Sulfur Dioxide Degradation by Composite Photocatalysts Prepared by Recycled Fine Aggregates and Nanoscale Titanium Dioxide
Abstract
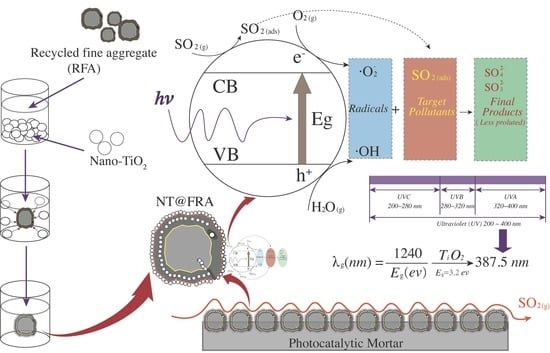
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
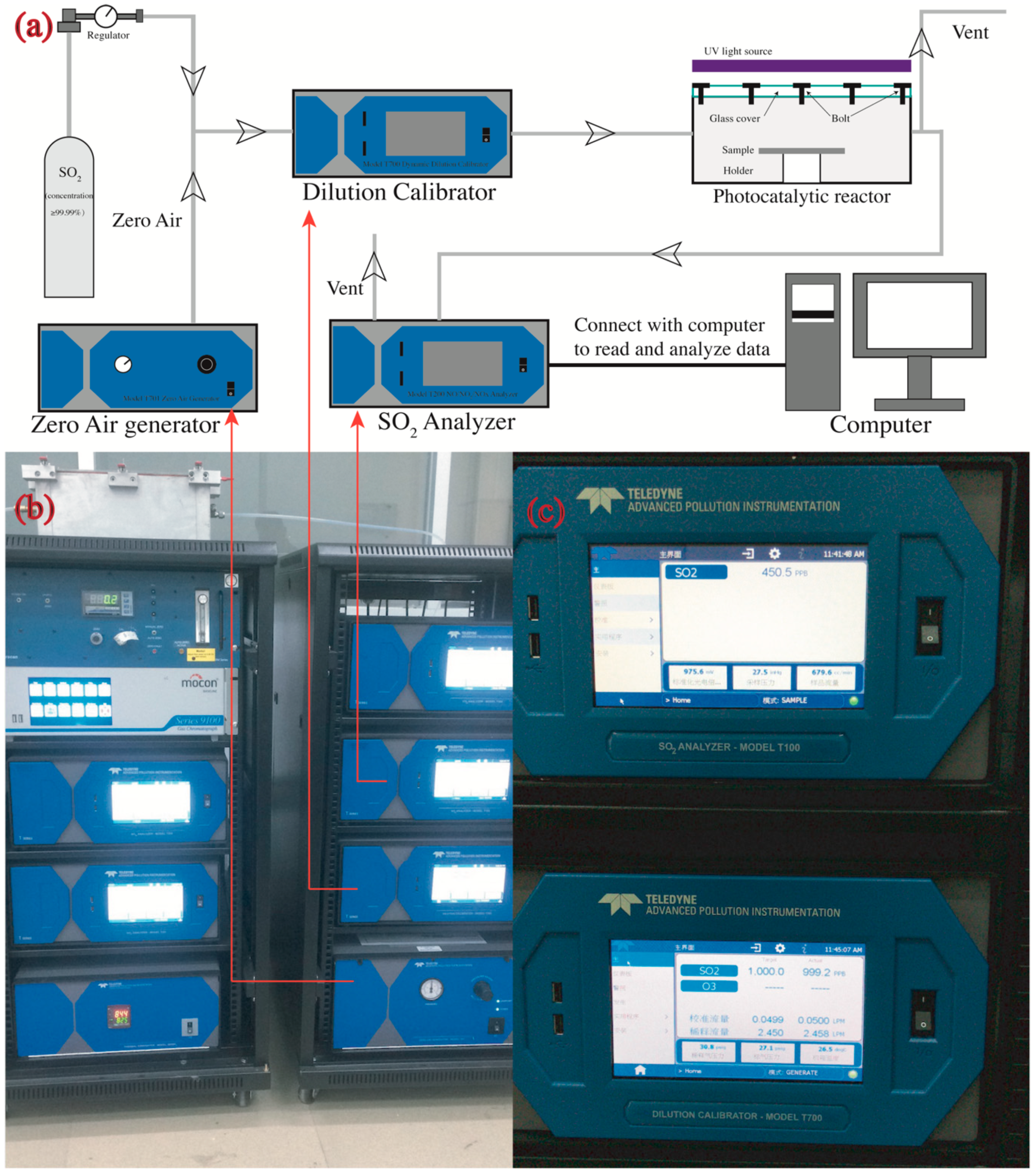
2.3. Testing
2.3.1. Compressive Strength
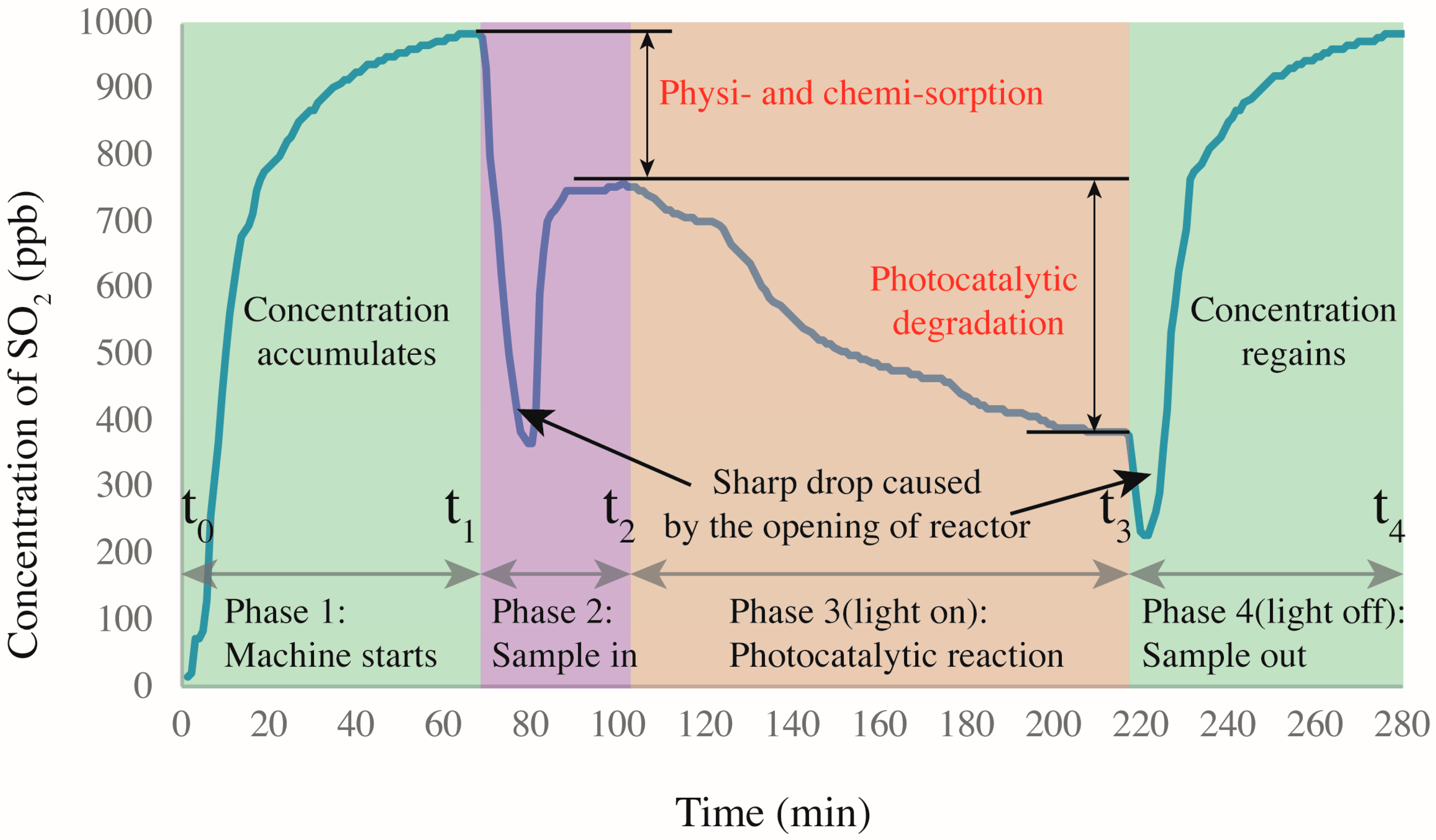
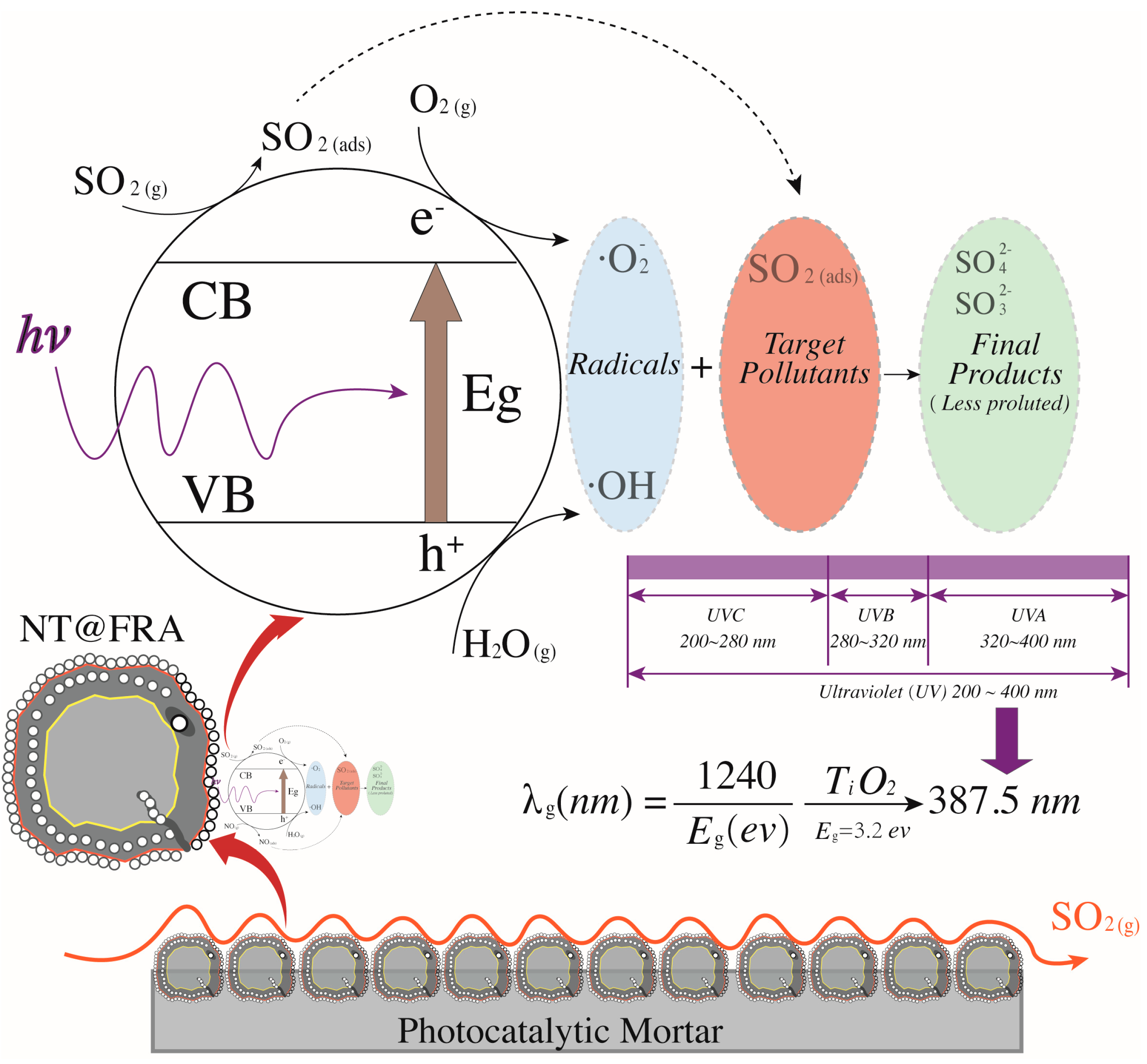
