In Vitro and In Vivo Antioxidant Activity of the New Magnetic-Cerium Oxide Nanoconjugates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation Techniques
2.3. Synthesis of Nanoparticles
2.3.1. Synthesis of Core-Shell Cerium Oxide Nanoparticles (CeNP) Covered with PEI Crosslinked (CePEI-GA)
- (a)
- Synthesis of CeNP coated with PEI (CePEI) was performed following a previously reported method with some modifications [23]. Briefly, 10 mL (1M) solution of polyethylenimine (PEI) was added under magnetic stirring to a 30 mL (25%) solution of NH4OH. Then, 5 mL (1M) solution of Ce(NO3)3∙6H2O was added drop wise, under magnetic stirring, to the previous solution. The reaction took place for 24 h at room temperature. The resulting yellow precipitate was separated by centrifugation and washed several times with water in order to remove the NH4OH and unbounded PEI chains. These CeNPs covered with PEI are stabilised by physical forces.
- (b)
- Synthesis of core-shell CeNP coated with PEI crosslinked by GA (CePEI-GA). In order to stabilise the PEI shell onto the CePEI, PEI was crosslinked with GA. Briefly, 24 mL (24%) GA solution was added drop wise, under magnetic stirring, to 36 mL (10 mg/mL) of an aqueous solution of previously synthetised CePEI nanoparticles. Reaction took place in 3 h, at room temperature, and the excess of GA was removed by washing the product several times with water. To insure that GA is totally reacted and all of the aldehyde groups are inactivated, 20 mL solution of PEI (10% in water) was added and reacted overnight. The final product was washed with water several times in order to remove the unreacted PEI, and then it was stored as a solution at 4 °C.
2.3.2. Synthesis of MNP Covered with PEI (MPEI)
- (a)
- Synthesis of bare magnetic nanoparticles (MNPs) was performed following the classical method of co-precipitation without modifications [30]. Briefly, 20 mL (25%) ammonium solution was added to a mixture of ferric and ferrous salts aqueous solutions, under nitrogen flow and mechanical stirring, having the Fe2+/Fe3+ = 1/2 molar ratio. The mixture became black immediately, and was stirred for another 30 min at 70 °C under nitrogen flow. The obtained MNPs were washed several times with water and ethanol. The bare MNPs were stored in ethanol at 5 °C in the refrigerator prior to use.
- (b)
- Preparation of core-shell MNP coated with PEI (MPEI) was done by a previously reported method, without any modification [6]. In brief, 10 mL of PEI (10%, 1.8 kDa) solution was added to 8 mL suspension of fresh MNP (50 mg/mL) in deionised water, sonicated for 5 min and stirred for 24 h. Excess of PEI was removed by washing the product five times with water using magnetic decantation. The MPEI conjugates can be stored for a long period of time in deionised water before use in subsequent reaction steps.
2.3.3. Synthesis of Hybrid Nanoparticles (MCePEI-GA)
- (a)
- Crosslinking of the PEI layer of MPEI nanoparticles and activation with aldehyde–aldehyde groups. PEI shell of MPEI was reticulated with GA by the same protocol presented above for CePEI-GA, except that, after the GA elimination from the supernatant, aldehyde–aldehyde groups from the PEI shell of MPEI reacted with GA were not deactivated by a subsequent reaction with PEI. At this stage, MNP remains covered with PEI crosslinked with GA and activated by inherently remaining free aldehyde groups. This product cannot be stored for a long time, and should be used quickly in subsequent reactions.
- (b)
- Synthesis of magnetite-nanoceria nanoparticles. 10 mL liquid dispersion of freshly activated MPEI with GA (100 mg) was added to 10 mL CePEI-GA of 100 mg, prior sonicated (1 min) and allowed to react under stirring at room temperature for 24 h. Afterwards, in order to deactivate the remaining aldehyde groups, the reaction was stopped by adding 20 mL of 10% PEI (1.8 kDa), and allowed to react for another 24 h in the same conditions. After these procedures, only the fraction that separated magnetically was collected. NPs which resulted from magnetic separation were washed with water five times in order to remove the unreacted PEI and were then stored as dispersions in water at 4 °C.
