1. Introduction
In biotechnological processes, the preparation of immobilized enzymes is a key step in optimizing the operational performance of an enzyme in aqueous or non-aqueous media. The most frequently used enzyme immobilization techniques are noncovalent adsorption or deposition [
1,
2], covalent attachment [
3,
4], entrapment in a polymeric device [
5,
6], and enzyme cross-linking [
7,
8]. To some extent, the recovery and recycling of an immobilized enzyme can lower expenses, however, immobilization processes can suffer from low enzyme loading, weak binding ability and difficulties in keeping the enzyme fixed to the carrier, and limited enzyme recovery. New techniques are needed to avoid such issues. In one particularly promising strategy, ferritin nanoparticles based on magnetite (Fe
3O
4) and maghemite (γ-Fe
2O
3), referred to as magnetoferritin, are used as the supporting material for enzyme immobilization. This material protects the enzyme and allows for simple recovery through the application of a magnetic field.
Ferritin is an iron storage protein with a spherical structure that plays an important role in regulating cellular iron content. Channels created by the symmetrical self-assembly of ferritin monomers into cage-like structures can guide Fe(II) ions toward catalytic centers [
9]. Twenty-four identical polypeptide subunits assemble into a ferritin cage with an outer diameter of 12 nm and an inner diameter of 8 nm. This protein shell is capable of storing up to 4500 Fe(III) atoms [
10,
11]. Furthermore, apoferritin, which is void of iron ions in the cavity, can be used to synthesize Fe oxide magnetic nanoparticles, referred to as magnetoferritin [
12]. Magnetoferritin was chosen as a possible paramagnetic nanoparticle due to its homogeneous dispersion in aqueous solution, sub-10 nm feature size, and response to magnetic filtration. Magnetoferritin is also relatively biocompatible and flexible, and has been used in a variety of biomedical applications [
13], magnetic resonance imaging [
14], and for radioactive ion separation in water treatment [
15]. In addition, magnetoferritin nanoparticles can be arranged into well-ordered periodic arrays free of unwanted aggregation [
16]. The immobilization of enzymes onto nanosized magnetic ferritin particles would allow for better separation from a reaction mixture with the use of an external magnetic field, thereby, simplifying reuse and recycling.
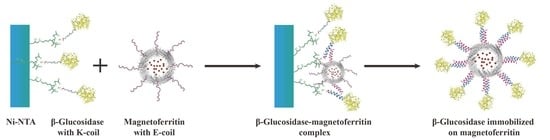
A novel immobilization method, employing a noncovalent anchoring moiety to link the enzyme to the exterior surface of magnetic ferritin, was demonstrated herein and is shown in
Figure 1. The coiled coil is a protein structural motif consisting of two or more α-helices that are wrapped around each other in a superhelical fashion. The primary structure of coiled coil-forming proteins is characterized by a distinct seven-residue repeat, denoted as (abcdefg)
n where n is the number of repeats. Positions “a” and “d” are occupied by hydrophobic residues that form the hydrophobic core of coiled coils, whereas positions “e” and “g” contain complementary charged side-chain functional groups that interact to form stabilizing salt bridges. The motivation of burying hydrophobic faces provides the primary driving force for self-assembly. Charged residues beside the hydrophobic faces can provide additional stability by forming intra- or interhelical salt bridges, and can help match the orientation of interacting peptide chains [
17]. Electrostatic interactions can play an important role in the heterodimer of a coiled-coil structure [
18,
19], however, for our purposes, we employed a heterodimeric coiled coil to avoid the dimerization of ferritins or enzymes. To eliminate the need to chemically link two proteins, a heterodimeric coiled-coil protein, consisting of E- and K-coils and having a small dissociation constant (K
d = 7 × 10
−8 M), was used as a noncovalent anchor [
20]. In this study, heterodimer formation was achieved by the placement of charged residues at the “e” and “g” positions of the heptad repeat; the E-coil contains glutamic acid residues, and the K-coil contains lysine residues, at these respective positions. Since the outer surface of ferritin exhibits a relatively low isoelectric point (pI = 5.3) [
21], the negatively charged E-coil was chosen as the attachment point for ferritin, ensuring that the coiled coil does not stick to the ferritin surface, and thus making it more efficient with respect to binding the K-coil. In this study, the complete amino acid sequences of the two peptides were (EIAALEK)
3 for the E-coil linked to human H chain ferritin and (KIAALKE)
3 for the K-coil linked to
β-glucosidase (
Table 1).
