Cultivar-Dependent Anticancer and Antibacterial Properties of Silver Nanoparticles Synthesized Using Leaves of Different Olea Europaea Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Leaves Extract
2.2. Synthesis of Green AgNPs
2.3. Characterization of Green AgNPs
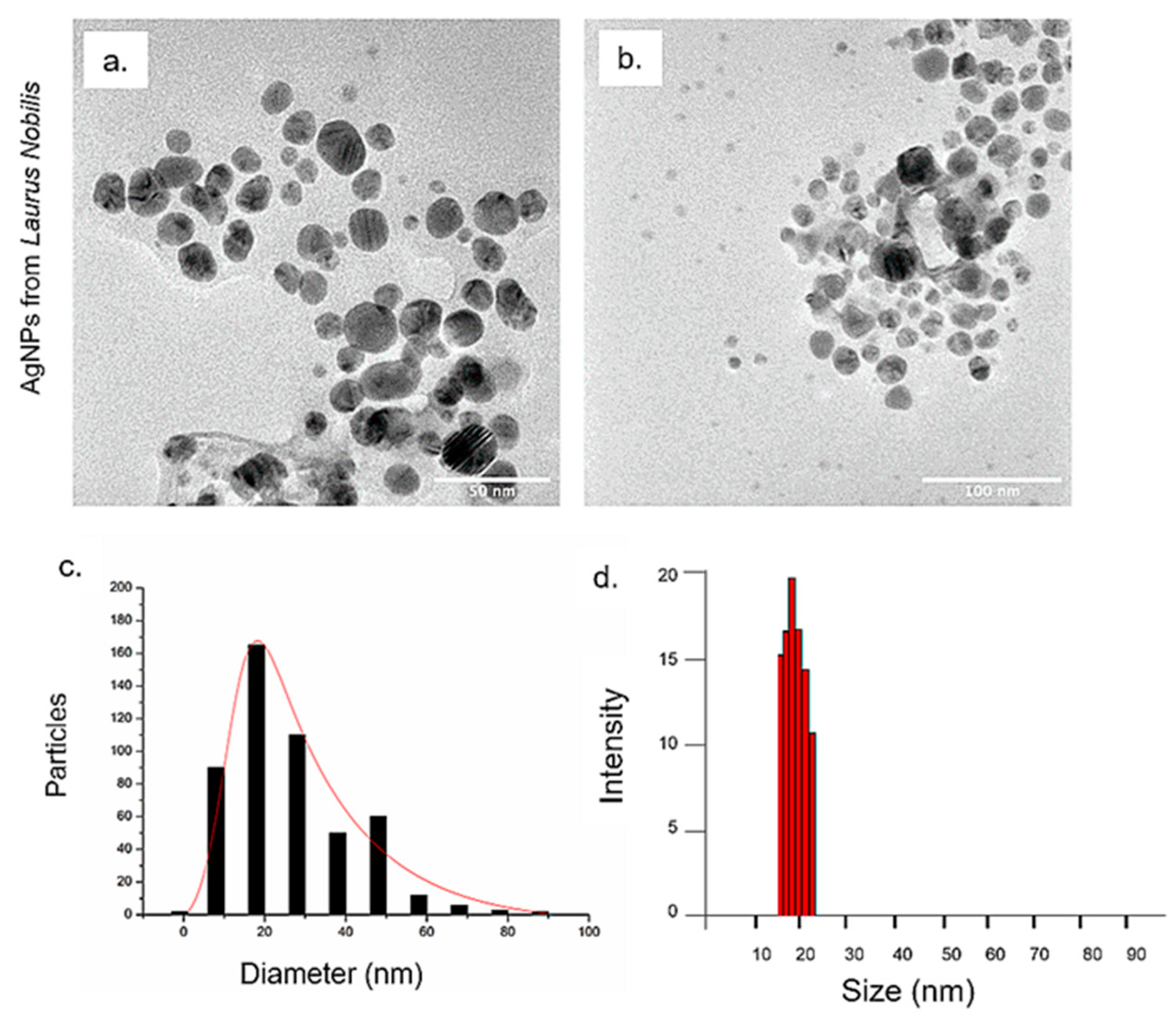
2.3.1. Transmission Electron Microscopy (TEM), Dynamic Light Scattering (DLS) and ζ-Potential
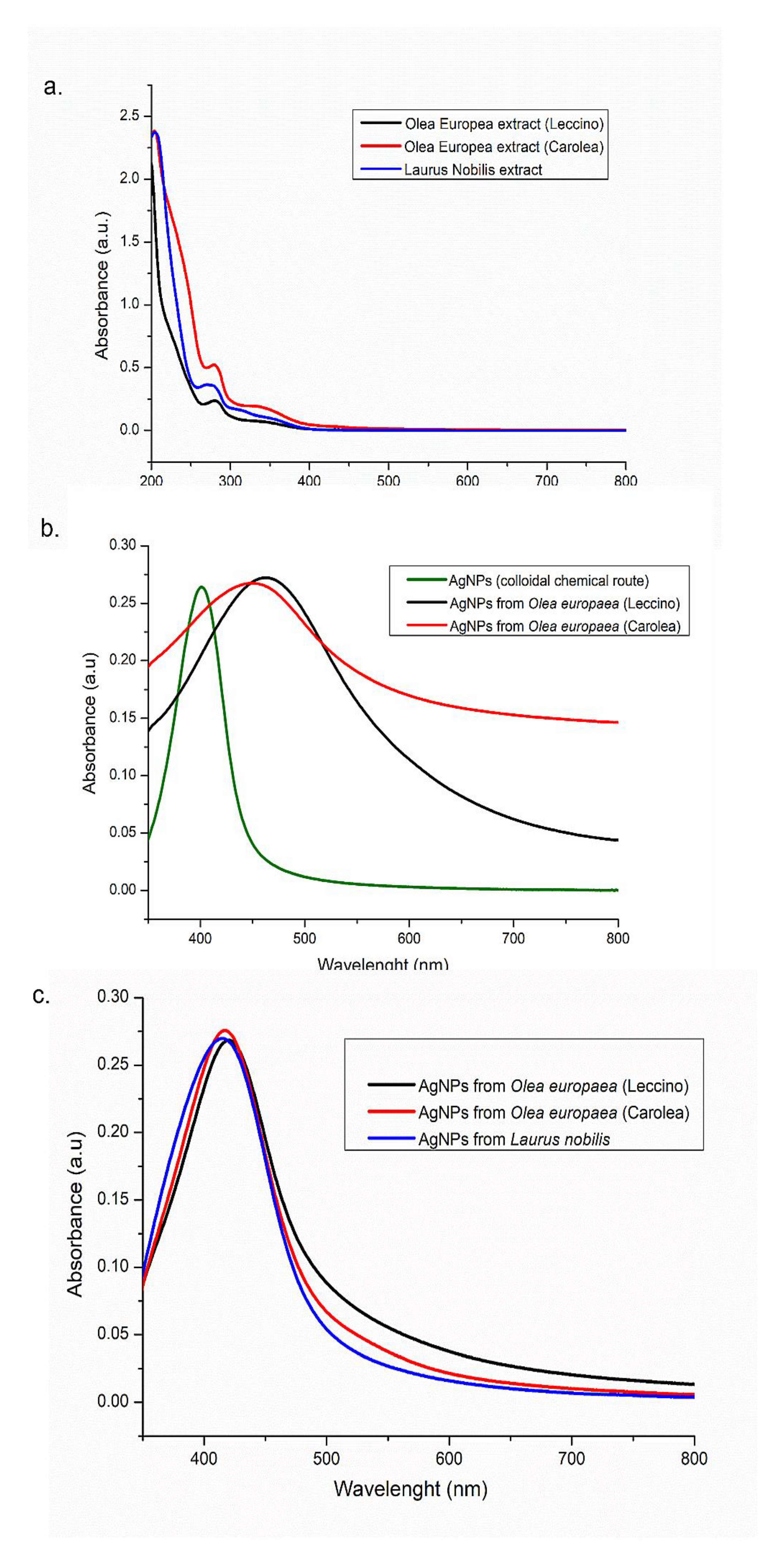
2.3.2. UV-Vis Spectroscopy
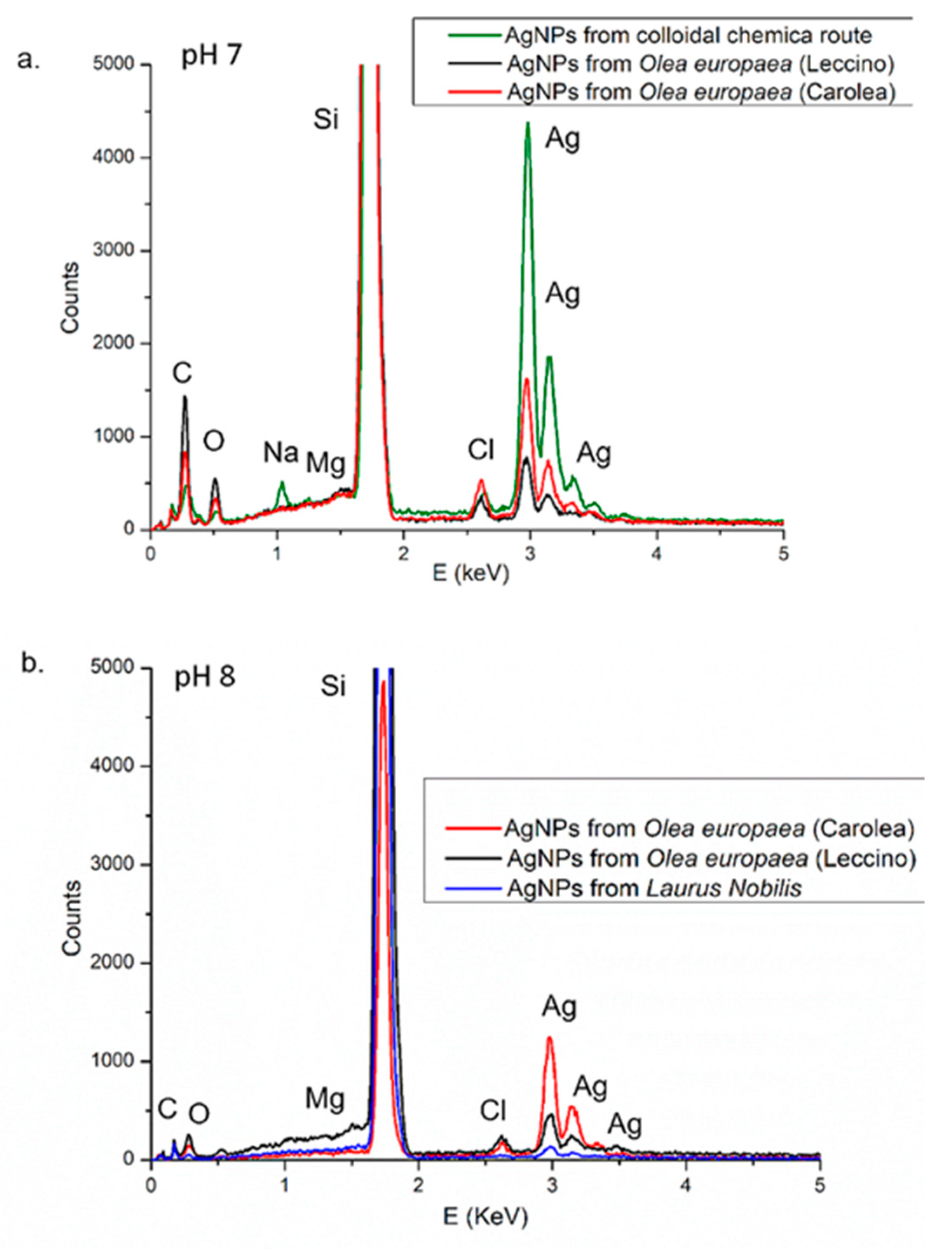
2.3.3. Energy-Dispersive X-ray Spectroscopy (EDS)
2.3.4. Attenuated Total Reflection (ATR) Fourier Transform Infrared Spectroscopy (FTIR)
2.3.5. Cell Culture
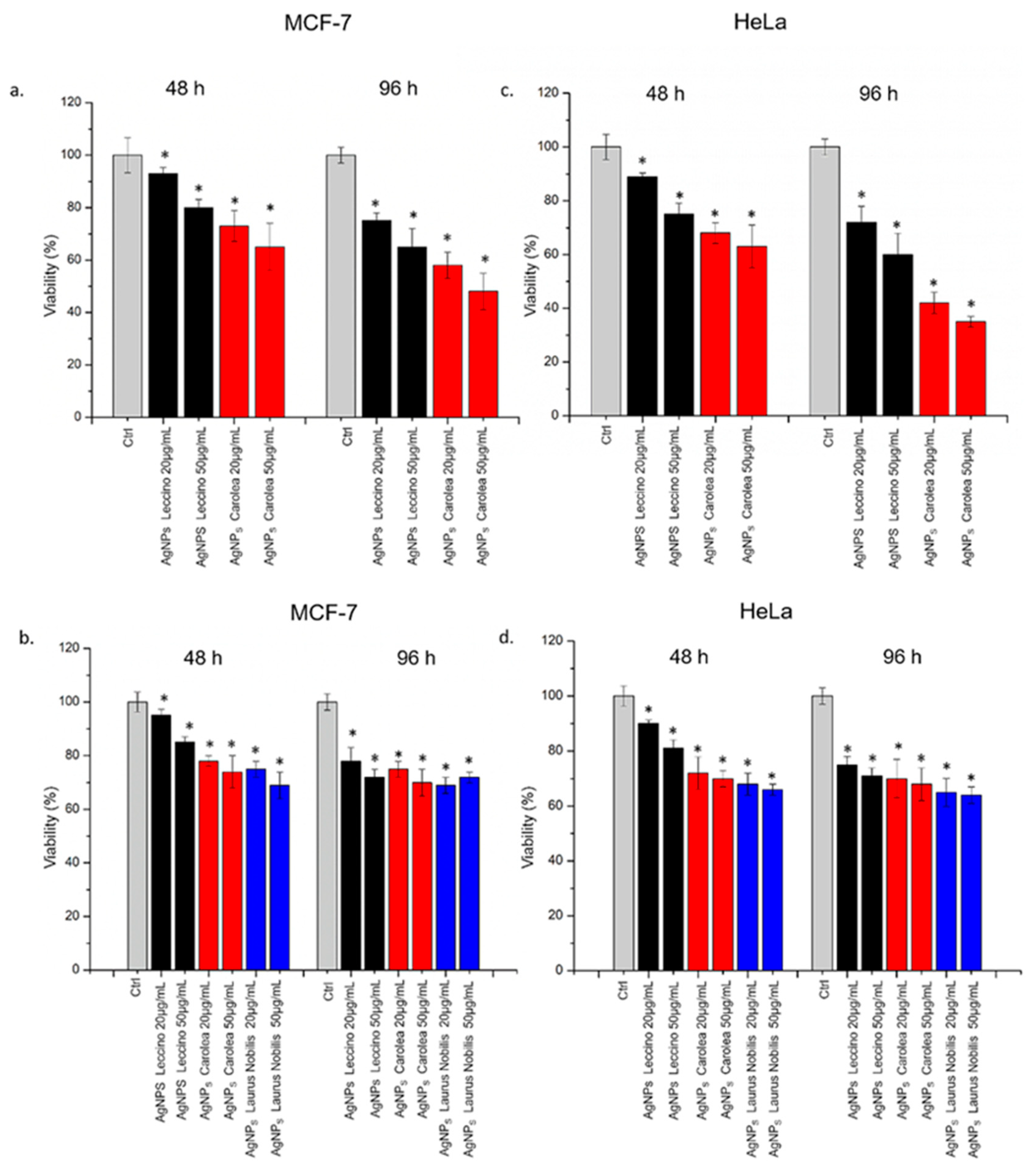
2.3.6. WST-8 Assay
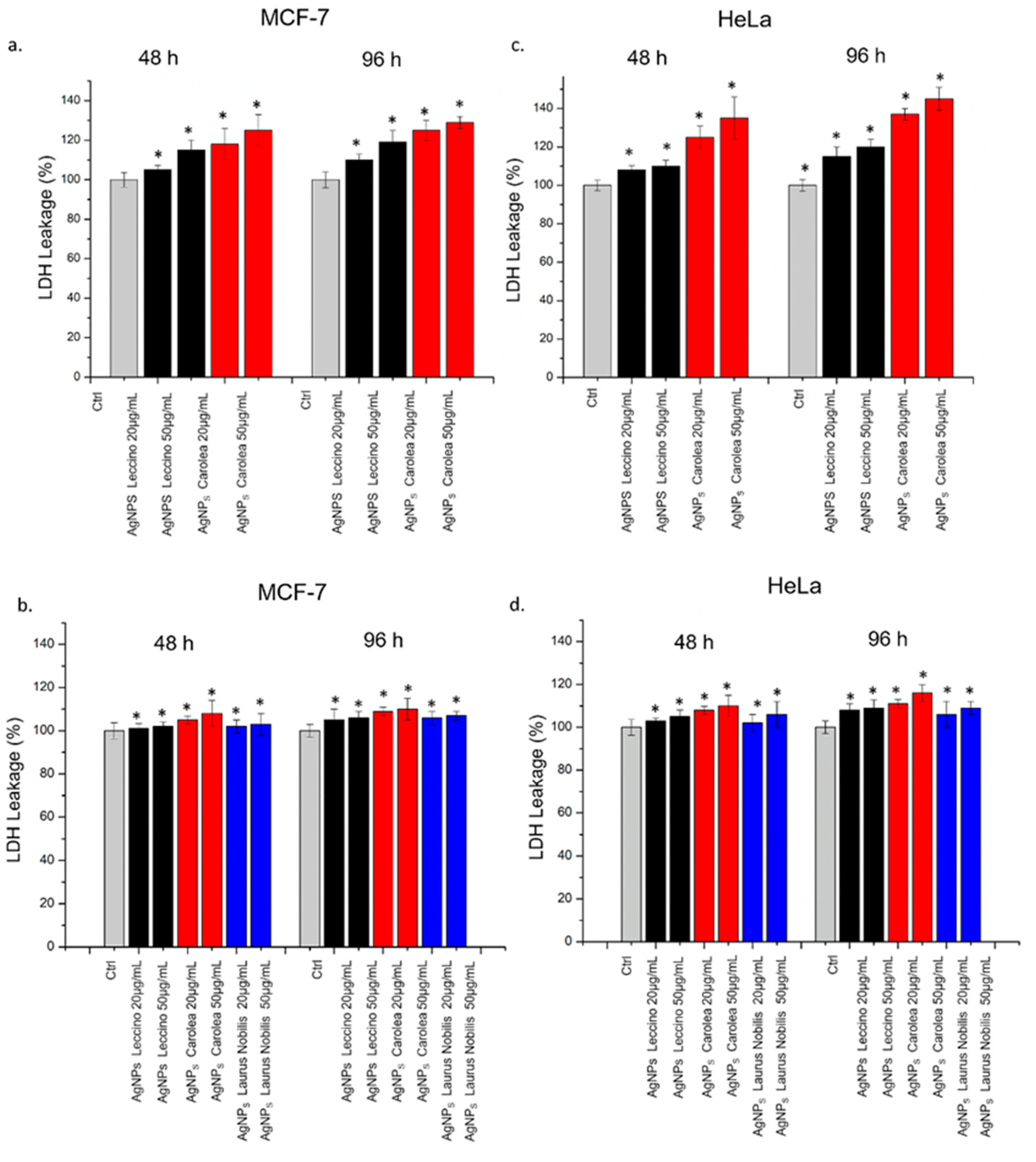
2.3.7. Lactate Dehydrogenase (LDH) Assay
2.3.8. Determination of the Intracellular Uptake of Green AgNPS by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES)
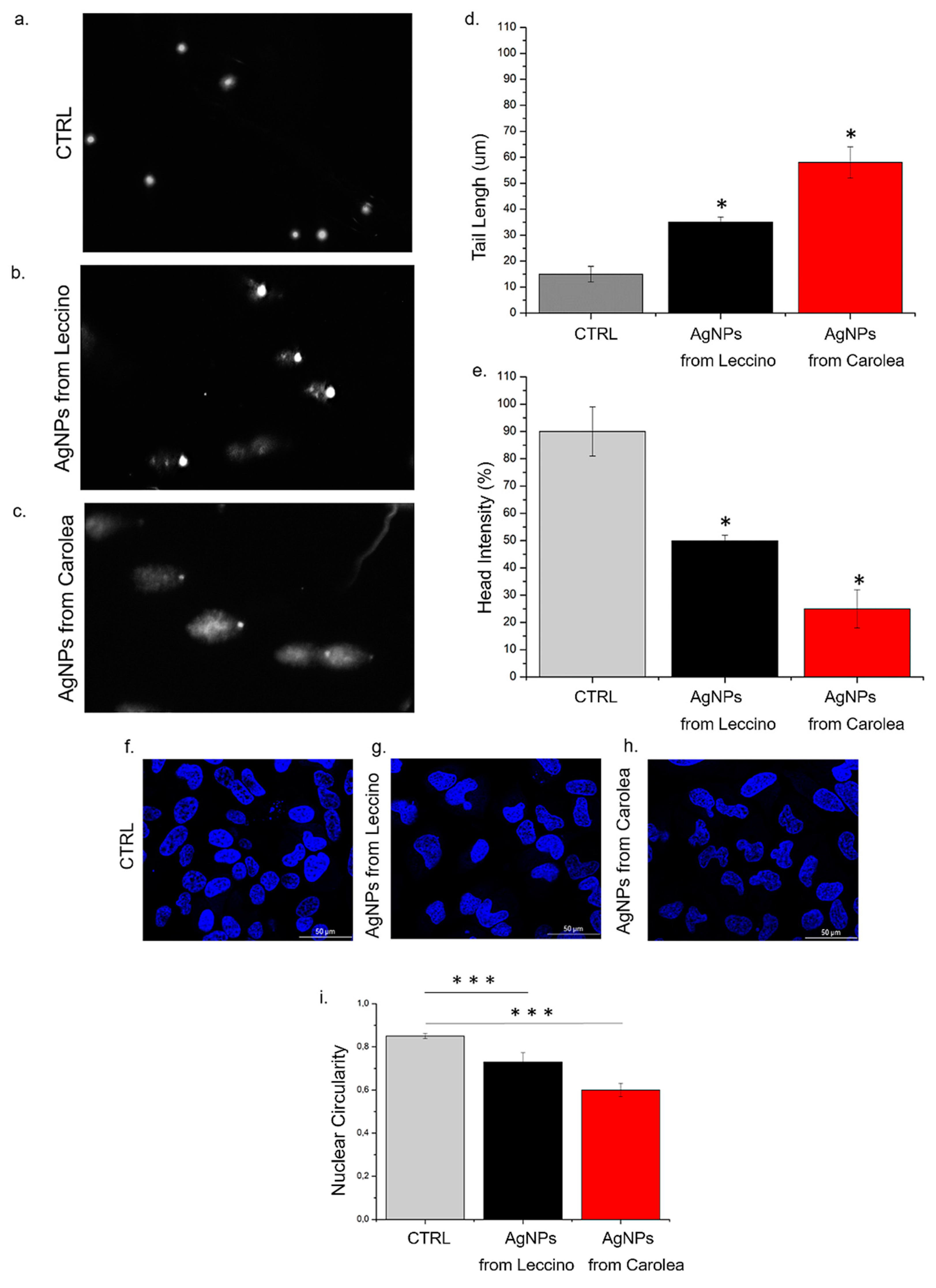
2.3.9. Comet Assay (Single Gel Electrophoresis)
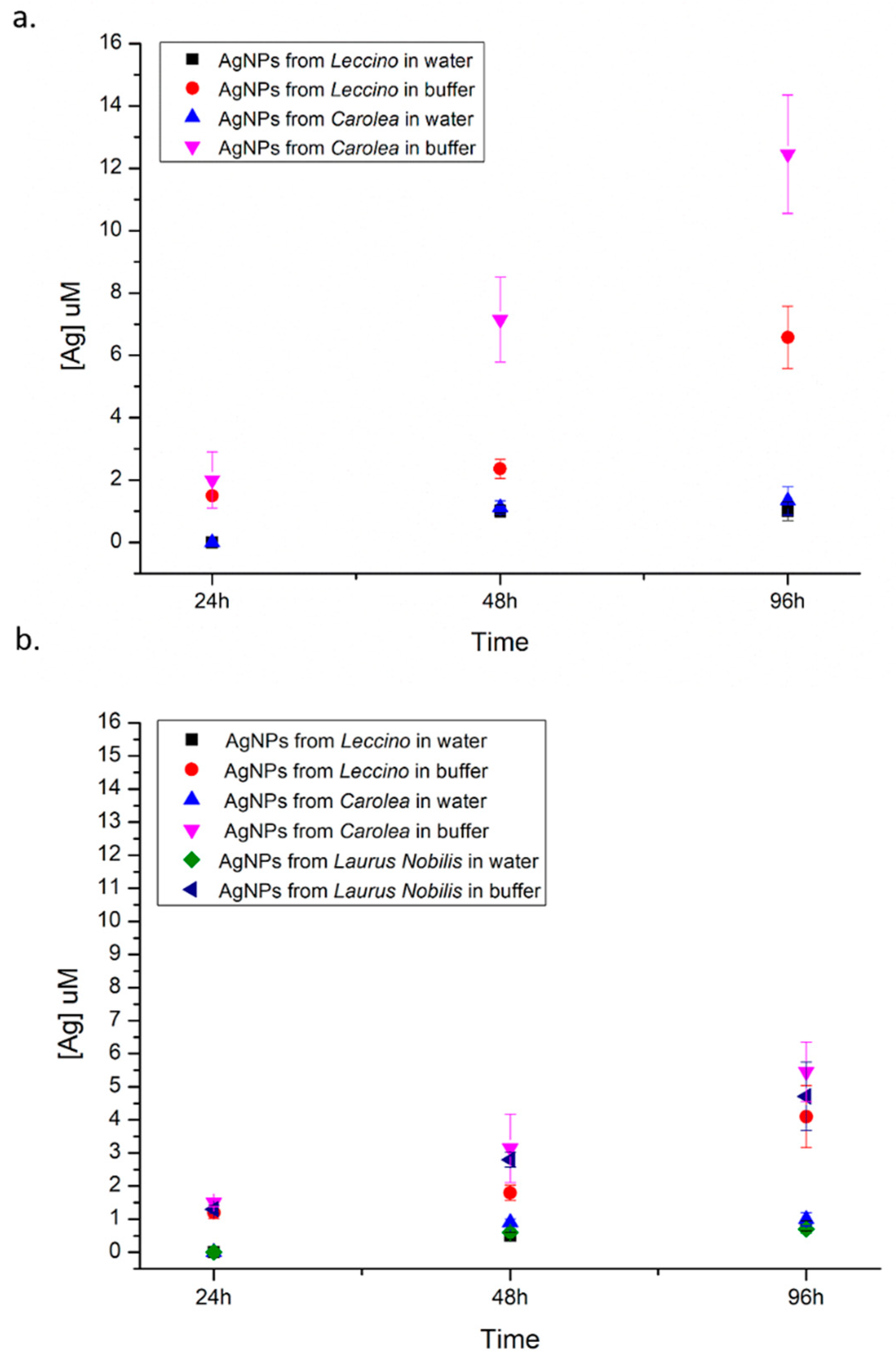
2.3.10. Determination of Ag+ Release
2.3.11. Confocal Measurements
2.4. Antibacterial Activity of Green AgNPs
Collection of Water Samples
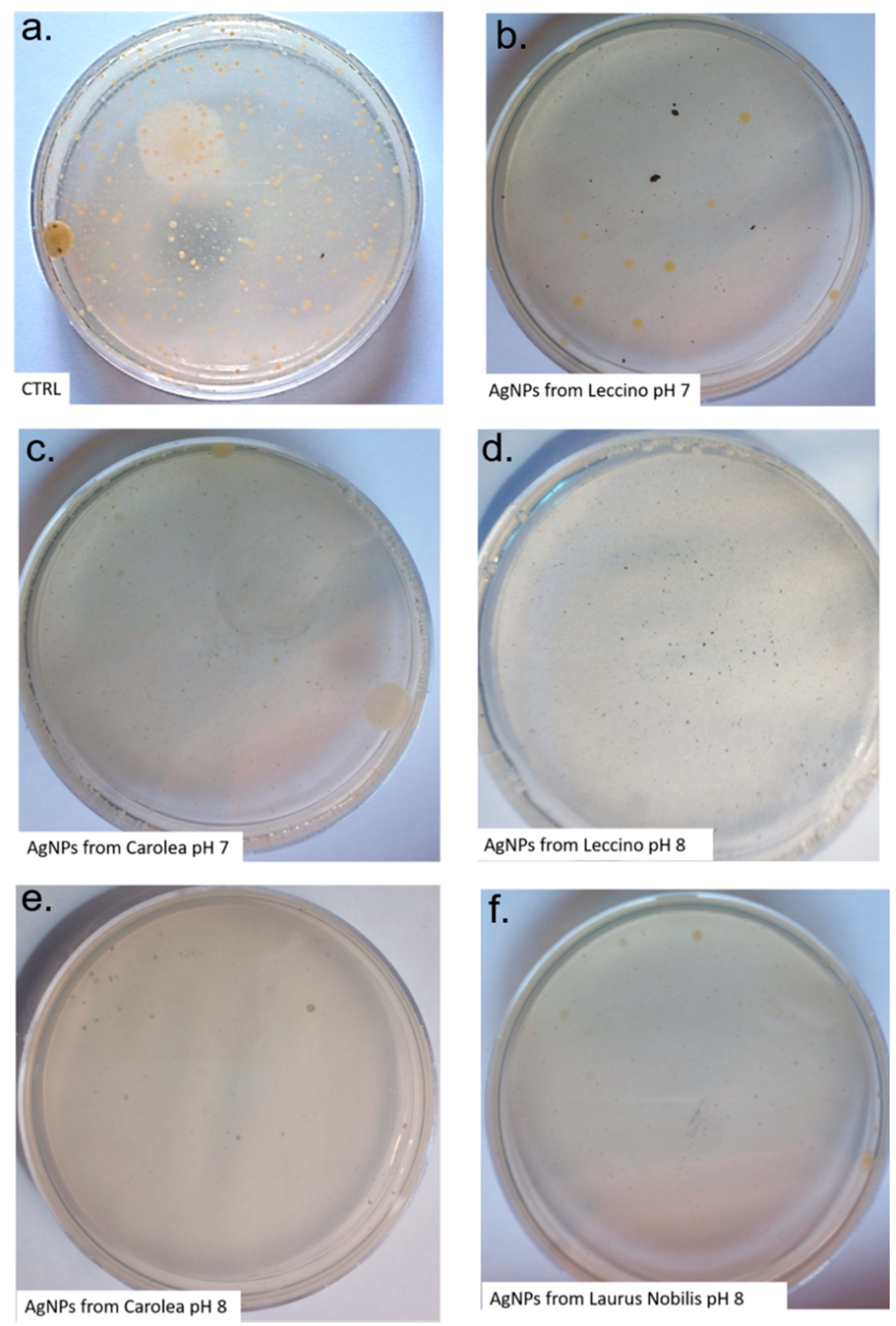
2.5. Total Bacteria Detection by Plate Count Techniques
2.6. Most Probable Number (MPN) to detect Coliforms and Faecal Coliforms
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marzo, J.L.; Jornet, J.M.; Pierobon, M. Nanotechnology Derived Nanotools in Biomedical Perspectives: An Update. Curr. Drug Targets 2019, 20, 800–807. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Johnson, M.E.; Bustos, A.R.M.; Murphy, K.E.; Winchester, M.R.; Baudrit, J.R.V. Silver Nanoparticles: Technological Advances, Societal Impacts, and Metrological Challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Zhang, Q.; Carmichael, P.L.; Boekelheide, K.; Andersen, M.E. Toxicity Testing in the 21st Century: Defining New Risk Assessment Approaches Based on Perturbation of Intracellular Toxicity Pathways. PLoS ONE 2011, 6, e20887. [Google Scholar] [CrossRef] [PubMed]