2.4. In Vitro Studies
2.4.1. Ex-Vivo Radical Scavenging Activity
2.4.2. Cytotoxicity Assay
2.5. In Vivo Studies
2.5.1. Animals
2.5.2. Experimental Design
2.5.3. In Vivo Antioxidant Tests
2.5.4. Data Analysis
3. Results and Discussion
3.1. Synthesis of Nanoconjugates with Antioxidant Properties
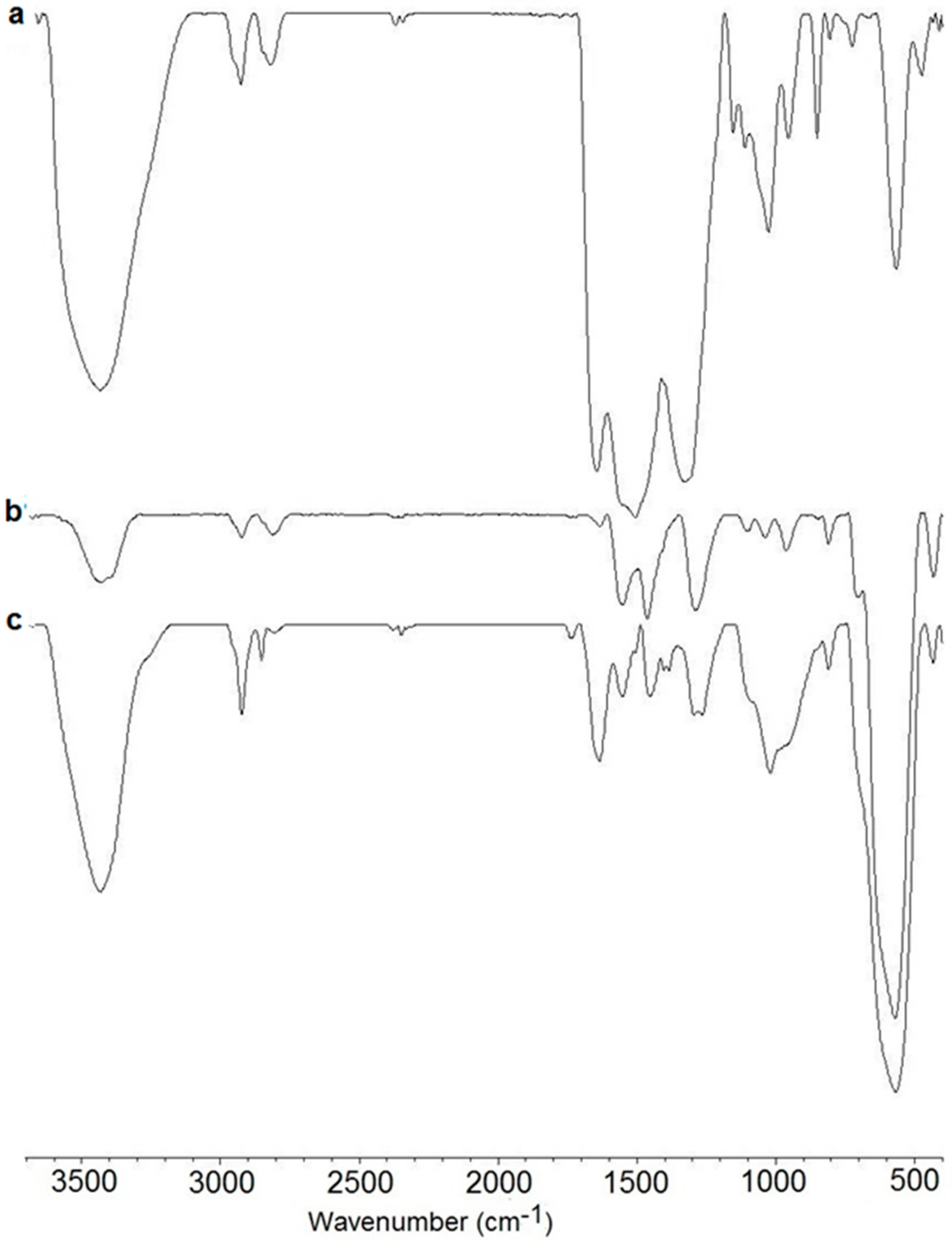
3.2. FT-IR Study of MPEI, CePEI-GA and MCePEI-GA Nanoparticles
3.3. Raman Study of MCePEI-GA
3.4. EDX Characterisation
3.5. XPS Measurements
3.6. Size and Morphology Studies
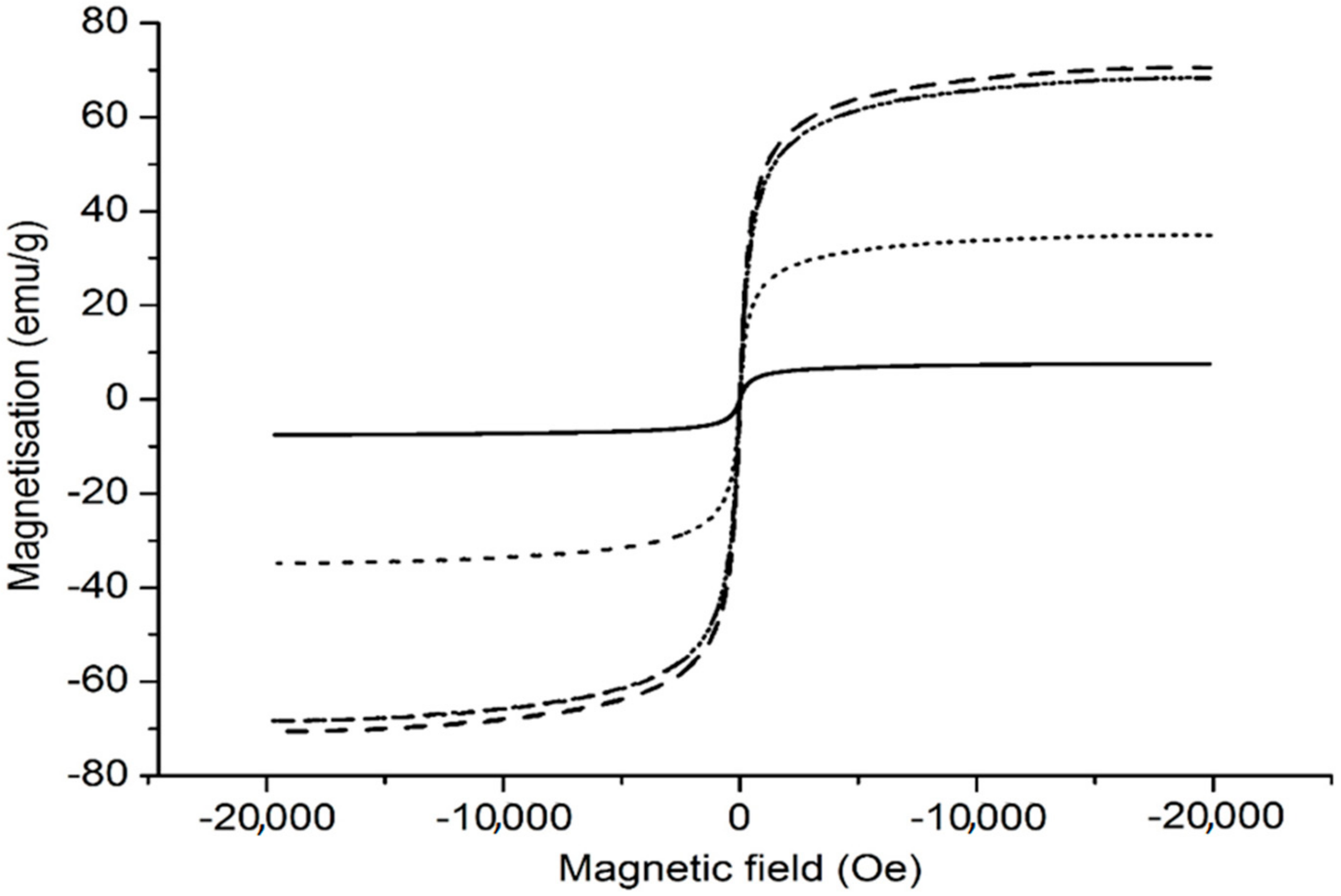
3.7. Magnetic Properties
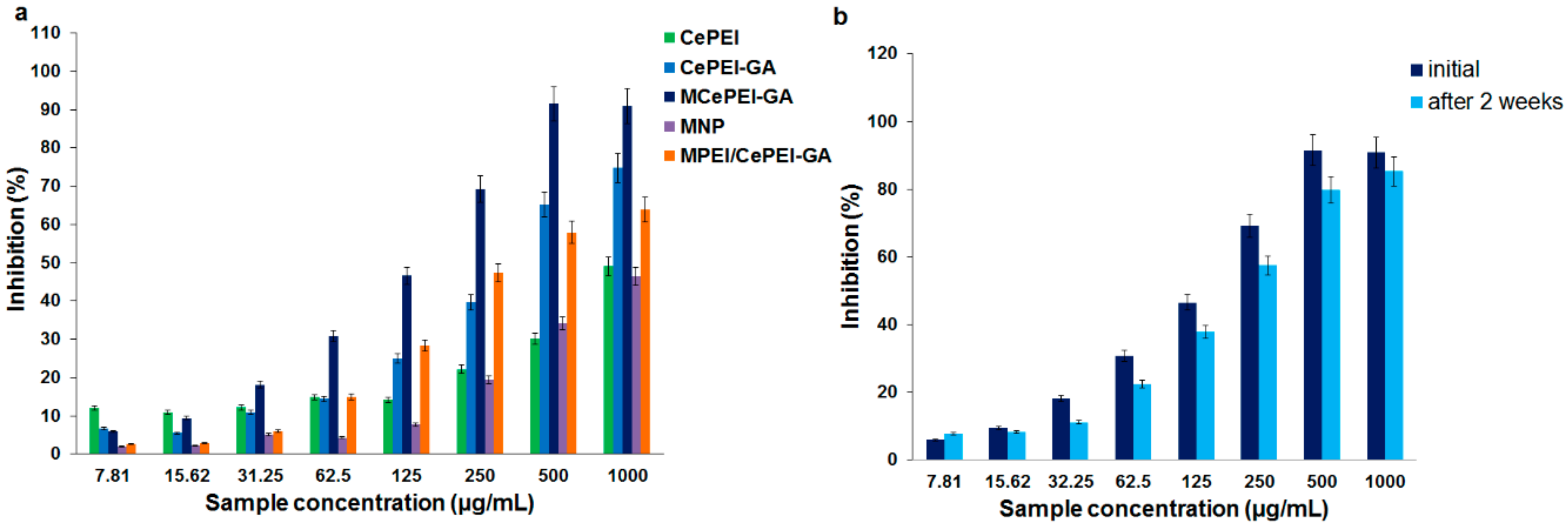
3.8. In Vitro Radical Scavenging Activity
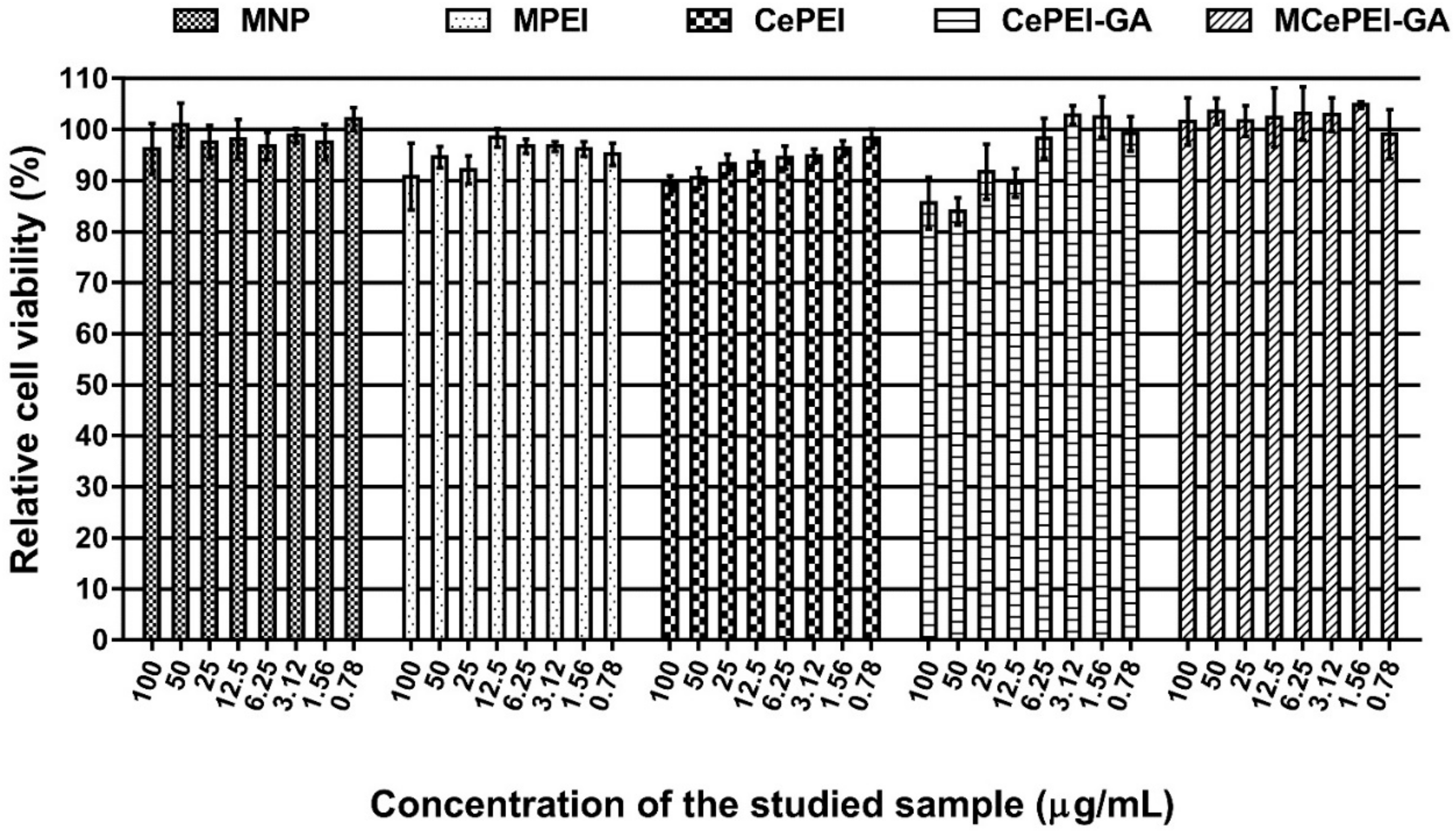
3.9. In Vitro Cytotoxicity Studies
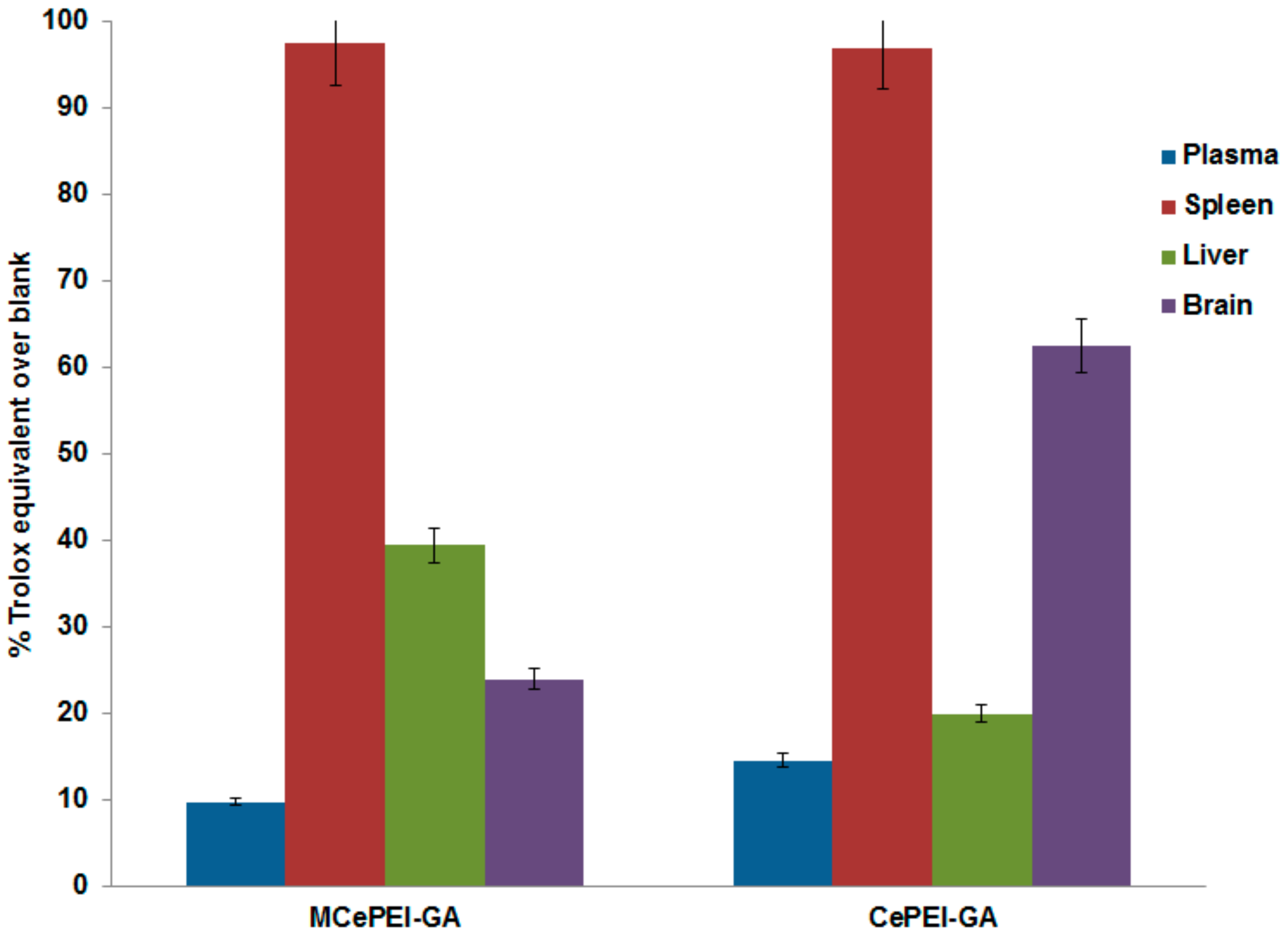
3.10. In Vivo Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Ethics Committee Approval and Patient Consent
Abbreviations
| MNP | magnetic nanoparticles |
| CeNP | cerium oxide nanoparticles |
| PEI | Polyethyleneimine |
| GA | Glutaraldehyde |
| MPEI | magnetic nanoparticles coated with polyethyleneimine |
| CePEI | cerium oxide nanoparticles coated with polyethyleneimine |
| CePEI-GA | cerium oxide nanoparticles coated with polyethyleneimine and reticulated with glutaraldehyde |
| MCePEI-GA | hybrid iron oxide—cerium oxide nanoparticles coated with polyethyleneimine and reticulated with glutaraldehyde |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS, ABTS+• | 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid and corresponding radical cation |
| NHDF | normal human dermal fibroblasts |
References
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Bhattarai, S.R. Theranostic nanoparticles: A recent breakthrough in nanotechnology. J. Nanomed. Nanotechol. 2012, 3, e114. [Google Scholar] [CrossRef]