The carboxyl terminus, including the E-helix, is not essential for proper folding of the monomer subunit and assembly of ferritin [
22]. Interestingly, human H chain ferritin nanocages can assemble into two conformations: “flip”, where the E helix of the carboxy terminus points toward the cavity, and “flop”, where the E-helix of the carboxyl terminus points outside [
22]. In general, the flip conformation is more common than the flop conformation in the construction of a native ferritin shell, however, fusion of the C-terminus of human H chain ferritin with a certain peptide or protein will result in a flop conformation, according to the observations of Cesareni et al. [
22]. Whether the conformation is flip or flop depends on whether the volume inside the cage is sufficient to contain the 24 C-terminal peptides [
23]. Thus, it is possible to attach enzymes to the outer surface of the ferritin by exploiting the electrostatic interactions between the E- and K-coils.
The objective of this work was to immobilize enzyme onto the outer surface of the magnetoferritin through the heterodimeric coiled-coils and recycle the enzyme under the magnetic field. Because the E-coil binds to the K-coil in a parallel fashion, plasmids of this E and K fusion systems were designed separately, and an E-coil was introduced into the C-terminus of human H chain ferritin without the sequence of a His-tag (named HE). In addition, a K-coil was introduced to the N-terminus of
β-glucosidase (named KG), which contains an N-terminal His-tag (
Table S1). Magnetoferritin of HE was synthesized and characterized and KG was immobilized onto the surface of the magnetoferritin of HE. The enzymatic properties and kinetics of KG and KG-HE were compared which showed that the immobilization has almost no effect on enzymes. Under the magnetic field, immobilized enzyme showed good retention efficiency after three times usages. The developed immobilization strategy can be applied to many other objectives.
2. Materials and Methods
2.1. Construction of HE and KG in Vector pET-20b
The original sequence of human H chain ferritin (HFtn) was synthesized previously in our lab. In order to construct the genetically engineered HFtn with the C-terminal E-coil sequence was designed, and the E-coil was fused to the C-terminus of HFtn using polymerase chain reaction (PCR).
The pET-20b vector containing the human H chain ferritin sequence was used as the template. To obtain plasmid of HE, primers were designed according to the sequence of target protein. These designed primers (
Table S1) were synthesized and used for PCR. After PCR amplification, the products were purified by agarose gel electrophoresis. The chains containing the target gene were obtained and the cyclization was performed using T4 DNA ligase (TaKaRa, Dalian, China). The resulting plasmids were transformed into
E. coli Top10 cells, and the DNA was extracted and the sequence of the HFtn with C-terminal E-coil was confirmed by DNA sequencing. The plasmid was then transformed into
E. coli BL21 (DE3) cells, which were used for the production of the HFtn with E-coil (HE).
The primers for the construction of the
β-glucosidase with N-terminal K-coil sequence were designed based on the sequences of
β-glucosidase and K-coil (
Table S1). The KG gene was amplified using PCR technique. Agarose gel electrophoresis was used to purify the PCR product. The plasmids were transformed into
E. coli Top10 cells, and the sequence of the
β-glucosidase with N-terminal K-coil (
KG) was confirmed by DNA sequencing. The plasmids were transformed into
E. coli BL21 (DE3) cells, which were used for the production of protein KG.
2.2. Expression of HE and KG
Plasmids harboring the gene of HE or KG were transformed into E. coli BL21 (DE3) (Novagen) cells. The LB medium (5 mL) containing 0.1 g/L of ampicillin was inoculated and incubated overnight at 150 rpm, 37 °C. The culture was then used to inoculate 200 mL of LB medium containing ampicillin (0.1 g/L) and grown at 37 °C. Protein expression was induced when OD600 reached 0.6 to 0.8, followed by addition of IPTG to a final concentration of 0.5 mM and incubated at 30 °C for 6 h.