- Osório, I.; Igreja, R.; Franco, R.; Cortez, J. Incorporation of silver nanoparticles on textile materials by an aqueous procedure. Mater. Lett. 2012, 75, 200–203. [Google Scholar] [CrossRef]
- Von Goetz, N.; Fabricius, L.; Glaus, R.; Weitbrecht, V.; Hungerbühler, K. Migration of silver from commercial plastic food containers and implications for consumer exposure assessment. Food Addit. Contam. Part A 2013, 303, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A 2008, 25, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Karim, N.; Afroj, S.; Tan, S.; Novoselov, K.S.; Yeates, S.G. All Inkjet-Printed Graphene-Silver Composite Ink on Textiles for Highly Conductive Wearable Electronics Applications. Sci. Rep. 2019, 8035. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.; Troc, N.; Cottancin, E.; Pellarin, M.; Weissker, H.; Lermé, J.; Kociak, M.; Hillenkamp, M. Plasmonic quantum size effects in silver nanoparticles are dominated by interfaces and local environments. Nat. Phys. 2019, 15, 275–280. [Google Scholar] [CrossRef]
- Barnard, A.S. Size, Shape, Stability, and Color of Plasmonic Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Health Mater. 2018, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V.; Rizzello, L.; Di Bello, M.P.; Rinaldi, R. One-step synthesis, toxicity assessment and degradation in tumoral pH environment of SiO2@Ag core/shell nanoparticles. J. Nanoparticle Res. 2017, 19, 14. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Mirmohammadi and BZ. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Farooqi, Z.H.; Khalid, R.; Begum, R.; Farooq, U.; Wu, Q.; Wu, W.; Ajmal, M.; Irfan, A.; Naseem, K. Facile synthesis of silver nanoparticles in a crosslinked polymeric system by in situ reduction method for catalytic reduction of 4-nitroaniline. Environ. Technol. 2019, 40, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Jime, V.M. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31. [Google Scholar]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 1–21. [Google Scholar] [CrossRef]
- Beach, E.S.; Cui, Z.; Anastas, P.T. Green Chemistry: A design framework for sustainability. Energy Environ. Sci. 2009, 2, 1038–1049. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Khandel, P.; Yadaw, R.K.; Soni, D.K.; Kanwar, L.; Shahi, S.K. Biogenesis of metal nanoparticles and their pharmacological applications: Present status and application prospects. J. Nanostructure Chem. 2018, 8, 217–254. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Prabha, S.; Lahtinen, M.; Sillanpää, M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar]
- Bose, D.; Chatterjee, S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl. Nanosci. 2016, 6, 895–901. [Google Scholar] [CrossRef]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 79–84. [Google Scholar]
- Yilmaz, M.; Turkdemir, H.; Kiliç, M.A.; Bayram, E.; Cicek, A.; Mete, A.; Ulug, B.; Yılmaz, M. Biosynthesis of silver nanoparticles using leaves of Stevia rebaudiana. Mater. Chem. Phys. 2011, 130, 1195–1202. [Google Scholar] [CrossRef]
- Roopan, S.M.; Rohit; Madhumitha, G.; Rahuman, A.; Kamaraj, C.; Bharathi, A.; Surendra, T.; Kamaraj, D. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind. Crop. Prod. 2013, 43, 631–635. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Raut Rajesh, W.; Lakkakula Jaya, R.; Kolekar Niranjan, S.; Mendhulkar Vijay, D.; Kashid Sahebrao, B. Phytosynthesis of Silver Nanoparticle Using Gliricidia sepium (Jacq.). Curr. Nanosci. 2009, 5, 117–122. [Google Scholar]
