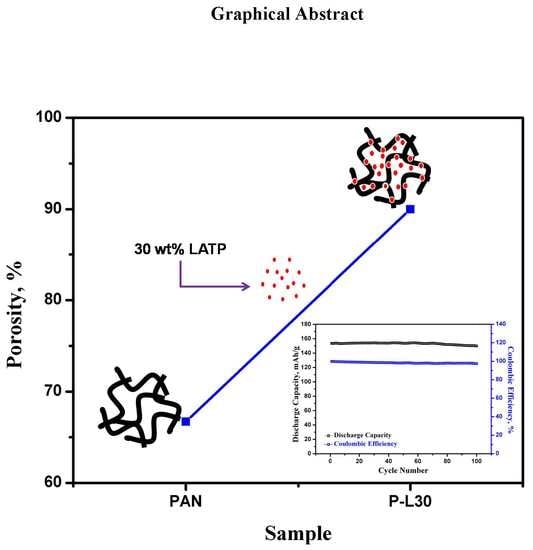
Preparation of Highly Porous PAN-LATP Membranes as Separators for Lithium Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
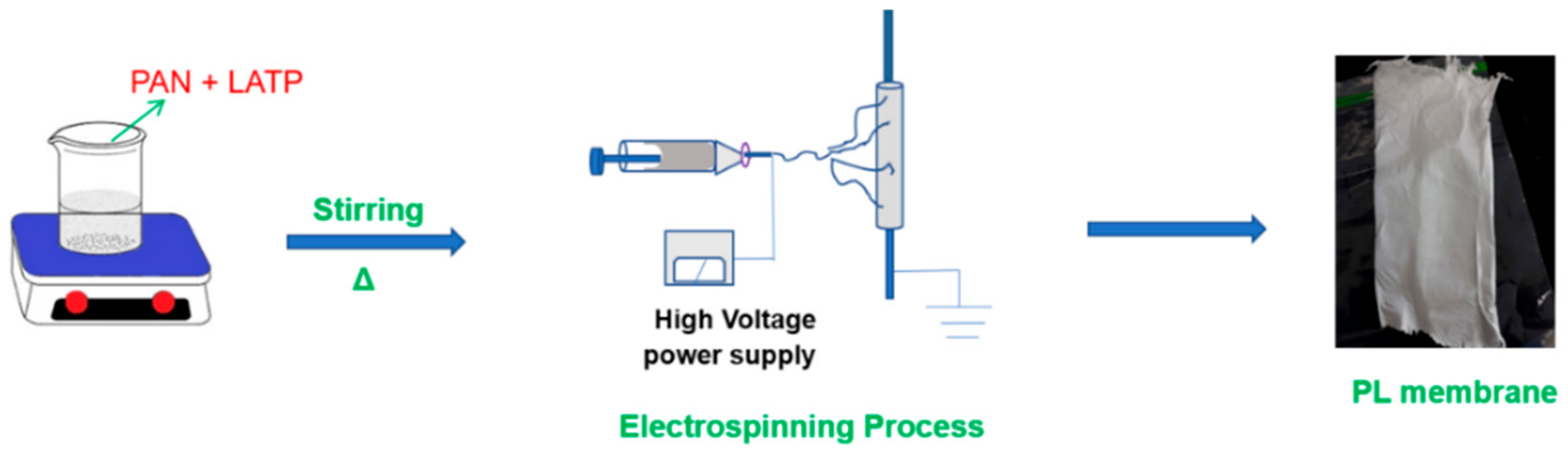
2.1. Fabrication of PL Membranes
2.2. Characterization of PL Membranes
2.3. Electrochemical Characterization
3. Results and Discussion
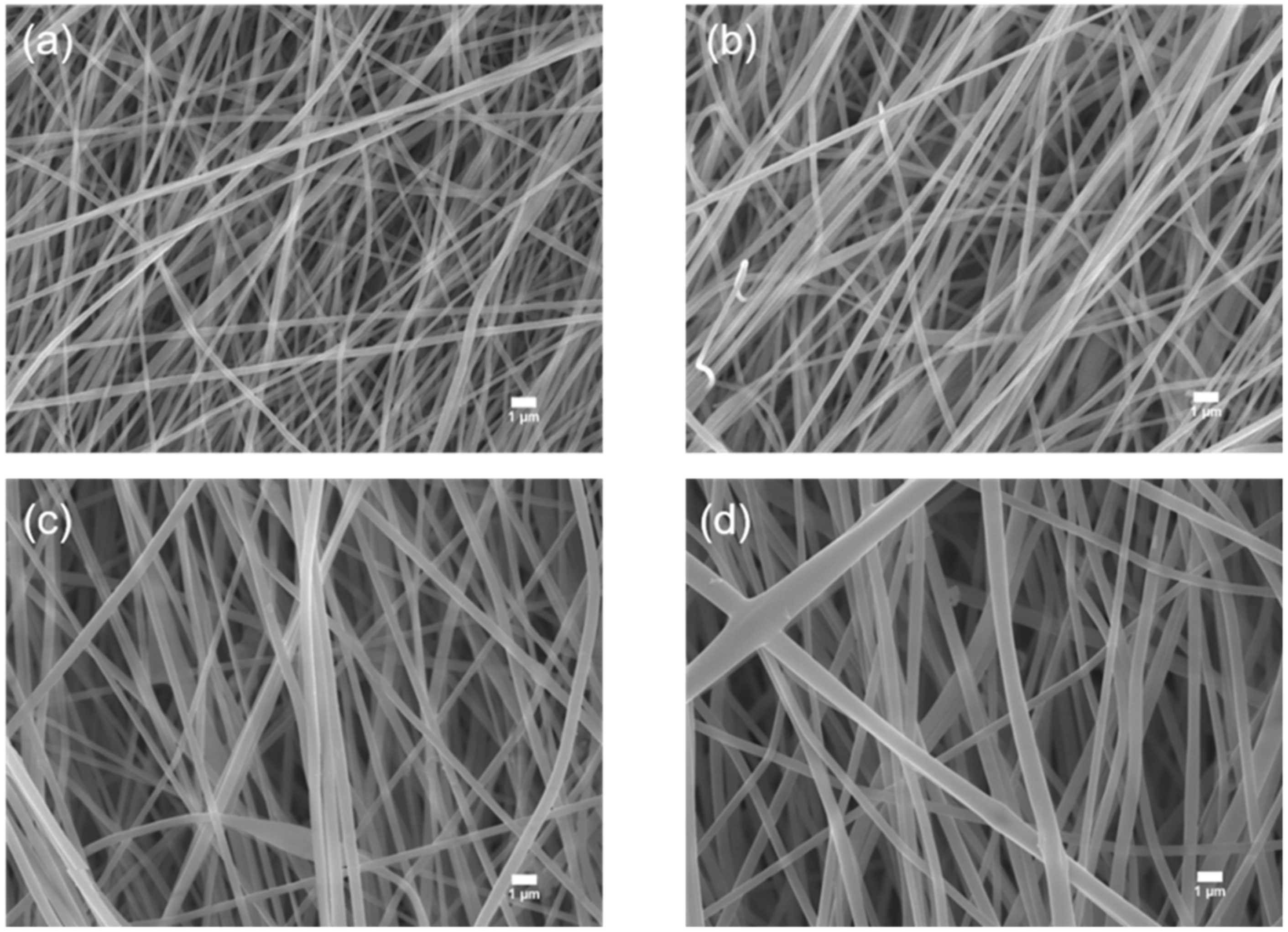
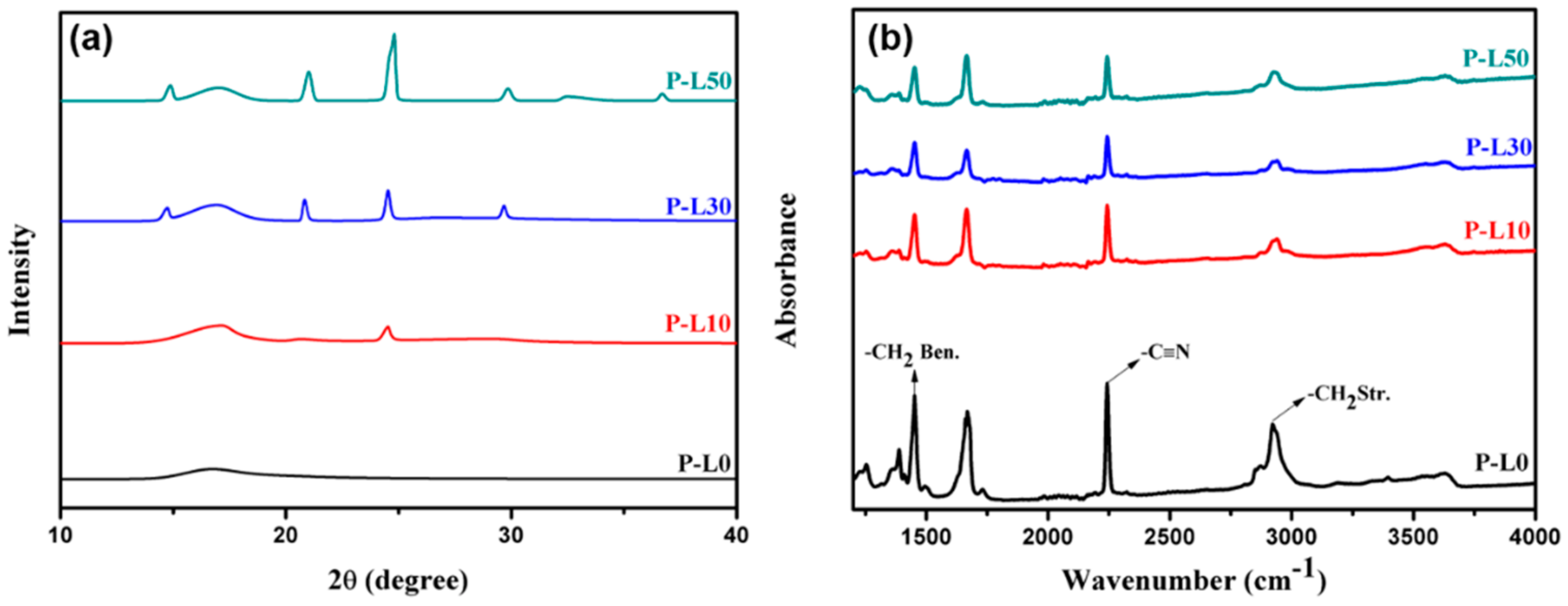
3.1. Morphology and Phase Change of PL Membranes
3.2. Porosity, Electrolyte Uptake, Thermal Stability, and Ionic Conductivity of PL Membranes
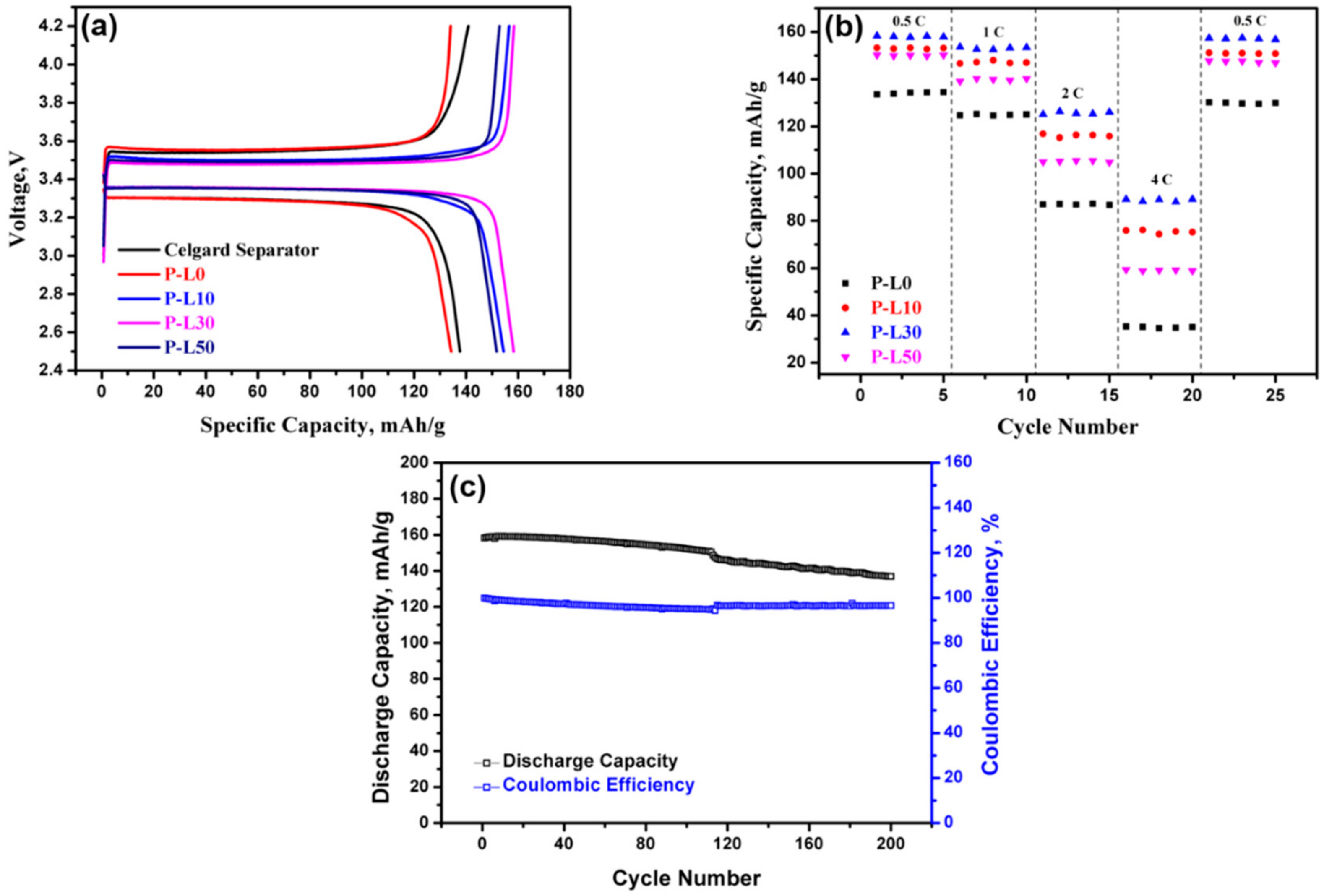
3.3. Electrochemical Investigation of PL Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [Green Version]
- Feng, K.; Li, M.; Liu, W.; Kashkooli, A.G.; Xiao, X.; Cai, M.; Chen, Z. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Qin, X.; Zou, J.; Luo, L.; Wang, Y.; Wu, M.; Zhu, H.; Chen, G.; Kang, F.; Li, B. Electrosprayed silicon-embedded porous carbon microspheres as lithium-ion battery anodes with exceptional rate capacities. Carbon 2018, 127, 424–431. [Google Scholar] [CrossRef]
- Ko, H.S.; Park, H.W.; Kim, G.J.; Lee, J.D. Electrochemical characteristics of lithium-excess cathode material (Li1+ x Ni0.9 Co0.05 Ti0.05 O2) for lithium-ion batteries. Korean J. Chem. Eng. 2019, 36, 620–624. [Google Scholar] [CrossRef]
- Kim, S.C.; Park, Y.K.; Kim, B.H.; Kim, H.; Lee, W.J.; Lee, H.; Jung, S.C. Facile precipitation of tin oxide nanoparticles on graphene sheet by liquid phase plasma method for enhanced electrochemical properties. Korean J. Chem. Eng. 2018, 35, 750–756. [Google Scholar] [CrossRef]
- Choi, S.I.; Lee, Y.M.; Jeong, H.C.; Jung, E.J.; Lee, M.S.; Kim, J.; Kim, Y.H.; Won, Y.S. Preparation of Si/C anode with PVA nanocomposite for Lithium-ion battery using electrospinning method. Korean Chem. Eng. Res. 2018, 56, 139–142. [Google Scholar]
- Kim, Y.S.; Oh, S.Y.; Kim, E.; Kim, D.; Kim, S.; Chu, C.H.; Park, K. Iron—Chrome crossover through Nafion membrane in Iron—Chrome redox flow battery. Korean Chem. Eng. Res. 2018, 56, 24–28. [Google Scholar]