- Tancredi, P.; da Costa, L.S.; Calderón, S.; Moscoso-Londoño, O.; Socolovsky, L.M.; Ferreira, P.; Muraca, D.; Zanchet, D.; Knobel, M. Exploring the synthesis conditions to control the morphology of gold-iron oxide heterostructures. Nano Res. 2019, 12, 1781–1788. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Serpooshan, V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano 2012, 6, 2656–2664. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile method to synthesize magnetic iron oxides/TiO2 hybrid nanoparticles and their photodegradation application of methylene blue. Nanoscale Res. Lett. 2011, 6, 533. [Google Scholar] [CrossRef]
- Lungoci, A.L.; Turin-Moleavin, I.-A.; Corciova, A.; Mircea, C.; Arvinte, A.; Fifere, A.; Marangoci, N.L.; Pinteala, M. Multifunctional magnetic cargo-complexes with radical scavenging properties. Mater. Sci. Eng. C 2019, 94, 608–618. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, D.; Song, C.G.; Kang, P.M. Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 2015, 10, 2709–2723. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Overview of reactive oxygen species. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; Royal Society of Chemistry: Cambridge, UK, 2016; pp. 1–21. [Google Scholar]
- Fu, S.; Wang, S.; Zhang, X.; Qi, A.; Liu, Z.; Yu, X.; Chen, C.; Li, L. Structural effect of Fe3O4 nanoparticles on peroxidase-like activity for cancer therapy. Colloids Surf. B Biointerfaces 2017, 154, 239–245. [Google Scholar] [CrossRef]
- Durdureanu-Angheluta, A.; Ardeleanu, R.; Pinteala, M.; Harabagiu, V.; Chiriac, H.; Simionescu, B.C. Silane covered magnetite particles, preparation and characterization. Dig. J. Nanomater. Bios. 2008, 3, 33–40. [Google Scholar]
- Durdureanu-Angheluta, A.; Ignat, M.-E.; Maier, S.S.; Pricop, L.; Coroaba, A.; Fifere, A.; Pinteala, M.; Chiriac, A. Lipolytic biocatalyst based on recyclable magnetite-polysiloxane nanoparticles. Appl. Surf. Sci. 2014, 292, 898–905. [Google Scholar] [CrossRef]
- Estelrich, J.; Escribano, E.; Queralt, J.; Busquets, M.A. Iron oxide nanoparticles for magnetically-guided and magnetically-responsive drug delivery. Int. J. Mol. Sci. 2015, 16, 8070. [Google Scholar] [CrossRef] [PubMed]