2.3.2. Photocatalytic Characterization
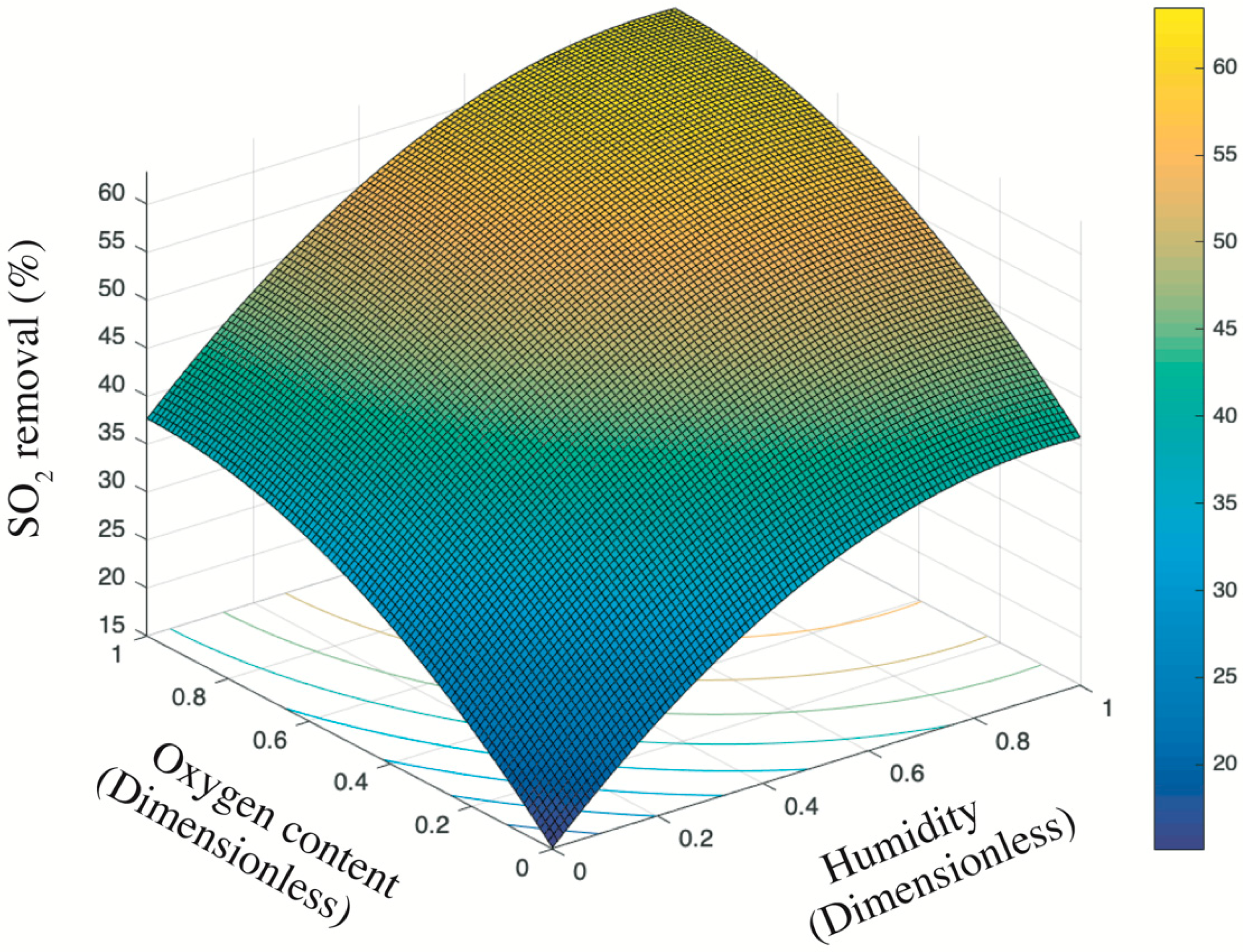
2.3.3. Response Surface Methodology
2.3.4. Environmental Scanning Electron Microscope (ESEM)
2.3.5. Microstructure Analysis
2.3.6. Weathering Test
3. Results and Discussion
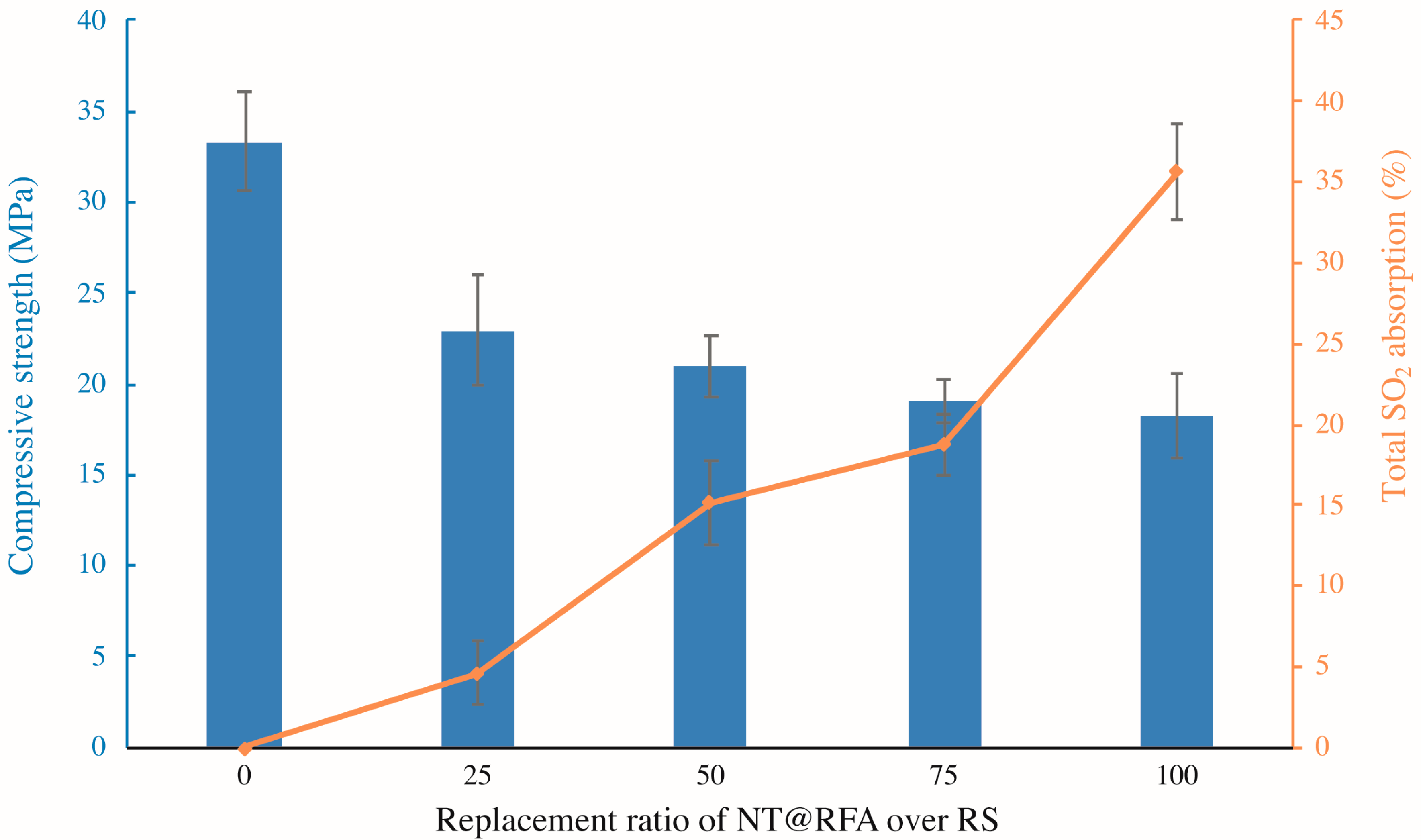
3.1. Effect of NT@RFA Content on Mechanical Strength and Total SO2 Degradation of Photocatalytic Mortar
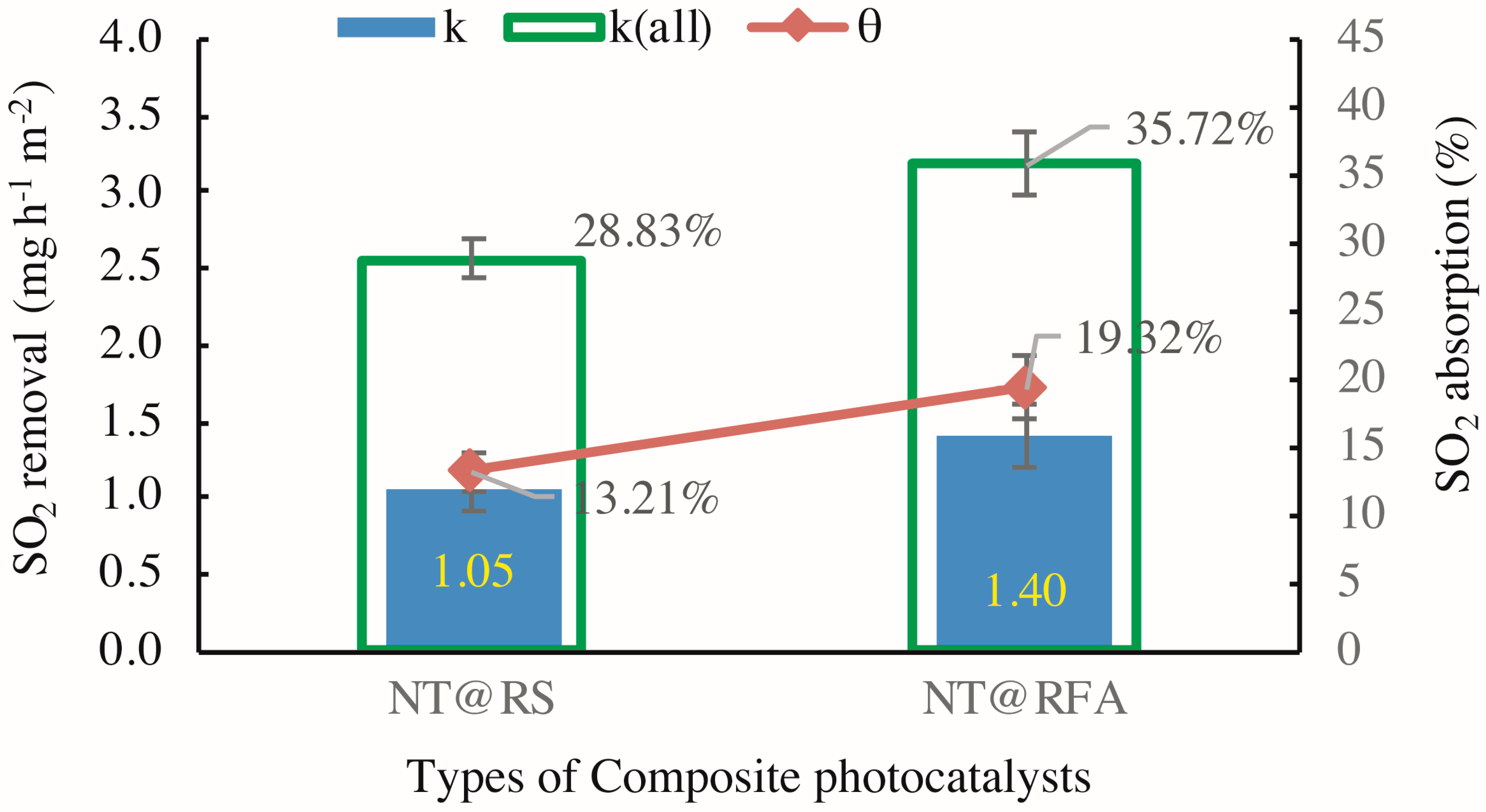