- Costa, C.M.; Kundu, M.; Dias, J.C.; Nunes-Pereira, J.; Botelho, G.; Silva, M.M.; Lanceros-Méndez, S. Mesoporous poly (vinylidene fluoride-co-trifluoroethylene) membranes for lithium-ion battery separators. Electrochim. Acta 2019, 301, 97–106. [Google Scholar] [CrossRef]
- Costa, C.M.; Kundu, M.; Cardoso, V.F.; Machado, A.V.; Silva, M.M.; Lanceros-Méndez, S. Silica/poly (vinylidene fluoride) porous composite membranes for lithium-ion battery separators. J. Membr. Sci. 2018, 564, 842–851. [Google Scholar] [CrossRef]
- Guo, T.; Song, J.; Jin, Y.; Sun, Z.; Li, L. Thermally stable and green cellulose-based composites strengthened by styrene-co-acrylate latex for lithium-ion battery separators. Carbohydr. Polym. 2019, 206, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of lithium-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef]
- Yan, S.; Deng, J.; Bae, C.; Xiao, X. Thermal expansion/shrinkage measurement of battery separators using a dynamic mechanical analyzer. Polym. Test. 2018, 71, 65–71. [Google Scholar] [CrossRef]
- Zhang, T.W.; Tian, T.; Shen, B.; Song, Y.H.; Yao, H.B. Recent advances on biopolymer fiber based membranes for lithium-ion battery separators. Compos. Commun. 2019, 14, 7–14. [Google Scholar] [CrossRef]
- Wang, E.; Wu, H.P.; Chiu, C.H.; Chou, P.H. The effect of battery separator properties on thermal ramp, overcharge and short circuiting of rechargeable Li-Ion batteries. J. Electrochem. Soc. 2019, 166, A125–A131. [Google Scholar] [CrossRef]
- Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv. 2013, 3, 11404–11417. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional nanofibers for environmental applications. J. Mater. Chem. 2008, 18, 5326–5334. [Google Scholar] [CrossRef]
- Li, J.; Zhong, Q.; Yao, Y.; Bi, S.; Zhou, T.; Guo, X.; Wu, M.; Feng, T.; Xiang, R. Electrochemical performance and thermal stability of the electrospun PTFE nanofiber separator for lithium-ion batteries. J. Appl. Polym. Sci. 2018, 135, 46508. [Google Scholar] [CrossRef]