- Durdureanu-Angheluta, A.; Uritu, C.M.; Coroaba, A.; Minea, B.; Doroftei, F.; Calin, M.; Maier, S.S.; Pinteala, M.; Simionescu, M.; Simionescu, B.C. Heparin-anthranoid conjugates associated with nanomagnetite particles and their cytotoxic effect on cancer cells. J. Biomed. Nanotechnol. 2014, 10, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron oxide nanoparticles—Current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.S.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, size- and structure-controlled synthesis and biocompatibility of iron oxide nanoparticles for magnetic theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Ratschker, T.; Nguyen, K.; Zschiesche, L.; Tietze, R.; Lyer, S.; Alexiou, C. Functionalized superparamagnetic iron oxide nanoparticles (SPIONs) as platform for the targeted multimodal tumor therapy. Front. Oncol. 2019, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Magnetic nanoparticles for MR imaging: Agents, techniques and cardiovascular applications. Basic Res. Cardiol. 2008, 103, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Caizer, C. Computational study on superparamagnetic hyperthermia with biocompatible SPIONs to destroy the cancer cells. J. Phys. 2014, 521, 012015. [Google Scholar] [CrossRef]
- Godage, O.; Bucharskaya, A.; Navolokin, N.; Maslyakova, G.; German, S.; Gorin, D. The magnetite nanoparticles in theranostic applications. J. Nanomed. Res. 2017, 5, 1–10. [Google Scholar]
- Corciova, A.; Ciobanu, C.; Poiata, A.; Mircea, C.; Nicolescu, A.; Dobrota, M.; Varganici, C.-D.; Pinteala, T.; Marangoci, N. Antibacterial and antioxidant properties of hesperidin: β-cyclodextrin complexes obtained by different techniques. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 71–84. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, B.; Ferreira, I. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef]
- Balasaheb Nimse, S.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Perez, J.M.; Asati, A.; Nath, S.; Kaittanis, C. Synthesis of Biocompatible Dextran-Coated Nanoceria with pH-Dependent Antioxidant Properties. Small 2008, 4, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef] [PubMed]