3.2. Effect of Different CPs on the SO2 Removal
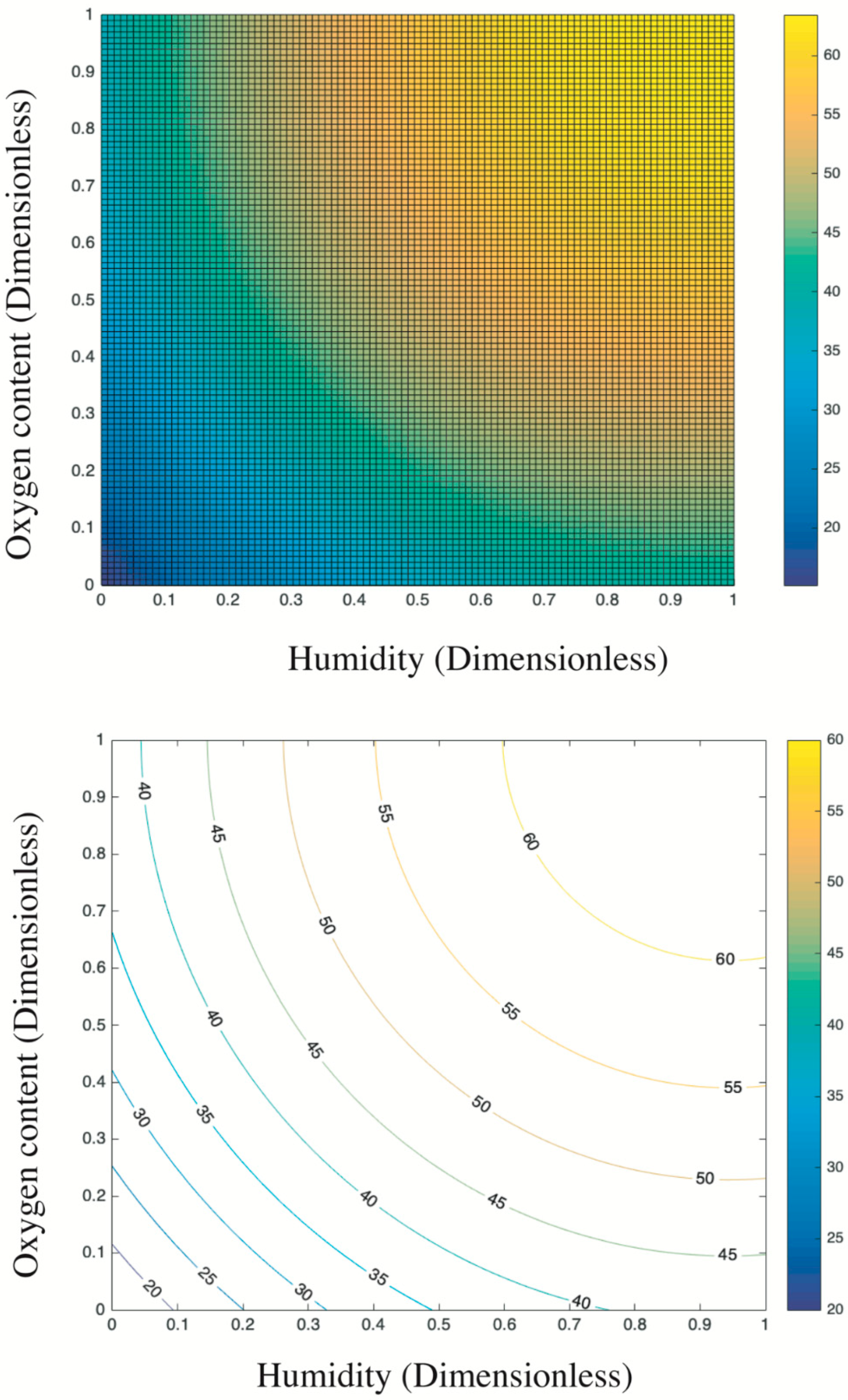
3.3. Integrated Effect of Oxygen Content and Humidity on the SO2 Removal
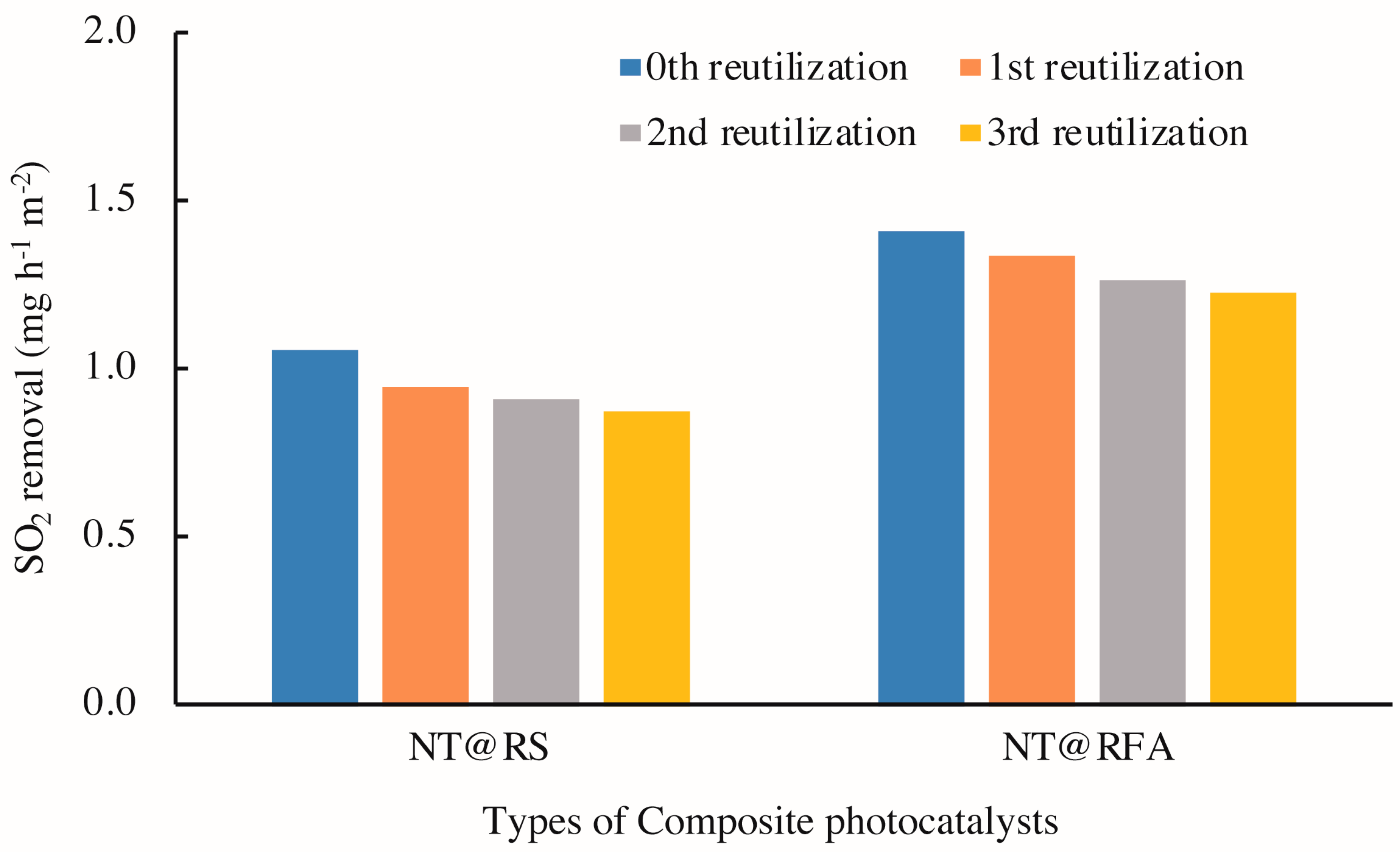
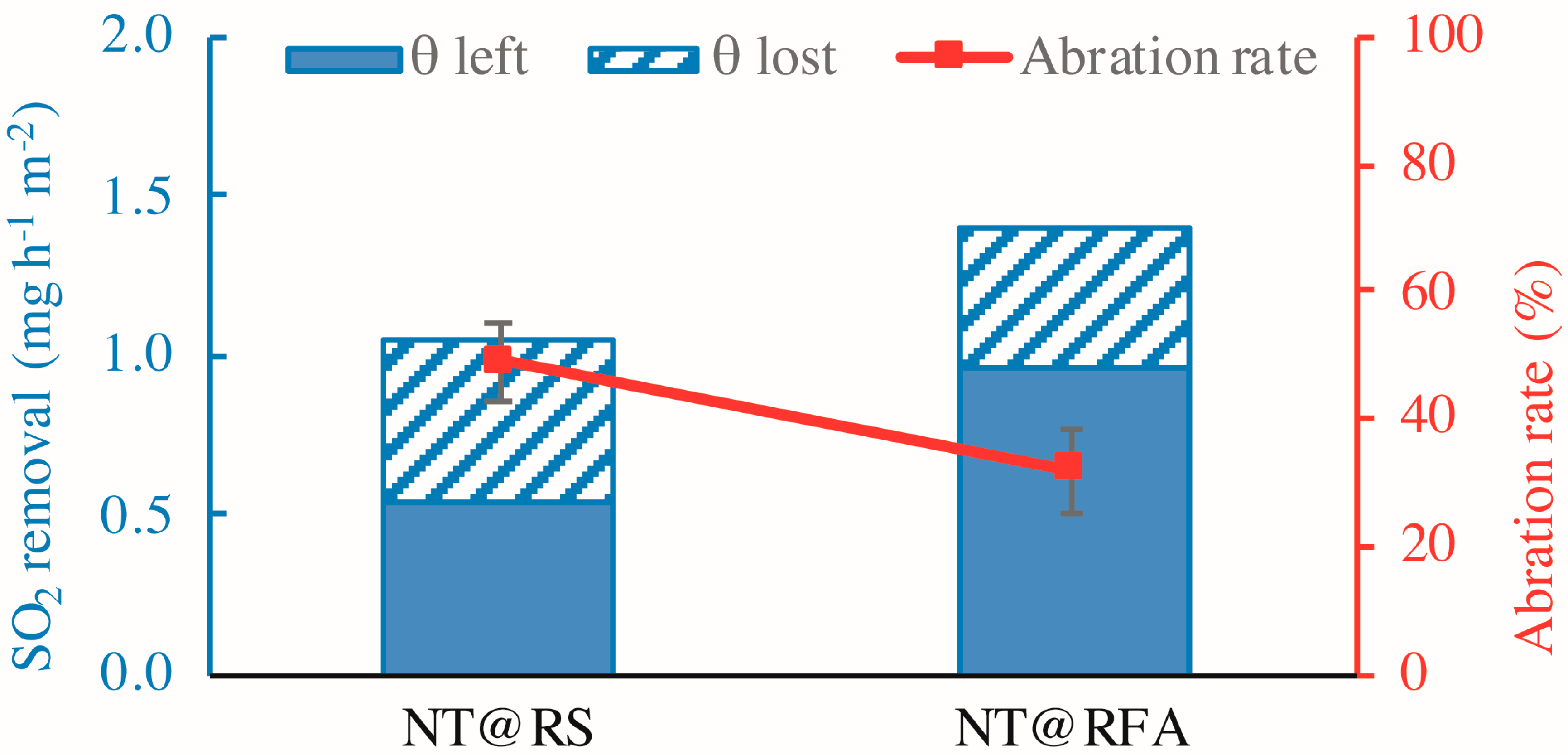
3.4. Reutilization and Durability of CPs
4. Conclusions
- (1)
- The prepared CPs increased the use value of C&DW because they endowed the waste materials with photocatalytic capability. In addition, the CPs facilitated the dispersion of NT within cementitious materials. This avoided agglomeration and provided a relatively larger surface area for effective UV irradiation.
- (2)
- The SO2 degradation comprised two processes of physiochemical absorption followed by photocatalytic removal, because the acidic SO2 could react with the alkaline new mortar and old mortar attached to RFA.
- (3)
- The sample containing NT@RFA achieved ~46% higher adsorption and ~24% higher total degradation than that with NT@RS because of its advantageous high alkalinity and high porosity.
- (4)
- Both reutilization and abrasion decreased the SO2 degradation capacities of all CPs. However, NT@RFA, because of its high porosity, showed relatively higher tolerance to weathering.
