29 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eseev, M.; Goshev, A.; Kapustin, S.; Tsykareva, Y. Creation of Superhydrophobic Coatings Based on MWCNTs Xerogel. Nanomaterials 2019, 9, 1584. https://doi.org/10.3390/nano9111584
Eseev M, Goshev A, Kapustin S, Tsykareva Y. Creation of Superhydrophobic Coatings Based on MWCNTs Xerogel. Nanomaterials. 2019; 9(11):1584. https://doi.org/10.3390/nano9111584
Chicago/Turabian StyleEseev, Marat, Andrey Goshev, Sergey Kapustin, and Yuliana Tsykareva. 2019. "Creation of Superhydrophobic Coatings Based on MWCNTs Xerogel" Nanomaterials 9, no. 11: 1584. https://doi.org/10.3390/nano9111584
APA StyleEseev, M., Goshev, A., Kapustin, S., & Tsykareva, Y. (2019). Creation of Superhydrophobic Coatings Based on MWCNTs Xerogel. Nanomaterials, 9(11), 1584. https://doi.org/10.3390/nano9111584