- Dowding, J.M.; Seal, S.; Self, W.T. Cerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO−). Drug Deliv. Transl. Res. 2013, 3, 375–379. [Google Scholar] [CrossRef]
- Charbgoo, F.; Ahmad, M.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomed. 2017, 12, 1401–1413. [Google Scholar] [CrossRef]
- Nguyen, A.; Abdelrasoul, G.; Lin, D.; Maadi, H.; Tong, J.; Chen, G.; Wang, R.; Anwar, A.; Shoute, L.; Fang, Q.; et al. Polyethylenimine-coated iron oxide magnetic nanoparticles for high efficient gene delivery. Appl. Nanosci. 2018, 8, 811–821. [Google Scholar] [CrossRef]
- Dascalu, A.I.; Ardeleanu, R.; Neamtu, A.; Maier, S.S.; Uritu, C.M.; Nicolescu, A.; Silion, M.; Peptanariu, D.; Calin, M.; Pinteala, M. Transfection-capable polycationic nanovectors which include PEGylated-cyclodextrin structural units: A new synthesis pathway. J. Mater. Chem. B 2017, 5, 7164–7174. [Google Scholar] [CrossRef]
- Ardeleanu, R.; Dascalu, A.; Neamtu, A.; Peptanariu, D.; Uritu, C.M.; Maier, S.S.; Nicolescu, A.; Simionescu, B.C.; Barboiu, M.; Pinteala, M. Multivalent polyrotaxane vectors as adaptive cargo complexes for gene therapy. Polym. Chem. 2018, 9, 845–859. [Google Scholar] [CrossRef]
- Durdureanu-Angheluta, A.; Pricop, L.; Stoica, I.; Peptu, C.-A.; Dascalu, A.; Marangoci, N.; Doroftei, F.; Chiriac, H.; Pinteala, M.; Simionescu, B.C. Synthesis and characterization of magnetite particles covered with α-trietoxysilil-polydimethylsiloxane. J. Magn. Magn. Mater. 2010, 322, 2956–2968. [Google Scholar] [CrossRef]
- Soren, S.; Jena, S.R.; Samanta, L.; Parhi, P. Antioxidant potential and toxicity study of the cerium oxide nanoparticles synthesized by microwave-mediated synthesis. Appl. Biochem. Biotechnol. 2015, 177, 148–161. [Google Scholar] [CrossRef]