- (5)
- The final product, viz. the photocatalytic mortar, can be used in any place in the building, such as the wall, floor, or even roof.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, L.; Wu, H.; Zhang, H.; Duan, H.; Wang, J.; Jiang, W.; Dong, B.; Liu, G.; Zuo, J.; Song, Q. Characterizing the generation and flows of construction and demolition waste in China. Constr. Build. Mater. 2017, 136, 405–413. [Google Scholar] [CrossRef]
- Wu, H.; Duan, H.; Zheng, L.; Wang, J.; Niu, Y.; Zhang, G. Demolition waste generation and recycling potentials in a rapidly developing flagship megacity of South China: Prospective scenarios and implications. Constr. Build. Mater. 2016, 113, 618–1007. [Google Scholar] [CrossRef]
- Jelić, I.; Šljivić-Ivanović, M.; Dimović, S.; Antonijević, D.; Jović, M.; Mirković, M.; Smičiklas, I. The applicability of construction and demolition waste components for radionuclide sorption. J. Clean. Prod. 2018, 171, 322–332. [Google Scholar] [CrossRef]
- Huang, B.; Wang, X.; Kua, H.; Geng, Y.; Bleischwitz, R.; Ren, J. Construction and demolition waste management in China through the 3R principle. Resour. Conserv. Recycl. 2018, 129, 36–44. [Google Scholar] [CrossRef]
- Chen, X.-F.; Kou, S.-C.; Xing, F. Effect of Agriculture and Construction Wastes on the Properties of Magnesium Oxychloride Cement Mortar with Tourmaline Powder. Materials 2019, 12, 115. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Banfill, P.F.G.; de Rojas, M.I.S.; Frías, M. Rheological and calorimetric behaviour of cements blended with containing ceramic sanitary ware and construction/demolition waste. Constr. Build. Mater. 2013, 40, 822–831. [Google Scholar] [CrossRef]
- Carro-López, D.; González-Fonteboa, B.; de Brito, J.; Martínez-Abella, F.; González-Taboada, I.; Silva, P. Study of the rheology of self-compacting concrete with fine recycled concrete aggregates. Constr. Build. Mater. 2015, 96, 491–501. [Google Scholar] [CrossRef]
- Panizza, M.; Natali, M.; Garbin, E.; Tamburini, S.; Secco, M. Assessment of geopolymers with Construction and Demolition Waste (CDW) aggregates as a building material. Constr. Build. Mater. 2018, 181, 119–133. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R. Mechanical properties and durability of mortar containing fine fraction of demolition wastes produced by selective demolition in South Italy. Compos. Part B Eng. 2017, 115, 43–50. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar] [CrossRef]
- Smazna, D.; Shree, S.; Polonskyi, O.; Lamaka, S.; Baum, M.; Zheludkevich, M.; Faupel, F.; Adelung, R.; Mishra, Y.K. Mutual interplay of ZnO micro-and nanowires and methylene blue during cyclic photocatalysis process. J. Environ. Chem. Eng. 2019, 7, 103016. [Google Scholar] [CrossRef]
- Park, S.J.; Das, G.S.; Schütt, F.; Adelung, R.; Mishra, Y.K.; Tripathi, K.M.; Kim, T.Y. Visible-light photocatalysis by carbon-nano-onion-functionalized ZnO tetrapods: Degradation of 2,4-dinitrophenol and a plant-model-based ecological assessment. NPG Asia Mater. 2019, 11, 8. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.-S. Photocatalytic Cementitious Materials: Influence of the Microstructure of Cement Paste on Photocatalytic Pollution Degradation. Environ. Sci. Technol. 2009, 43, 8948–8952. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.-C.; Zhang, H.; Wang, Y. Durability of recycled aggregate concrete—A review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.C.; Poon, C.S. Photocatalytic cement-based materials: Comparison of nitrogen oxides and toluene removal potentials and evaluation of self-cleaning performance. Build. Environ. 2011, 46, 1827–1833. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Chen, J.; Xia, M.; Wang, T.; Poon, C.S. Pathways of conversion of nitrogen oxides by nano TiO2 incorporated in cement-based materials. Build. Environ. 2018, 144, 412–418. [Google Scholar] [CrossRef]
- Krishnan, P.; Zhang, M.-H.; Cheng, Y.; Riang, D.T.; Yu, L.E. Photocatalytic degradation of SO2 using TiO2-containing silicate as a building coating material. Constr. Build. Mater. 2013, 43, 197–202. [Google Scholar] [CrossRef]
- Faraldos, M.; Kropp, R.; Anderson, M.A.; Sobolev, K. Photocatalytic hydrophobic concrete coatings to combat air pollution. Catal. Today 2016, 259, 228–236. [Google Scholar] [CrossRef]
- Fernández-Catalá, J.; Cazorla-Amorós, D.; Berenguer-Murcia, Á. Facile encapsulation of P25 (TiO2) in spherical silica with hierarchical porosity with enhanced photocatalytic properties for gas-phase propene oxidation. Appl. Catal. A Gen. 2018, 564, 123–132. [Google Scholar] [CrossRef]
- Chen, X.-F.; Lin, S.-R.; Kou, S.-C. Effect of composite photo-catalysts prepared with recycled clay brick sands and nano-TiO2 on methyl orange and NOx removal. Constr. Build. Mater. 2018, 171, 152–160. [Google Scholar] [CrossRef]