- Feng, G.; Li, Z.; Mi, L.; Zheng, J.; Feng, X.; Chen, W. Polypropylene/hydrophobic-silica-aerogel-composite separator induced enhanced safety and low polarization for lithium-ion batteries. J. Power Sources 2018, 376, 177–183. [Google Scholar] [CrossRef]
- Pan, R.; Cheung, O.; Wang, Z.; Tammela, P.; Huo, J.; Lindh, J.; Edström, K.; Strømme, M.; Nyholm, L. Mesoporous Cladophora cellulose separators for lithium-ion batteries. J. Power Sources 2016, 321, 185–192. [Google Scholar] [CrossRef]
- Rao, E.; McVerry, B.; Borenstein, A.; Anderson, M.; Jordan, R.S.; Kaner, R.B. Roll-to-roll functionalization of polyolefin separators for high-performance lithium-ion batteries. ACS Appl. Energy Mater. 2018, 1, 3292–3300. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Y.; Cao, H.; Zhao, L.; Huang, Y.; Song, A.; Lin, Y.; Wang, M.; Li, X. A novel polyacrylonitrile-based porous structure gel polymer electrolyte composited by incorporating polyhedral oligomeric silsesquioxane by phase inversion method. J. Solid State Electrochem. 2018, 22, 1771–1783. [Google Scholar] [CrossRef]
- Subramania, A.; Sundaram, N.K.; Kumar, G.V.; Vasudevan, T. New polymer electrolyte based on (PVA–PAN) blend for Li-ion battery applications. Ionics 2006, 12, 175–178. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.; Zhao, Z.; Zhao, Y.; Li, F.; Yang, L. PVDF/PAN/SiO2 polymer electrolyte membrane prepared by combination of phase inversion and chemical reaction method for lithium ion batteries. J. Solid State Electrochem. 2016, 20, 699–712. [Google Scholar] [CrossRef]
- Li, H.; Chao, C.Y.; Han, P.L.; Yan, X.R.; Zhang, H.H. Preparation and properties of gel-filled PVDF separators for lithium ion cells. J. Appl. Polym. Sci. 2017, 134, 44473. [Google Scholar] [CrossRef]
- Almuhamed, S.; Bonne, M.; Khenoussi, N.; Brendle, J.; Schacher, L.; Lebeau, B.; Adolphe, D.C. Electrospinning composite nanofibers of polyacrylonitrile/synthetic Na-montmorillonite. J. Ind. Eng. Chem. 2016, 35, 146–152. [Google Scholar] [CrossRef]