- Durdureanu-Angheluta, A.; Dascalu, A.; Fifere, A.; Coroaba, A.; Pricop, L.; Chiariac, H.; Tura, B.; Pinteala, M.; Simionescu, B.C. Progress in the synthesis and characterization of magnetite nanoparticles with amino groups on the surface. J. Magn. Magn. Mater. 2012, 324, 1679–1689. [Google Scholar] [CrossRef]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Jamal, A. Exploration of CeO2 nanoparticles as a chemi-sensor and photo-catalyst for environmental applications. Sci. Total Environ. 2011, 409, 2987–2992. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, P.; Nie, T.; Wei, H.; Cui, Z. Characterization of a polyamine microsphere and its adsorption for protein. Int. J. Mol. Sci. 2013, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, C.; Vandenberghe, R.E.; Markova-Deneva, I.; Nedkov, I. Raman spectroscopy investigation of magnetite nanoparticles in ferrofluids. J. Magn. Magn. Mater. 2010, 322, 1904–1911. [Google Scholar] [CrossRef]
- Grumezescu, A.M.; Grumezescu, A.M.; Andronescu, E.; Holban, A.M.; Ficai, A.; Ficai, D.; Voicu, G.; Grumezescu, V.; Balaure, P.C.; Chifiriuc, C.M. Water dispersible cross-linked magnetic chitosan beads for increasing the antimicrobial efficiency of aminoglycoside antibiotics. Int. J. Pharm. 2013, 454, 233–240. [Google Scholar] [CrossRef]
- Lu, W.; Lu, W.; Ling, M.; Jia, M.; Huang, P.; Li, C.; Yan, B. Facile synthesis and characterization of polyethylenimine-coated Fe3O4 superparamagnetic nanoparticles for cancer cell separation. Mol. Med. Rep. 2014, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Coey, J.M.D. Noncollinear spin arrangement in ultrafine ferrimagnetic crystallites. Phys. Rev. Lett. 1971, 27, 1140–1142. [Google Scholar] [CrossRef]
- Mørup, S. Mössbauer spectroscopy studies of suspensions of Fe3O4 microcrystals. J. Magn. Magn. Mater. 1983, 39, 45–47. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. High impact of in situ dextran coating on biocompatibility, stability and magnetic properties of iron oxide nanoparticles. Mater. Sci. Eng. C 2017, 75, 947–956. [Google Scholar] [CrossRef]