- Mtarfi, N.H.; Rais, Z.; Taleb, M.; Kada, K.M. Effect of fly ash and grading agent on the properties of mortar using response surface methodology. J. Build. Eng. 2017, 9, 109–116. [Google Scholar] [CrossRef]
- Francioso, V.; Moro, C.; Martinez-Lage, I.; Velay-Lizancos, M. Curing temperature: A key factor that changes the effect of TiO2 nanoparticles on mechanical properties, calcium hydroxide formation and pore structure of cement mortars. Cem. Concr. Compos. 2019, 104, 103374. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Q.; Guo, R.; Wang, Z.; Liu, X.; Shi, X.; Qiu, Z.; Qin, H.; Jia, P.; Qin, Y.; et al. Removal of NO by using sodium persulfate/limestone slurry: Modeling by response surface methodology. Fuel 2019, 254, 115612. [Google Scholar] [CrossRef]
- Popović, S.; Karadžić, M.; Cakl, J. Optimization of ultrafiltration of cutting oil wastewater enhanced by application of twisted tapes: Response surface methodology approach. J. Clean. Prod. 2019, 231, 320–330. [Google Scholar] [CrossRef]
- Rivera, J.F.; Cristelo, N.; Fernández-Jiménez, A.; de Gutiérrez, R.M. Synthesis of alkaline cements based on fly ash and metallurgic slag: Optimisation of the SiO2/Al2O3 and Na2O/SiO2 molar ratios using the response surface methodology. Constr. Build. Mater. 2019, 213, 424–433. [Google Scholar] [CrossRef]
- Hammoudi, A.; Moussaceb, K.; Belebchouche, C.; Dahmoune, F. Comparison of artificial neural network (ANN) and response surface methodology (RSM) prediction in compressive strength of recycled concrete aggregates. Constr. Build. Mater. 2019, 209, 425–436. [Google Scholar] [CrossRef]
- Shaw, M.C. Engineering Statistics. In Engineering Problem Solving; Shaw, M.C., Ed.; William Andrew Publishing: Norwich, NY, USA, 2001; pp. 375–407. ISBN 978-0-8155-1447-3. [Google Scholar]
- Guo, M.Z.; Poon, C.S. Photocatalytic NO removal of concrete surface layers intermixed with TiO2. Build. Environ. 2013, 70, 102–109. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Chen, H.; Feng, P.; Ye, S.; Sun, W. The coupling effect of calcium concentration and pH on early hydration of cement. Constr. Build. Mater. 2018, 185, 391–401. [Google Scholar] [CrossRef]
- Shaw, A.C.; Romero, M.A.; Elder, R.H.; Ewan, B.C.R.; Allen, R.W.K. Measurements of the solubility of sulphur dioxide in water for the sulphur family of thermochemical cycles. Int. J. Hydrog. Energy 2011, 36, 4749–4756. [Google Scholar] [CrossRef]
- Kato, S.; Hirano, Y.; Iwata, M.; Sano, T.; Takeuchi, K.; Matsuzawa, S. Photocatalytic degradation of gaseous sulfur compounds by silver-deposited titanium dioxide. Appl. Catal. B Environ. 2005, 57, 109–115. [Google Scholar] [CrossRef]
- Long, W.-J.; Zheng, D.; Duan, H.; Han, N.; Xing, F. Performance enhancement and environmental impact of cement composites containing graphene oxide with recycled fine aggregates. J. Clean. Prod. 2018, 194, 193–202. [Google Scholar] [CrossRef]
- Seo, D.S.; Choi, H.B. Effects of the old cement mortar attached to the recycled aggregate surface on the bond characteristics between aggregate and cement mortar. Constr. Build. Mater. 2014, 59, 72–77. [Google Scholar] [CrossRef]
- Liu, H.; Yu, X.; Yang, H. The integrated photocatalytic removal of SO2 and NO using Cu doped titanium dioxide supported by multi-walled carbon nanotubes. Chem. Eng. J. 2014, 243, 465–472. [Google Scholar] [CrossRef]
- Kowsari, E.; Abdpour, S. Investigation performance of rod-like ZnO/CdO composites, synthesized in ionic liquid medium as photocatalytic for degradation of air pollutants (SO2 and NOX). Opt. Int. J. Light Electron Opt. 2016, 127, 11567–11576. [Google Scholar] [CrossRef]
- Xia, D.; Hu, L.; He, C.; Pan, W.; Yang, T.; Yang, Y.; Shu, D. Simultaneous photocatalytic elimination of gaseous NO and SO2 in a BiOI/Al2O3-padded trickling scrubber under visible light. Chem. Eng. J. 2015, 279, 929–938. [Google Scholar] [CrossRef]
- Lettieri, M.; Colangiuli, D.; Masieri, M.; Calia, A. Field performances of nanosized TiO2 coated limestone for a self-cleaning building surface in an urban environment. Build. Environ. 2019, 147, 506–516. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Zhang, J. Photochemical Removal of SO2 over TiO2-Based Nanofibers by a Dry Photocatalytic Oxidation Process. Energy Fuels 2017, 31, 9905–9914. [Google Scholar] [CrossRef]
- Wang, H.; You, C. Photocatalytic removal of low concentration SO2 by titanium dioxide. Chem. Eng. J. 2016, 292, 199–206. [Google Scholar] [CrossRef]
- Wang, H.; You, C.; Tan, Z. Enhanced photocatalytic oxidation of SO2 on TiO2 surface by Na2CO3 modification. Chem. Eng. J. 2018, 350, 89–99. [Google Scholar] [CrossRef]