- Huai, Y.; Gao, J.; Deng, Z.; Suo, J. Preparation and characterization of a special structural poly (acrylonitrile)-based microporous membrane for lithium-ion batteries. Ionics 2010, 16, 603–611. [Google Scholar] [CrossRef]
- Vondrák, J.; Sedlaríková, M.; Reiter, J.; Kašpar, D. PMMA Based Gel Polymer Electrolytes. In Materials for Lithium-Ion Batteries; Julien, C., Stoynov, Z., Eds.; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- He, J.; Liu, J.; Li, J.; Lai, Y.; Wu, X. Enhanced ionic conductivity and electrochemical capacity of lithium ion battery based on PVDF-HFP/HDPE membrane. Mater. Lett. 2016, 170, 126–129. [Google Scholar] [CrossRef]
- Kang, D.W.; Kim, J.K. Characterization of fibrous gel polymer electrolyte for lithium polymer batteries with enhanced electrochemical properties. J. Electroanal. Chem. 2016, 775, 37–42. [Google Scholar] [CrossRef]
- Elia, G.A.; Ducros, J.B.; Sotta, D.; Delhorbe, V.; Brun, A.; Marquardt, K.; Hahn, R. Polyacrylonitrile separator for high-performance aluminum batteries with improved interface stability. ACS Appl. Mater. Interfaces 2017, 9, 38381–38389. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kolla, P.; Yang, R.; Wang, Z.; Zhao, Y.; Smirnova, A.L.; Fong, H. Electrospun polyacrylonitrile nanofibrous membranes with varied fiber diameters and different membrane porosities as lithium-ion battery separators. Electrochimca Acta 2017, 236, 417–423. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, J.; Chong, C.; Yu, X.; Wang, L.; Shi, Z. An electrospun lignin/polyacrylonitrile nonwoven composite separator with high porosity and thermal stability for lithium-ion batteries. RSC Adv. 2015, 5, 101115–101120. [Google Scholar] [CrossRef]
- Freitag, A.; Langklotz, U.; Rost, A.; Stamm, M.; Ionov, L. Ionically conductive polymer/ceramic separator for lithium-sulfur batteries. Energy Storage Mater. 2017, 9, 105–111. [Google Scholar] [CrossRef]