- Singhal, G.; Bhavesh, R.; Kasariya, K.; Sharma, A.R.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanoparticle Res. 2011, 13, 2981–2988. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Green Synthesis of Silver Nanoparticles Using Cycas Leaf Green Synthesis of Silver Nanoparticles Using Cycas Leaf. Int. J. Green Nanotechnol. Phys. Chem. 2010, 111–117. [Google Scholar]
- Parvaiz, M.; Hussain, K.; Shoaib, M.; William, G. A Review: Therapeutic Significance of Olive Olea europaea L. (Oleaceae Family). Glob. J. Pharmacol. 2013, 7, 333–336. [Google Scholar]
- Khan, Y.; Panchal, S.; Vyas, N.; Butani, A.; Kumar, V. Olea europaea: A Phyto-Pharmacological Review. Pharmacogn. Rev. 2007, 1, 112–116. [Google Scholar]
- Bianco, A.; Uccella, N. Biophenolic components of olives. Food Res. Int. 2000, 33, 475–485. [Google Scholar] [CrossRef]
- Farag, R.S.; Basuny, A.M. Safety evaluation of olive phenolic compounds as natural antioxidants. Int. J. Food Sci. Nutr. 2009, 54, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Rice, S.B.; Chan, C.; Brown, S.C.; Eschbach, P.; Han, L.; Ensor, D.S.; Stefaniak, A.B.; Bonevich, J.; Vladár, A.E.; Walker, A.R.H.; et al. Particle size distributions by transmission electron microscopy: An interlaboratory comparison case study. Metrologia 2013, 50, 663–678. [Google Scholar] [CrossRef]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag + ion release in the cytosol. Nanomedicine Nanotechnology. Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef]
- Richard, F.; Micheal, G.M.; Mera, D. Water Waste and Health Management in Hot Climates; Ohn Wiley and Sons Ltd.: London, UK, 1979. [Google Scholar]
- Talaro, K.P.; Chess, B. Foundations in Microbiology, 2nd ed.; WCB/McGraw Hill: Boston, MA, USA, 1996. [Google Scholar]