E. coli cells containing HE or KG were harvested after expression by centrifugation at 5500× g, 4 °C for 5 min. The supernatant was discarded, and the pellet cells were resuspended in 8 mL lysis buffer (50 mM NaH2PO4, 10 mM imidazole and 300 mM NaCl, pH 8.0). The solution was sonicated for 5 s with a 5 s interval, and the process continued for 15 min. The samples were centrifuged at 8500× g for 30 min.
2.3. Purification of HE by Solid Ammonium Sulfate Precipitation
Solid ammonium sulfate (Nanjing Chemical Reagent Co., Ltd, Nanjing, China) was added into the supernatant of HE protein to make a final saturation of 20%. After stirring for 12 h at room temperature, precipitation was achieved and was harvested by centrifugation and the corresponding supernatant was added with solid ammonium sulfate again. The ammonium sulfate concentration was increased stepwise by 10% until the final saturation reached 90%.
2.4. Size Exclusion Chromatography (SEC) Analysis
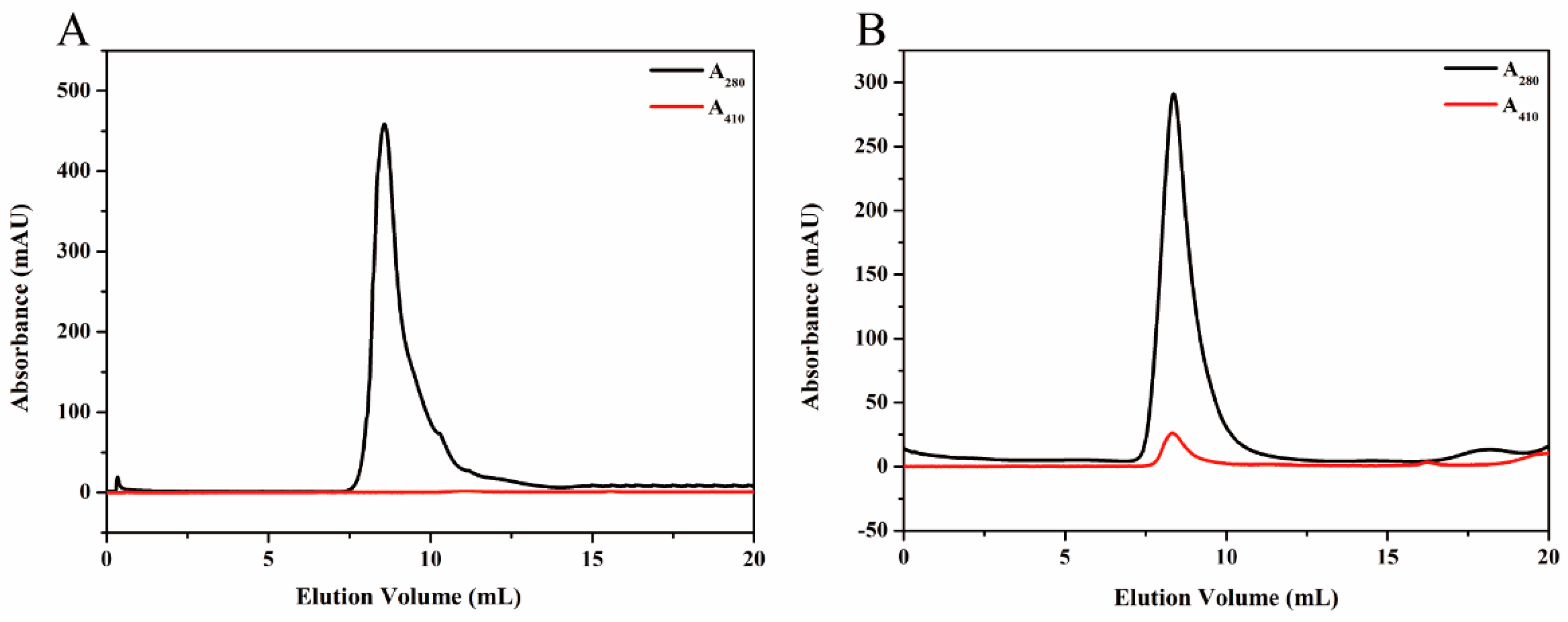
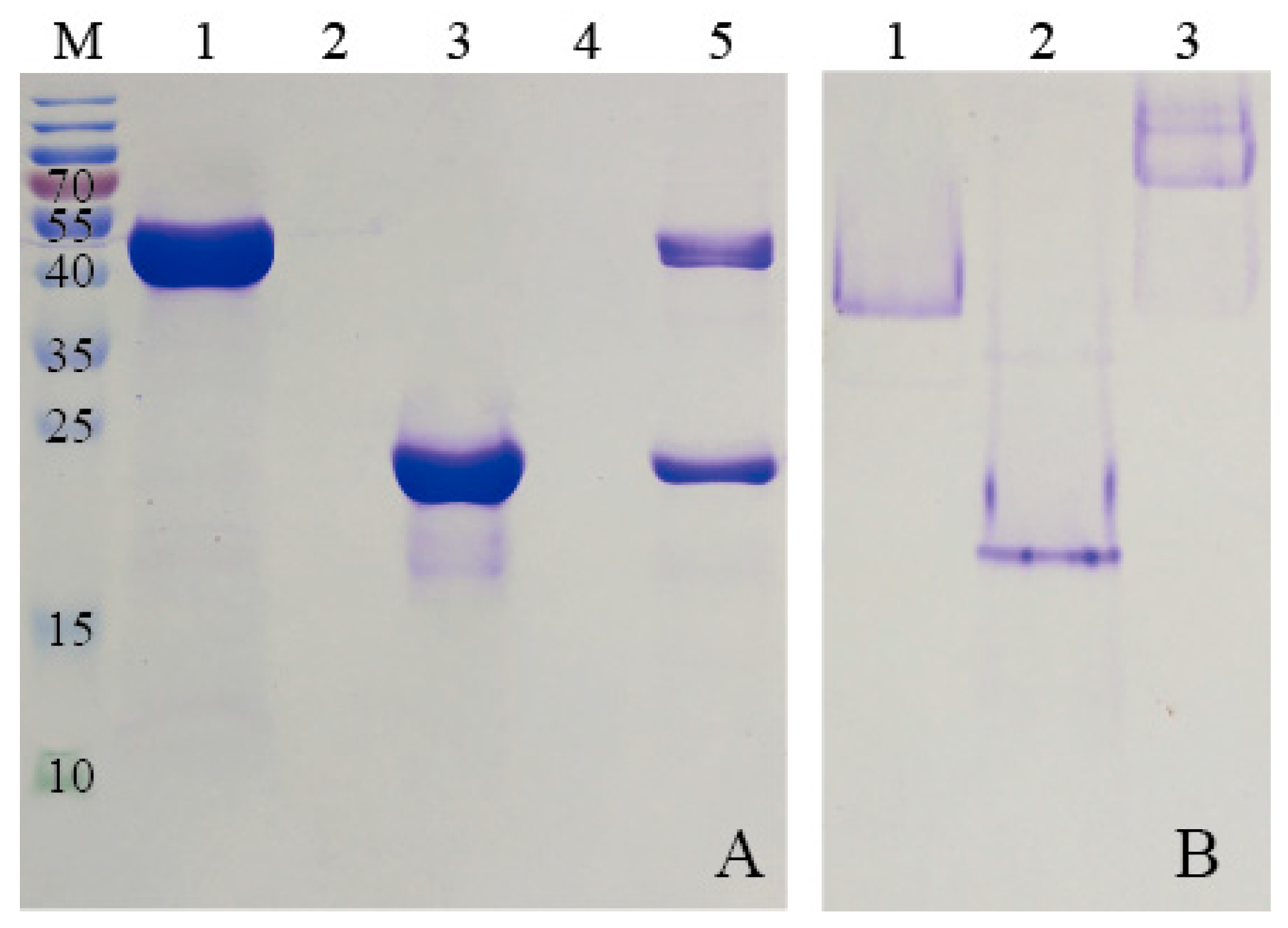
To purify HE protein, the pellets harvested in ammonium sulfate precipitation were re-dissolved in PBS buffer (2 mM KH2PO4, 8 mM Na2HPO4, 136 mM NaCl, 2.6 mM KCl, and pH 7.4), and the solution which contained target protein was dialyzed against