- Kumar, J.; Kichambare, P.; Rai, A.K.; Bhattacharya, R.; Rodrigues, S.; Subramanyam, G. A high performance ceramic-polymer separator for lithium batteries. J. Power Sources 2016, 301, 194–198. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, H.W.; Kim, Y.; Kim, J.K. Composite gel polymer electrolyte with ceramic particles for LiNi1/3Mn1/3Co1/3O2-Li4Ti5O12 lithium ion batteries. Electrochim. Acta 2017, 236, 394–398. [Google Scholar] [CrossRef]
- Duluard, S.; Paillassa, A.; Puech, L.; Vinatier, P.; Turq, V.; Rozier, P.; Lenormand, P.; Taberna, P.L.; Simon, P.; Ansart, F. Lithium conducting solid electrolyte Li1.3Al0.3Ti1.7(PO4)3 obtained via solution chemistry. J. Eur. Ceram. Soc. 2013, 33, 1145–1153. [Google Scholar] [CrossRef]
- Kou, Z.; Miao, C.; Wang, Z.; Mei, P.; Zhang, Y.; Yan, X.; Jiang, Y.; Xiao, W. Enhanced ionic conductivity of novel composite polymer electrolytes with Li1.3Al0.3Ti1.7(PO4)3 NASICON-type fast ion conductor powders. Solid State Ion. 2019, 338, 138–143. [Google Scholar] [CrossRef]
- Nairn, K.M.; Best, A.S.; Newman, P.J.; Macfarlane, D.R.; Forsyth, M. Ceramic-polymer interface in composite electrolytes of lithium aluminium titanium phosphate and polyetherurethane polymer electrolyte. Solid State Ion. 1999, 121, 115–119. [Google Scholar] [CrossRef]
- Liang, X.; Han, D.; Wang, Y.; Lan, L.; Mao, J. Preparation and performance study of a PVDF–LATP ceramic composite polymer electrolyte membrane for solid-state batteries. RSC Adv. 2018, 8, 40498–40504. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, Z.; Qiu, Y.; Zhang, X. Fabrication and characterization of LATP/PAN composite fiber-based lithium-ion battery separators. Electrochimica Acta 2011, 56, 6474–6480. [Google Scholar] [CrossRef]
- Lee, S.S.; Lim, Y.J.; Kim, H.W.; Kim, J.K.; Jung, Y.G.; Kim, Y. Electrochemical properties of a ceramic-polymer-composite-solid electrolyte for Li-ion batteries. Solid State Ion. 2016, 284, 20–24. [Google Scholar] [CrossRef]