- Zhang, W.; Deng, L.; Wang, G.; Guo, X.; Li, Q.; Zhang, J.; Khashab, N. Low-magnetization magnetic microcapsules: A synergistic theranostic platform for remote cancer cells therapy and imaging. Part. Part. Syst. Charact. 2014, 31, 985–993. [Google Scholar] [CrossRef]
- Baldim, V.; Bedioui, F.; Mignet, N.; Margaill, I.; Berret, J.-F. The enzyme-like catalytic activity of cerium oxide nanoparticles and its dependency on Ce3+ surface area concentration. Nanoscale 2018, 10, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.G.; Jeong, H.G.; Kang, D.W.; Nam, M.J.; Kim, C.K.; Kim, D.Y.; Choi, I.-Y.; Ki, S.K.; Kim, S.; Han, J.H.; et al. Customized lipid-coated magnetic mesoporous silica nanoparticle doped with ceria nanoparticles for theragnosis of intracerebral hemorrhage. Nano Res. 2018, 11, 3582–3592. [Google Scholar] [CrossRef]
- Orozco, L.M.; Renz, M.; Corma, A. Cerium oxide as a catalyst for the ketonization of aldehydes: Mechanistic insights and a convenient way to alkanes without the consumption of external hydrogen. Green Chem. 2017, 19, 1555–1569. [Google Scholar] [CrossRef]
- Kotora, P.; Kotora, P.; Šeršeň, F.; Filo, J.; Loos, D.; Gregáň, J.; Gregáň, F. The scavenging of DPPH, galvinoxyl and ABTS radicals by imine analogs of resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef] [PubMed]
- Sundgea, S.; Vibhuteb, Y. Solvent-free, environmentally benign synthesis of some imines and antioxidant activity. J. Chem. Pharm. Res. 2016, 8, 338–346. [Google Scholar]
- Pulido-Reyes, G.; Rodea-Palomares, I.; Das, S.; Sakthivel, T.S.; Leganes, F.; Rosal, R.; Seal, S.; Fernández-Piñas, F. Untangling the biological effects of cerium oxide nanoparticles: The role of surface valence states. Sci. Rep. 2015, 5, 15613. [Google Scholar] [CrossRef]
- Hirst, S.M.; Karakoti, A.; Singh, S.; Self, W.; Tyler, R.; Seal, S.; Reilly, C.M. Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ. Toxicol. 2013, 28, 107–118. [Google Scholar] [CrossRef]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Straatman, M.; Monti, D.; Stahl, W.; Sies, H.; et al. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087. [Google Scholar] [CrossRef]