GFC buffer three times at 6 h intervals. Subsequently, the solution was centrifuged at 5500× g for 5 min to remove any denatured proteins. The supernatant was concentrated using the Amicon Ultra-4 10K centrifugal filter device (Millipore, Billerica, MA, USA). After centrifugation at 3000× g for 20 min, the target protein was purified by SEC. The samples were stored at 4 °C for later use.
KG was purified by Ni-NTA agarose (QIAGEN, Hilden, Germany) prior to the SEC analysis. Briefly, the supernatant, mentioned previously, was incubated with Ni-NTA agarose beads for 2 h at 4 °C to ensure that KG was bound to the beads. Subsequently, the flow-through was collected and the Ni-NTA column was washed with different concentrations of imidazole buffers. The purified KG was then eluted from the agarose beads by using elution buffer (50 mM NaH2PO4, 250 mM imidazole and 300 mM NaCl, and pH 8.0). The KG solution was dialyzed against GFC buffer for 24 h to remove the excess imidazole and a Amicon Ultra-4 10K centrifugal filter device was used to condense KG for further SEC analysis.
Proteins were further purified by SEC experiments on an ÄKTApurifier (GE Healthcare, Uppsala, Sweden) which was equipped with a Superdex
TM 200 10/300 GL column. The proteins were eluted by GFC buffer (50 mM Na