- Bicy, K.; Suriyakumar, S.; Anu, A.S.; Kalarikkal, N.; Stephen, A.M.; Geethamma, V.G.; Rouxel, D.; Thomas, S. Highly lithium ion conductive, Al2O3 decorated electrospun P(VDF-TrFE) membranes for lithium ion battery separators. New J. Chem. 2018, 42, 19505–19520. [Google Scholar]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Nemavhola, F. Structural morphology and electronic conductivity of blended Nafion®-polyacrylonitrile/zirconium phosphate nanofibres. Int. J. Mech. Mater. Eng. 2019, 14. Available online: https://link.springer.com/article/10.1186/s40712-019-0098-1 (accessed on 7 November 2019). [CrossRef]
- Shi, X.; Ma, N.; Wu, Y.; Lu, Y.; Xiao, Q.; Li, Z.; Lei, G. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery. Solid State Ion. 2018, 325, 112–119. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Chen, Y.; Qiao, L.; Lu, X.; Tian, X. Modified polypropylene/cotton fiber composite nonwoven as lithium-ion battery separator. Mater. Chem. Phys. 2018, 219, 368–375. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, N.; Yan, J.; Kang, W.; Ju, J.; Wang, L.; Li, Z.; Cheng, B. Effect of OctaphenylPolyhedral oligomeric silsesquioxane on the electrospun Poly-m-phenylene isophthalamid separators for lithium-ion batteries with high safety and excellent electrochemical performance. Chem. Eng. J. 2019, 356, 11–21. [Google Scholar] [CrossRef]
- Li, L.; Liu, P.; Fu, Q.S.; Gong, Y.; Zhang, S.R.; He, H.J.; Chen, J. Study on preparation of polyacrylonitrile/polyimide composite lithium-ion battery separator by electrospinning. J. Mater. Res. 2019, 34, 642–651. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Li, X. Porous polyetherimide membranes with tunable morphology for lithium-ion battery. J. Membr. Sci. 2018, 565, 42–49. [Google Scholar] [CrossRef]
- Waqas, M.; Ali, S.; Lv, W.; Chen, D.; Boateng, B.; He, W. High-Performance PE-BN/PVDF-HFP bilayer separator for lithium-ion batteries. Adv. Mater. Interfaces 2019, 6, 1801330. [Google Scholar] [CrossRef]