- Bartram, J.; Ballance, R. (Eds.) Water Quality Monitoring—A Practical Guide to the Design and Implementation of Freshwater Quality Studies and Monitoring Programmes; United Nations Environment Programme and the World Health Organization: Geneva, Switzerland, 1996. [Google Scholar]
- Hajna, A.A.; Perry, C.A.; Sc, D. Comparative Study of Presumptive and the Coliform Group and for Fecal Streptococci. Am. J. Public Health Nations Health 1943, 33, 550–556. [Google Scholar] [CrossRef]
- Rand, M.C.; Greenberg, A.E.; Taras, M.J. Standard Methods for the Examination of Water and Wastewater, 14th ed.; American Public Health Association: Washington, DC, USA, 1976; ISBN 0875530788. [Google Scholar]
- Fishbein, M.; Surkiewicz, B.F. Comparison of the Recovery of Escherichia coli from Frozen Foods and Nutmeats by Confirmatory Incubation in EC Medium at 44.5 and 45.5 C. Appl. Microbiol. 1964, 12, 127–131. [Google Scholar] [PubMed]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.B. A Study of the Protective Properties of Iraqi Olive Leaves against Oxidation and Pathogenic Bacteria in Food Applications. Antioxidants 2017, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Baldoni, L.; Tosti, N.; Ricciolini, C.; Belaj, A.; Arcioni, S.; Pannelli, G.; Germana, M.A.; Mulas, M.; Porceddu, A. Genetic Structure of Wild and Cultivated Olives in the Central Mediterranean Basin. Ann. Bot. 2006, 98, 935–942. [Google Scholar] [CrossRef]
- Fabbri, A.; Galaverna, G.; Ganino, T. Polyphenol composition of olive leaves with regard to cultivar, time of collection and shoot type. Acta Hortic. 2008. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Kumar, P.; Selvi, S.S.; Govindaraju, M. Seaweed-mediated biosynthesis of silver nanoparticles using Gracilaria corticata for its antifungal activity against Candida spp. Appl. Nanosci. 2013, 3, 495–500. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Qi, C.; Musetti, S.; Fu, L.-H.; Zhu, Y.-J.; Huang, L. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications. Chem. Soc. Rev. 2019, 48, 2698–2737. [Google Scholar] [CrossRef]