2HPO
4, 150 mM NaCl, and pH 7.2) with a flow rate of 0.5 mL min
−1 as detected at 280 nm and 410 nm. The column was calibrated using six well-characterized protein standards, as previous described [
9].
2.5. Synthesis and Characterization of Magnetoferritin
Magnetoferritin was synthesized using the method previously described [
24]. Prior to transferring into the anaerobic chamber, all solutions were carefully deoxygenated with nitrogen. Solution of ferritin (1 mg/mL, 10 mL) dissolved in 100 mM Tris-HCl was added to the reaction vessel. The temperature of the vessel was maintained at 65 °C, and the pH value was stabilized at 8.5 that was adjusted by 100 mM NaOH. Freshly prepared H
2O
2 (4.17 mM) and Fe(II) (12.5 mM (NH
4)
2Fe(SO
4)
2∙6H
2O) were added into the vessel with the stoichiometric equivalents (1:3 = H
2O
2: Fe
2+) according to the equation below:
After 50 min, 300 mM sodium citrate was added to chelate additional Fe2+. To remove the extra Fe irons precipitated outside the protein shell and the HE clusters, the synthesized ferrimagnetic HE was further purified to acquire the intact protein cages. Furthermore, the sample was centrifuged for 10 min at 8500× g to remove the aggregates, followed by gel filtration using SuperdexTM 200 10/300 GL as a column.