- Arifeen, W.U.; Kim, M.; Choi, J.; Yoo, K.; Kurniawan, R.; Ko, T.J. Optimization of porosity and tensile strength of electrospun polyacrylonitrile nanofibrous membranes. Mater. Chem. Phys. 2019, 229, 310–318. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Zhu, J.; Zhang, X. Silica/polyacrylonitrile hybrid nanofiber membrane separators via sol-gel and electrospinning techniques for lithium-ion batteries. J. Power Sources 2016, 313, 205–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Xiang, H.; Shi, P.; Wang, H. A thin inorganic composite separator for lithium-ion batteries. J. Membrane Sci. 2016, 509, 19–26. [Google Scholar] [CrossRef]
- Chao, C.Y.; Feng, Y.F.; Hua, K.; Li, H.; Wu, L.J.; Zhou, Y.S.; Dong, Z.W. Enhanced wettability and thermal stability of polypropylene separators by organic–inorganic coating layer for lithium-ion batteries. J. Appl. Polym. Sci. 2018, 135, 46478. [Google Scholar] [CrossRef]
- Sun, G.; Dong, G.; Kong, L.; Yan, X.; Tian, G.; Qi, S.; Wu, D. Robust polyimide nanofibrous membrane with porous-layer-coated morphology by in situ self-bonding and micro-crosslinking for lithium-ion battery separator. Nanoscale 2018, 10, 22439–22447. [Google Scholar] [CrossRef]
- Kong, L.; Yan, Y.; Qiu, Z.; Zhou, Z.; Hu, J. Robust fluorinated polyimide nanofibers membrane for high-performance lithium-ion batteries. J. Membr. Sci. 2018, 549, 321–331. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Zhang, X. Polymethylmethacrylate/polyacrylonitrile membranes via centrifugal spinning as separator in Li-ion batteries. Polymers 2015, 7, 629–643. [Google Scholar] [CrossRef]
- Xu, H.; Li, D.; Liu, Y.; Jiang, Y.; Li, F.; Xue, B. Preparation of halloysite/polyvinylidene fluoride composite membrane by phase inversion method for lithium ion battery. J. Alloy. Compd. 2019, 790, 305–315. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, P.; Gou, L.; Hou, Z.; Huang, B. Enhancement on the thermostability and wettability of lithium-ion batteries separator via surface chemical modification. Mater. Lett. 2017, 208, 98–101. [Google Scholar] [CrossRef]