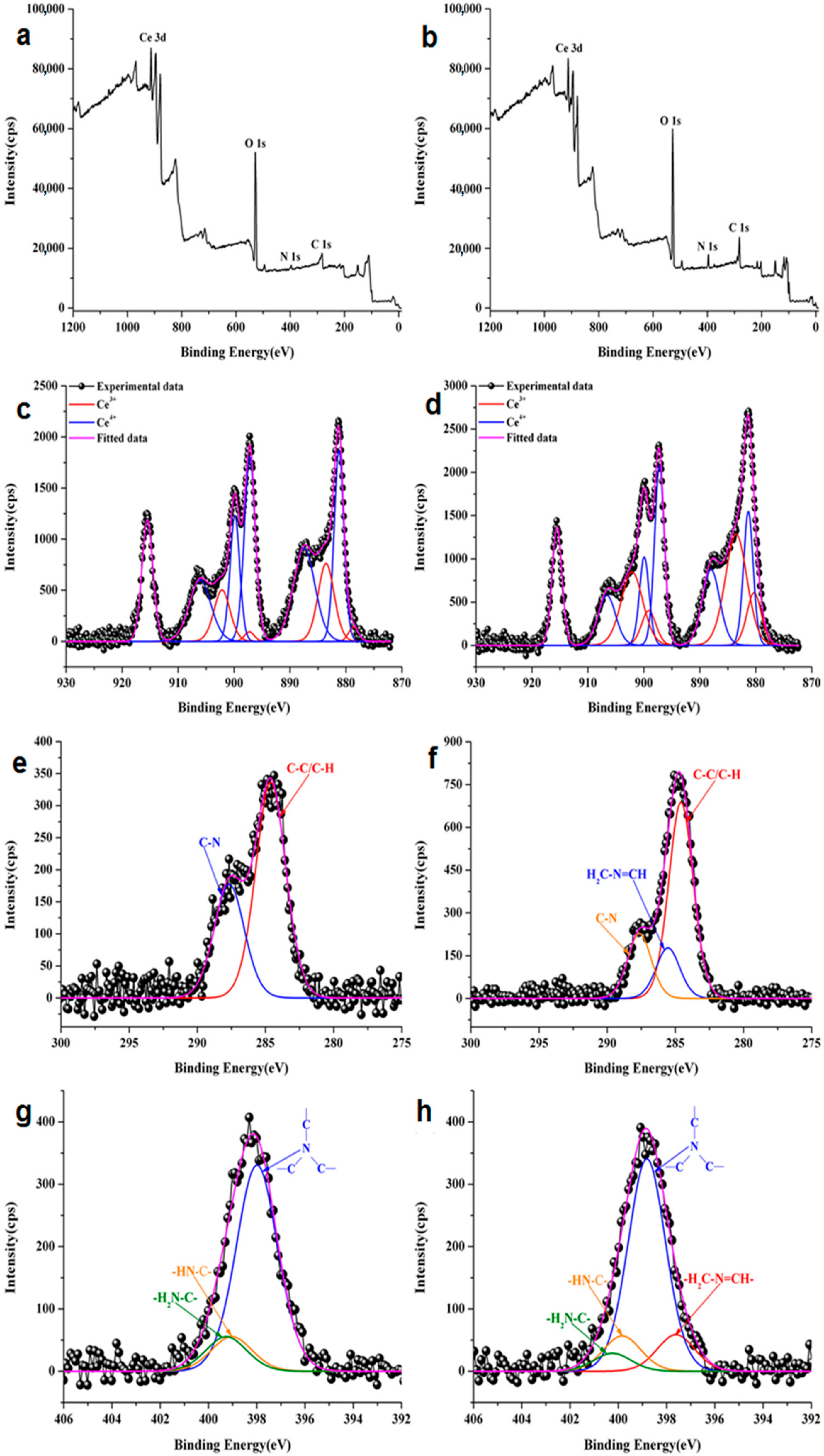

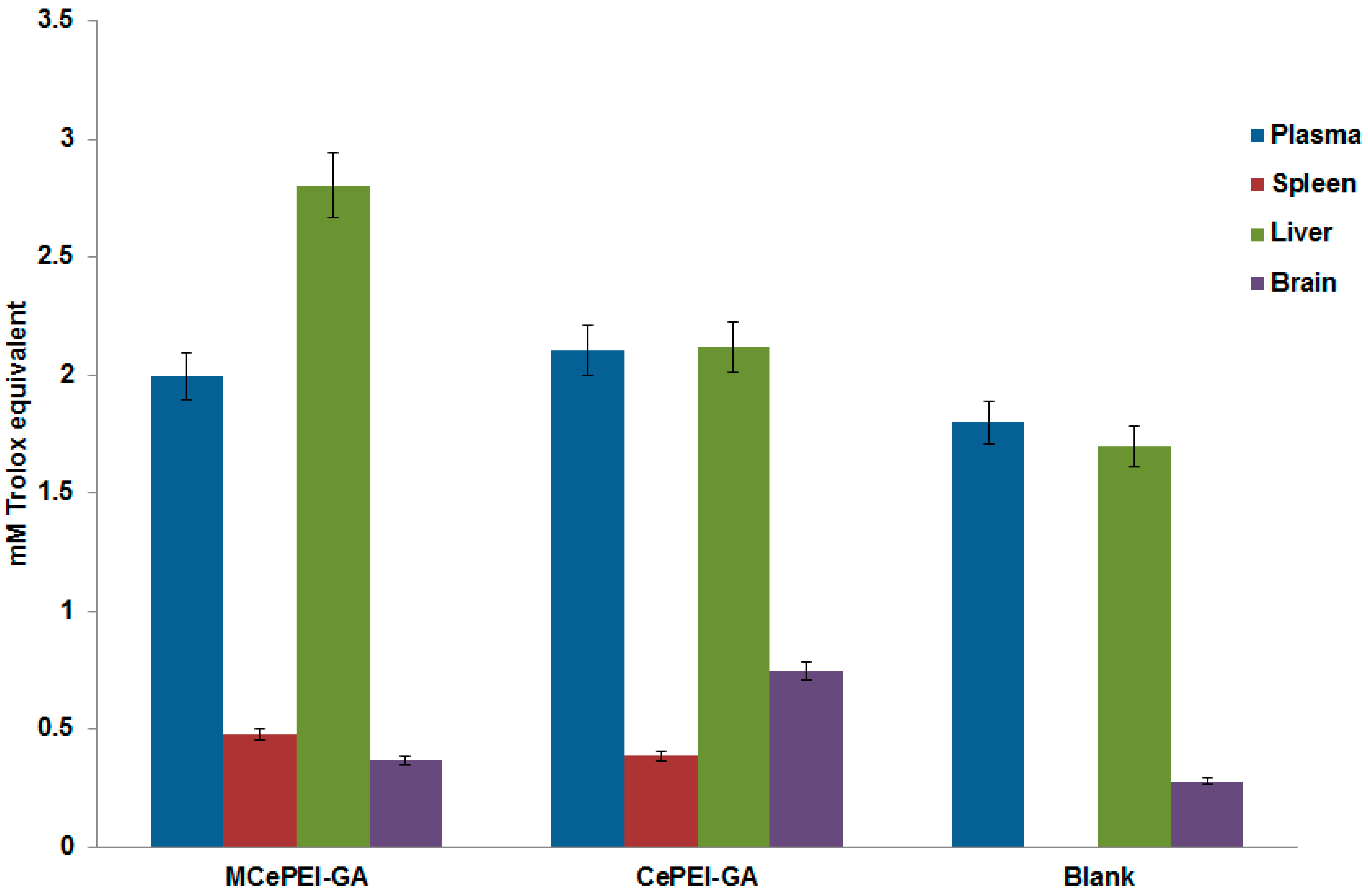
| Assignment | Binding Energy (eV) | CePEI Relative Concentration (%) | Total | Binding Energy (eV) | CePEI-GA Relative Concentration (%) | Total |
|---|---|---|---|---|---|---|
| Ce3+ | 878.7 | 1.9 | 19.1% | 880.3 | 10.0 | 43.9% |
| 897.3 | 898.9 | |||||
| 883.5 | 17.2 | 883.5 | 33.9 | |||
| 902.1 | 902.1 | |||||
| Ce4+ | 881.2 | 25.1 | 80.9% | 881.3 | 15.1 | 56.1% |
| 899.8 | 899.9 | |||||
| 887.5 | 27.8 | 888.0 | 17.6 | |||
| 906.1 | 906.6 | |||||
| 897.2 | 28.0 | 897.2 | 23.4 | |||
| 915.8 | 915.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turin-Moleavin, I.-A.; Fifere, A.; Lungoci, A.-L.; Rosca, I.; Coroaba, A.; Peptanariu, D.; Nastasa, V.; Pasca, S.-A.; Bostanaru, A.-C.; Mares, M.; et al. In Vitro and In Vivo Antioxidant Activity of the New Magnetic-Cerium Oxide Nanoconjugates. Nanomaterials 2019, 9, 1565. https://doi.org/10.3390/nano9111565
Turin-Moleavin I-A, Fifere A, Lungoci A-L, Rosca I, Coroaba A, Peptanariu D, Nastasa V, Pasca S-A, Bostanaru A-C, Mares M, et al. In Vitro and In Vivo Antioxidant Activity of the New Magnetic-Cerium Oxide Nanoconjugates. Nanomaterials. 2019; 9(11):1565. https://doi.org/10.3390/nano9111565
Chicago/Turabian StyleTurin-Moleavin, Ioana-Andreea, Adrian Fifere, Ana-Lacramioara Lungoci, Irina Rosca, Adina Coroaba, Dragos Peptanariu, Valentin Nastasa, Sorin-Aurelian Pasca, Andra-Cristina Bostanaru, Mihai Mares, and et al. 2019. "In Vitro and In Vivo Antioxidant Activity of the New Magnetic-Cerium Oxide Nanoconjugates" Nanomaterials 9, no. 11: 1565. https://doi.org/10.3390/nano9111565
APA StyleTurin-Moleavin, I.-A., Fifere, A., Lungoci, A.-L., Rosca, I., Coroaba, A., Peptanariu, D., Nastasa, V., Pasca, S.-A., Bostanaru, A.-C., Mares, M., & Pinteala, M. (2019). In Vitro and In Vivo Antioxidant Activity of the New Magnetic-Cerium Oxide Nanoconjugates. Nanomaterials, 9(11), 1565. https://doi.org/10.3390/nano9111565