2.5.1. Sucrose Density Gradient (SDG)
Sucrose density gradient (SDG) ultracentrifugation was performed to purify the synthesized magnetoferritin. A total gradient volume of solution (4 mL) containing magnetoferritin (1.23 mL) and sucrose gradients (20%, 35%, 50%, and 65%) were added to the centrifugation tube accordingly. Centrifugation was carried out at 2000× g for 5 h, at 4 °C. Distribution of magnetoferritin was determined by analysis of the absorption at 280 nm and 410 nm.
2.5.2. TEM Analysis
Transmission electron microscopy (TEM) was used for further analysis. A small volume of Fe3O4-ferritin solution was placed onto a carbon-coated copper grid for TEM observation using JEM-1200EX (JEOL, Tokyo, Japan) with and without 1% uranyl acetate.
2.5.3. HRTEM and SAED Analysis
A Tecnai G2 F20 S-TWIN (200 KV) was used for high resolution transmission electronic microscopy (HRTEM) analysis. The magnetoferritin samples were dried on carbon-coated copper grid for TEM observations. To prevent electron beam damage, all observations and image acquisitions were performed using exposures of <1 s. Selected area electron diffraction (SAED) patterns and lattice imaging were recorded on Tecnai G2 F20 S-TWIN (200 kV) (FEI, Hillsboro, OR, USA).
2.5.4. Raman Spectra
A dried sample was prepared by freezing in liquid nitrogen and subsequently lyophilized for 12 h to 24 h. The 532 nm cw laser line irradiated through the sample of magnetoferritin to detect the intensities of the radiation which corresponded to the different wavelengths, and the Raman spectrum was collected by laser Raman spectrometer DXR532 (Thermo Fisher Scientific Inc., Asheville, NC, USA).
2.6. Preparation of KG-HE Complex
The E-coil is attached to the C-terminus of HE, which was added in excess to the column containing bound KG. This allows KE to bind to the modified β-glucosidase. Before washing away any uncombined proteins, the modified β-glucosidase and ferritin were incubated in the Ni- nitrilotriacetic acid (Ni-NTA) column for over 1 h to bind KG and HE. Unbound excess HE was eluted using buffers containing low concentrations of imidazole. High concentrations of imidazole were used to elute the protein complex from the Ni-NTA column. The KG-HE complex was then dialyzed in a pH 7.5 buffer to remove the imidazole.