- Shang, J.; Zhu, Y.; Du, Y.; Xu, Z. Comparative Studies on the Deactivation and Regeneration of TiO2 Nanoparticles in Three Photocatalytic Oxidation Systems: C7H16, SO2, and C7H16–SO2. J. Solid State Chem. 2002, 166, 395–399. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Zou, S.C.; Mak, C.L. Inhibition effect of SO2 on NOx and VOCs during the photodegradation of synchronous indoor air pollutants at parts per billion (ppb) level by TiO2. Appl. Catal. B Environ. 2004, 49, 187–193. [Google Scholar] [CrossRef]
- Liang, H.; Li, X.; Yang, Y.; Sze, K. Effects of dissolved oxygen, pH, and anions on the 2,3-dichlorophenol degradation by photocatalytic reaction with anodic TiO2 nanotube films. Chemosphere 2008, 73, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Kansal, S.K.; Sood, S.; Umar, A.; Mehta, S.K. Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J. Alloys Compd. 2013, 581, 392–397. [Google Scholar] [CrossRef]
- Wendt, S.; Matthiesen, J.; Schaub, R.; Vestergaard, E.K.; L\aegsgaard, E.; Besenbacher, F.; Hammer, B. Formation and Splitting of Paired Hydroxyl Groups on Reduced TiO2 (110). Phys. Rev. Lett. 2006, 96, 066107. [Google Scholar] [CrossRef] [PubMed]
- Henderson, M.A. A surface science perspective on photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Pettibone, J.; Grassian, V.H. Sulfur dioxide adsorption and photooxidation on isotopically-labeled titanium dioxide nanoparticle surfaces: Roles of surface hydroxyl groups and adsorbed water in the formation and stability of adsorbed sulfite and sulfate. Phys. Chem. Chem. Phys. 2012, 14, 6957–6966. [Google Scholar] [CrossRef]
- Munter, R. ChemInform Abstract: Advanced Oxidation Processes: Current Status and Prospects. ChemInform 2001, 32, 73–78. [Google Scholar] [CrossRef]
- Vorontsov, A.V.; Lion, C.; Savinov, E.N.; Smirniotis, P.G. Pathways of photocatalytic gas phase destruction of HD simulant 2-chloroethyl ethyl sulfide. J. Catal. 2003, 220, 414–423. [Google Scholar] [CrossRef]
- Poon, C.S.; Cheung, E. NO removal efficiency of photocatalytic paving blocks prepared with recycled materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Li, J.-S.; Poon, C.S. Improved photocatalytic nitrogen oxides removal using recycled glass-nano-TiO2 composites with NaOH pre-treatment. J. Clean. Prod. 2019, 209, 1095–1104. [Google Scholar] [CrossRef]

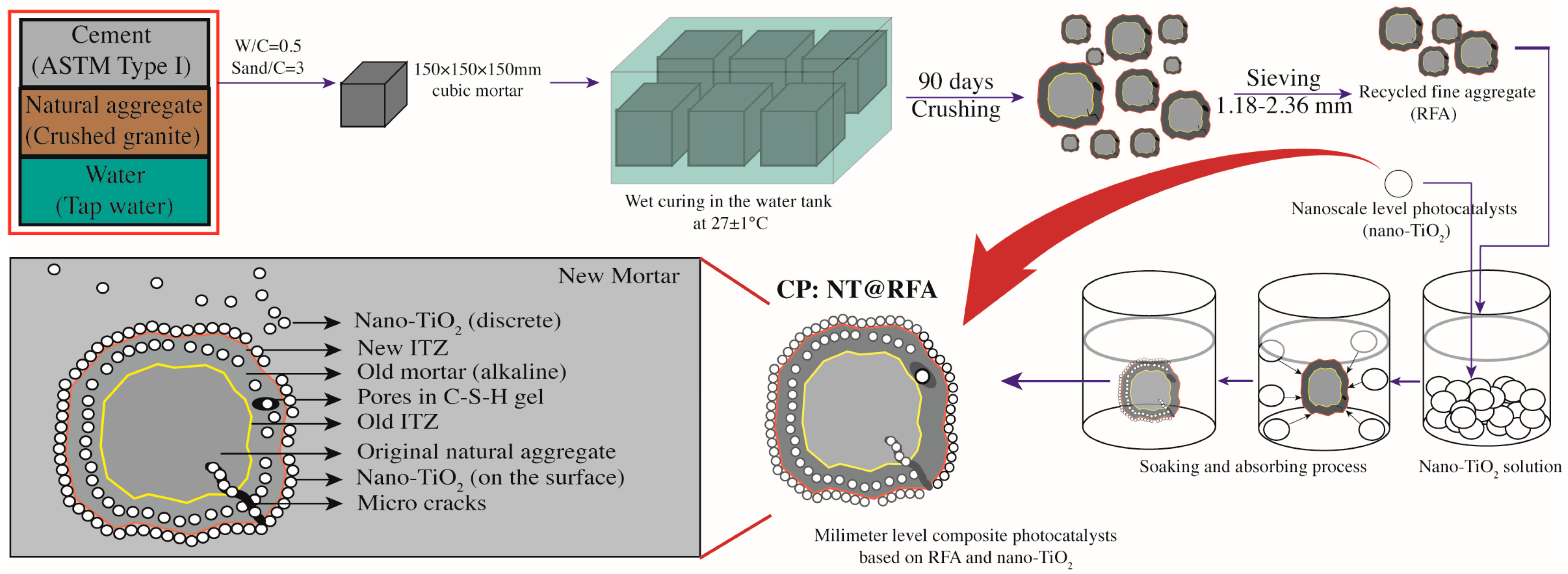
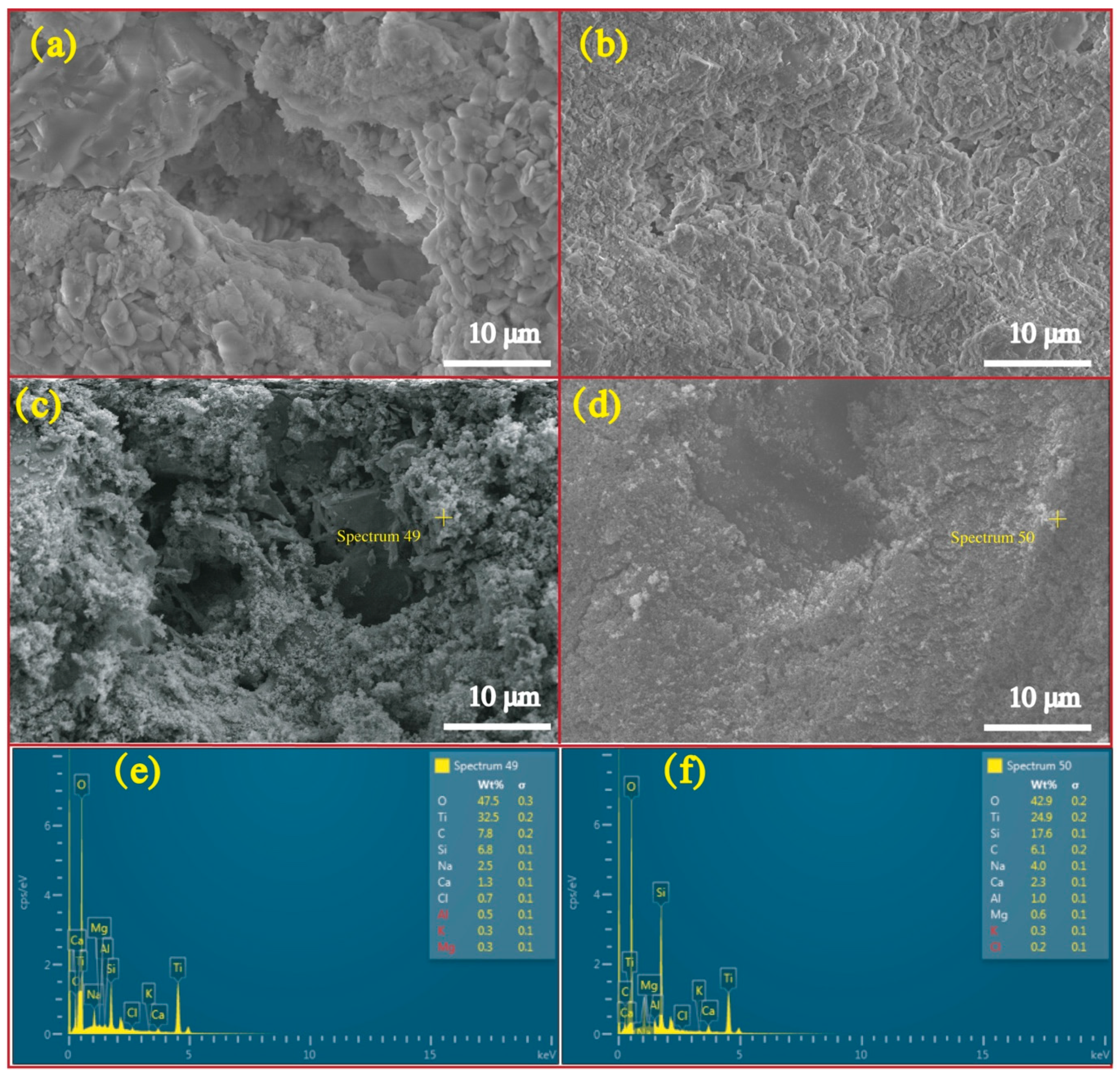
| Materials | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | LoI * | Density | Water Absorption | Size |
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (kg/m3) | (%) | (mm) | |
| Cement | 19.61 | 3.32 | 7.33 | 63.15 | 2.54 | 2.13 | 1.92 | 3160 | - | - |
| RFA | 59.63 | 4.66 | 16.3 | 14.33 | 1.5 | 1.43 | 2.15 | 2387 | 6.8 | 1.18–2.36 |
| RS | 96.18 | 0.06 | 2.76 | - | - | - | 1.00 | 2651 | 0.85 | 1.18–2.36 |
| Mix. Notation | RFA (g) | RS (g) | NT (P25) (g) | Water (mL) | P25 Absorption (g) |
|---|---|---|---|---|---|
| NT@RFA | 80 | - | 1.0 | 100 | 0.0023 (per gram of RFA) |
| NT@RS | - | 80 | 1.0 | 100 | 0.0015 (per gram of RS) |
| Cement | Water | NT@RFA | NT@RS | Replacement Ratio of NT@RFA over NT@RS (%) | |
|---|---|---|---|---|---|
| M1 | 1 | 0.5 | - | 2.5 | 0 |
| M2 | 0.625 | 1.875 | 25 | ||
| M3 | 1.25 | 1.25 | 50 | ||
| M4 | 1.875 | 0.625 | 75 | ||
| M5 | 2.5 | 0 | 100 |
| Mortar Notation | Oxygen Content (ppb) | Test | |||||
|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 600 | 800 | 1000 | SO2 Removal | |
| M5-0 | 3 | 3 | 3 | 3 | 3 | 3 | 18 |
| M5-1 | 3 | 3 | 3 | 3 | 3 | 3 | 18 |
| M5-2 | 3 | 3 | 3 | 3 | 3 | 3 | 18 |
| M5-3 | 3 | 3 | 3 | 3 | 3 | 3 | 18 |
| M5-4 | 3 | 3 | 3 | 3 | 3 | 3 | 18 |
| M5-5 | 3 | 3 | 3 | 3 | 3 | 3 | 18 |
| Total | 18 | 18 | 18 | 18 | 18 | 18 | 108 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-F.; Kou, S.-C. Sulfur Dioxide Degradation by Composite Photocatalysts Prepared by Recycled Fine Aggregates and Nanoscale Titanium Dioxide. Nanomaterials 2019, 9, 1533. https://doi.org/10.3390/nano9111533
Chen X-F, Kou S-C. Sulfur Dioxide Degradation by Composite Photocatalysts Prepared by Recycled Fine Aggregates and Nanoscale Titanium Dioxide. Nanomaterials. 2019; 9(11):1533. https://doi.org/10.3390/nano9111533
Chicago/Turabian StyleChen, Xue-Fei, and Shi-Cong Kou. 2019. "Sulfur Dioxide Degradation by Composite Photocatalysts Prepared by Recycled Fine Aggregates and Nanoscale Titanium Dioxide" Nanomaterials 9, no. 11: 1533. https://doi.org/10.3390/nano9111533
APA StyleChen, X.-F., & Kou, S.-C. (2019). Sulfur Dioxide Degradation by Composite Photocatalysts Prepared by Recycled Fine Aggregates and Nanoscale Titanium Dioxide. Nanomaterials, 9(11), 1533. https://doi.org/10.3390/nano9111533