- Ma, L.; Chen, R.; Hu, Y.; Zhang, W.; Zhu, G.; Zhao, P.; Chen, T.; Wang, C.; Yan, W.; Wang, Y.; et al. Nanoporous and lyophilic battery separator from regenerated eggshell membrane with effective suppression of dendritic lithium growth. Energy Storage Mater. 2018, 14, 258–266. [Google Scholar] [CrossRef]
- Lee, H.; Lim, H.S.; Ren, X.; Yu, L.; Engelhard, M.H.; Han, K.S.; Lee, J.; Kim, H.T.; Xiao, J.; Liu, J.; et al. Detrimental effects of chemical crossover from the lithium anode to cathode in rechargeable lithium metal batteries. ACS Energy Lett. 2018, 3, 2921–2930. [Google Scholar] [CrossRef]
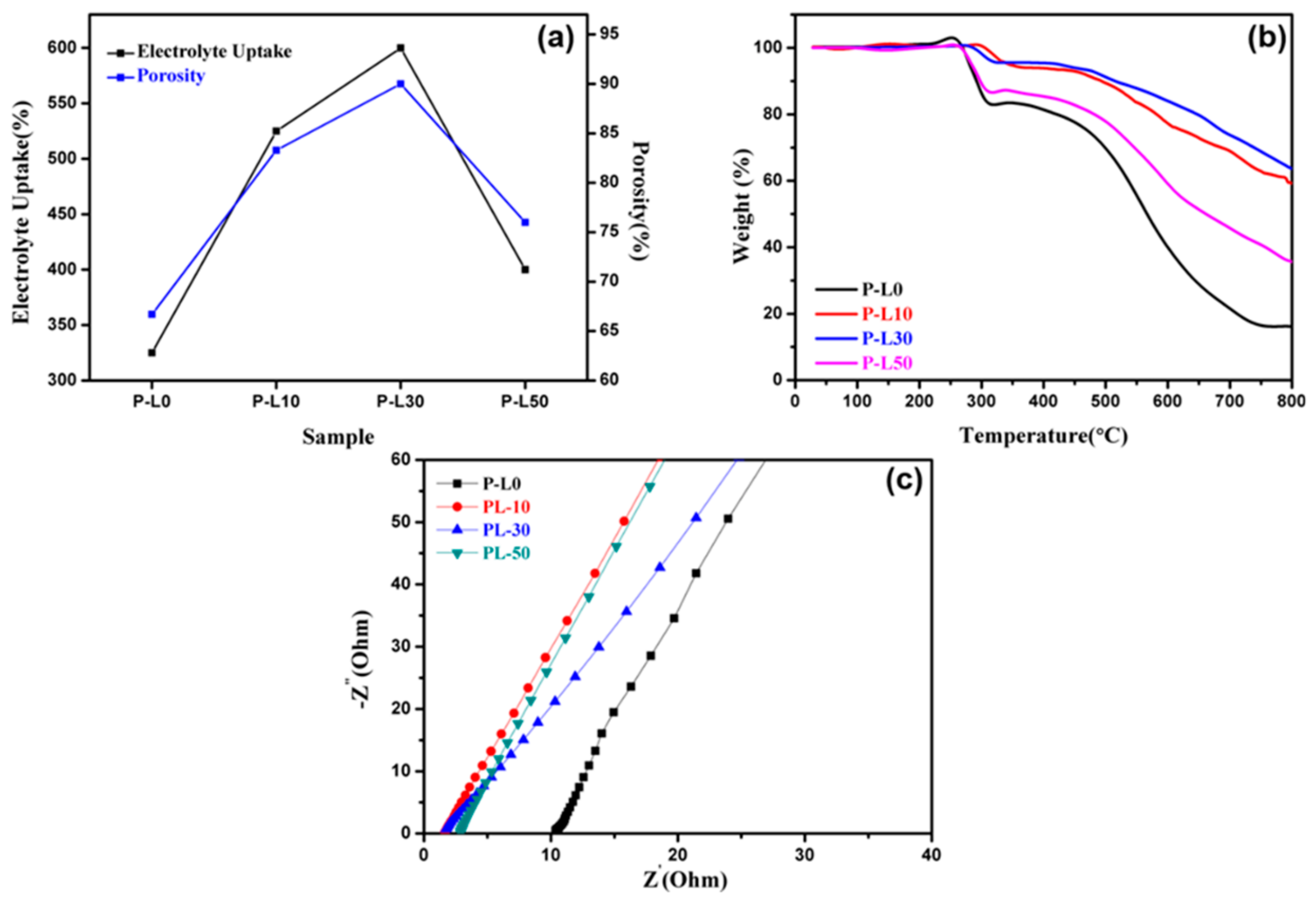
| Sample | Porosity (%) | Electrolyte Uptake (%) | Ionic Conductivity (mS/cm) |
|---|---|---|---|
| P-L0 | 66.7 | 325 | 0.22 |
| P-L10 | 83.3 | 525 | 1.4 |
| P-L30 | 90 | 600 | 1.7 |
| P-L50 | 76 | 400 | 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanta, J.; Kwon, O.H.; Choi, J.H.; Yun, Y.-M.; Kim, J.-K.; Jeong, S.M. Preparation of Highly Porous PAN-LATP Membranes as Separators for Lithium Ion Batteries. Nanomaterials 2019, 9, 1581. https://doi.org/10.3390/nano9111581
Mohanta J, Kwon OH, Choi JH, Yun Y-M, Kim J-K, Jeong SM. Preparation of Highly Porous PAN-LATP Membranes as Separators for Lithium Ion Batteries. Nanomaterials. 2019; 9(11):1581. https://doi.org/10.3390/nano9111581
Chicago/Turabian StyleMohanta, Jagdeep, O Hyeon Kwon, Jong Hyeok Choi, Yeo-Myeong Yun, Jae-Kwang Kim, and Sang Mun Jeong. 2019. "Preparation of Highly Porous PAN-LATP Membranes as Separators for Lithium Ion Batteries" Nanomaterials 9, no. 11: 1581. https://doi.org/10.3390/nano9111581
APA StyleMohanta, J., Kwon, O. H., Choi, J. H., Yun, Y.-M., Kim, J.-K., & Jeong, S. M. (2019). Preparation of Highly Porous PAN-LATP Membranes as Separators for Lithium Ion Batteries. Nanomaterials, 9(11), 1581. https://doi.org/10.3390/nano9111581