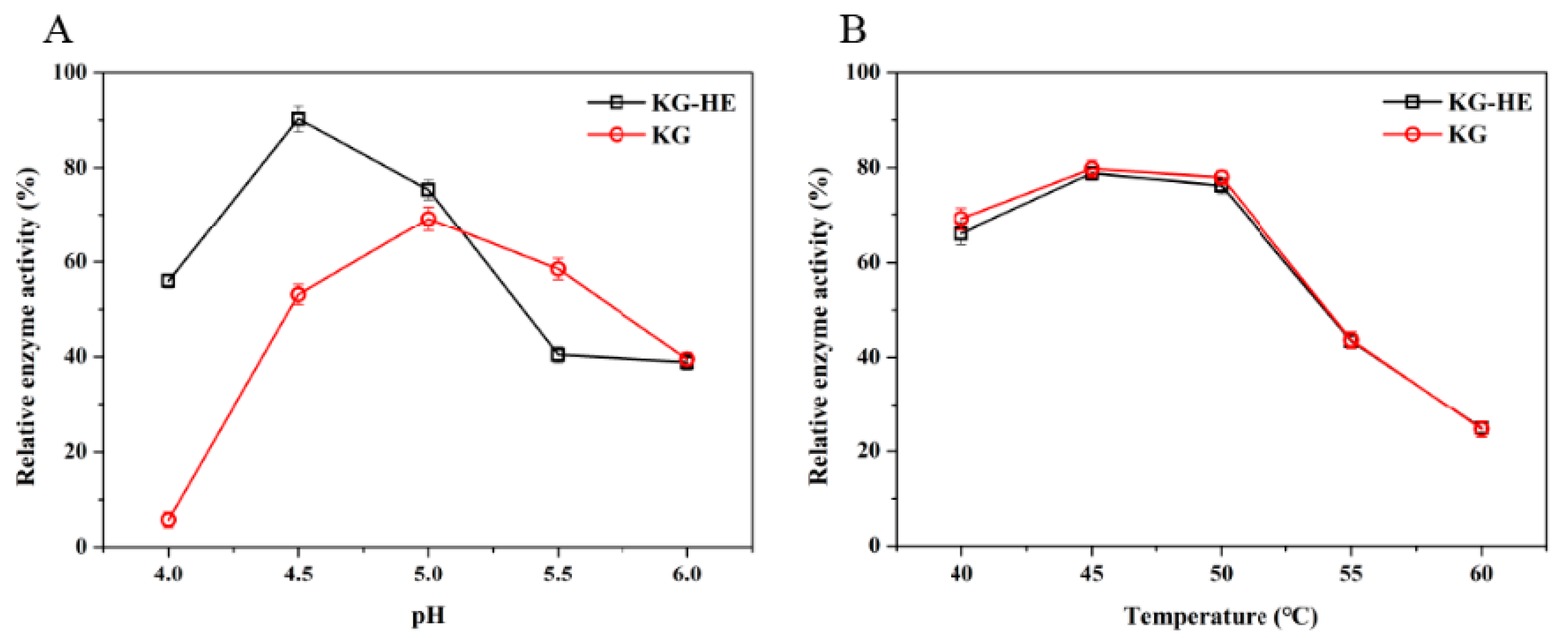
2.7. Comparison of Enzymatic Properties between Free Enzyme and Immobilized Enzyme
The properties of the
β-glucosidase activity were investigated with the chromogenic substrate
p-nitrophenyl-α-D-glucopyranoside (
pNPG) (Aladdin, Shanghai, China). Hydrolysis of
pNPG presented at a concentration of 5 mM, was assayed by the release of
p-nitrophenol (
pNP) spectrophotometrically at a wavelength of 400 nm [
25,
26]. Changes in absorbance were converted to mmol
pNP according to the calibration curve (
Figure S1) prepared with known
pNP concentrations. The enzyme (10 µL) was mixed with an equal volume of
pNPG in 180 µL of 50 mM imidazole-potassium acid phthalate buffer (pH 6.0), and incubated for 20 min at 45 °C. Subsequently, termination of reaction was performed by adding 600 µL of 1 M Na
2CO
3 to the reaction system. A total of 200 µL of solution was detected at 400 nm to calculate the relative enzyme activity.
For enzyme kinetic determination, reactions were performed depending on the optimal conditions of free or immobilized β-glucosidase. Reactions were carried out using 0.1 mM to 1.0 mM pNPG as the substrate in a total volume of 0.8 mL. The released p-nitrophenol was monitored continuously at 400 nm by a microplate reader (BioTek, Winooski, VT, USA).
2.8. Reusability of Immobilized Enzyme
To evaluate the effects of an applied magnetic field, the KG-HE complex solutions were placed within a magnetic field at 4 °C. All analyses were carried out in triplicate. The enzyme activity of the KG-HE complex prior to the introduction of the magnetic field was used as the baseline value. Magnetic immobilized enzymes were pulled to the magnetic field over 24 h and the supernatant, which should contain any nonmagnetic immobilized enzymes, was analyzed for residual enzymatic activity. After three usage cycles, each followed by exposure to a magnetic field, relative enzyme activities in the supernatant and magnetic immobilized enzymes were obtained, and the retention rates of relative enzyme activities were calculated.