- Cruz-Matías, I.; Ayala, D.; Hiller, D.; Gutsch, S.; Zacharias, M.; Estradé, S.; Peiró, F. Sphericity and roundness computation for particles using the extreme vertices model. J. Comput. Sci. 2019, 30, 28–40. [Google Scholar] [CrossRef]
- Singh, C.; Kumar, J.; Kumar, P.; Chauhan, B.S.; Tiwari, K.N.; Mishra, S.K.; Srikrishna, S.; Saini, R.; Nath, G.; Singh, J. Green synthesis of silver nanoparticles using aqueous leaf extract of Premna integrifolia (L.) rich in polyphenols and evaluation of their antioxidant, antibacterial and cytotoxic activity. Biotechnol. Biotechnol. Equip. 2019, 33, 359–371. [Google Scholar] [CrossRef]
- Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Van der Zande, M.; Undas, A.K.; Kramer, E.; Monopoli, M.P.; Peters, R.J.; Garry, D.; Antunes Fernandes, E.C.; Hendriksen, P.J.; Marvin, H.J.; Peijnenburg, A.A.; et al. Different responses of Caco-2 and MCF-7 cells to silver nanoparticles are based on highly similar mechanisms of action. Nanotoxicology 2016, 10, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Butler, M. Animal Cell Culture and Technology, 2nd ed.; Taylor & Francis: London, UK, 2003; ISBN 9780203427835. [Google Scholar]
- Lu, B.; Hu, M.; Liu, K.; Peng, J. Cytotoxicity of berberine on human cervical carcinoma HeLa cells through mitochondria, death receptor and MAPK pathways, and in-silico drug-target prediction. Toxicol. In Vitro 2010, 24, 1482–1490. [Google Scholar] [CrossRef]
- Moradhaseli, S.; Mirakabadi, A.Z.; Sarzaeem, A.; Kamalzadeh, M.; Hosseini, R.H. Cytotoxicity of ICD-85 NPs on Human Cervical Carcinoma HeLa Cells through Caspase-8 Mediated Pathway. Iran J. Pharm. Res. 2013, 12, 155–163. [Google Scholar] [PubMed]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S.S. Mechanisms of Nanoparticle Internalization and Transport across an Intestinal Epithelial Cell Model: Effect of Size and Surface Charge. Mol. Pharm. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Nambara, K.; Niikura, K.; Mitomo, H.; Ninomiya, T.; Takeuchi, C.; Wei, J.; Matsuo, Y.; Ijiro, K. Reverse Size Dependences of the Cellular Uptake of Triangular and Spherical Gold Nanoparticles. Langmuir 2016, 32, 12559–12567. [Google Scholar] [CrossRef]
- Helmy, I.M.; Azim, A.M.A. Efficacy of ImageJ in the assessment of apoptosis. Diagn. Pathol. 2012, 7, 15. [Google Scholar] [CrossRef]
- João, P.S. Cabral. Water Microbiology. Bacterial Pathogens and Water. Int. J. Environ. Res. Public Health 2010, 7, 3657–3703. [Google Scholar]
- Council of the European Union. Council Directive 80/778/EEC of 15 July 1980 relating to the quality of water intended for human consumption. Off. J.Eur. Communities 1980. [Google Scholar]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and Shape-Dependent Antibacterial Studies of Silver Nanoparticles Synthesized by Wet Chemical Routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]




| Green AgNPs | pH | Zeta Potential Value |
|---|---|---|
| AgNPs from Olea europaea (Leccino) | 7 | −15 ± 5 |
| AgNPs from Olea europaea (Carolea) | 7 | −20 ± 3 |
| AgNPs from Olea europaea (Leccino) | 8 | −30 ± 8 |
| AgNPs from Olea europaea (Carolea) | 8 | −18 ± 2 |
| AgNPs from Laurus Nobilis | 8 | −25 ± 2 |
| Green AgNPs | Circularity Value (pH 7) | Circularity Value (pH 8) |
|---|---|---|
| AgNPs from Olea Europaea–(Leccino) | 0.55 ± 4 | 0.88 ± 3 |
| AgNPs from Olea Europaea–(Carolea) | 0.28 ± 8 | 0.63 ± 6 |
| AgNPs from Laurus Nobilis | - | 0.65 ± 4 |
| Colonies and Coliforms | Contaminated Well Water | Well Water + 50µg/mL AgNPs from Leccino (pH 7) | Well Water + 50µg/mL AgNPs from Carolea (pH 7) | Well Water + 50 µg/mL AgNPs from Leccino (pH 8) | Well Water + 50 µg/mL AgNPs from Carolea (pH 8) | Well Water +50 µg/mL AgNPs from Laurus Nobilis (pH 8) |
|---|---|---|---|---|---|---|
| Total colonies (22 °C)(UCF/ml) 100 allowed | 120 | 108 | 105 | 79 | 82 | 84 |
| Total colonies (37 °C)(UCF/ml) 20 allowed | 28 | 12 | 6 | absent | 1 | 3 |
| Total Coliforms (MPN/100 mL) Absent allowed | 19 | 11 | 9 | absent | absent | absent |
| Total Faecal Coliforms (MPN/100 mL) Absent allowed | 7 | 6 | 6 | absent | absent | absent |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Matteis, V.; Rizzello, L.; Ingrosso, C.; Liatsi-Douvitsa, E.; De Giorgi, M.L.; De Matteis, G.; Rinaldi, R. Cultivar-Dependent Anticancer and Antibacterial Properties of Silver Nanoparticles Synthesized Using Leaves of Different Olea Europaea Trees. Nanomaterials 2019, 9, 1544. https://doi.org/10.3390/nano9111544
De Matteis V, Rizzello L, Ingrosso C, Liatsi-Douvitsa E, De Giorgi ML, De Matteis G, Rinaldi R. Cultivar-Dependent Anticancer and Antibacterial Properties of Silver Nanoparticles Synthesized Using Leaves of Different Olea Europaea Trees. Nanomaterials. 2019; 9(11):1544. https://doi.org/10.3390/nano9111544
Chicago/Turabian StyleDe Matteis, Valeria, Loris Rizzello, Chiara Ingrosso, Eva Liatsi-Douvitsa, Maria Luisa De Giorgi, Giovanni De Matteis, and Rosaria Rinaldi. 2019. "Cultivar-Dependent Anticancer and Antibacterial Properties of Silver Nanoparticles Synthesized Using Leaves of Different Olea Europaea Trees" Nanomaterials 9, no. 11: 1544. https://doi.org/10.3390/nano9111544
APA StyleDe Matteis, V., Rizzello, L., Ingrosso, C., Liatsi-Douvitsa, E., De Giorgi, M. L., De Matteis, G., & Rinaldi, R. (2019). Cultivar-Dependent Anticancer and Antibacterial Properties of Silver Nanoparticles Synthesized Using Leaves of Different Olea Europaea Trees. Nanomaterials, 9(11), 1544. https://doi.org/10.3390/nano9111544









