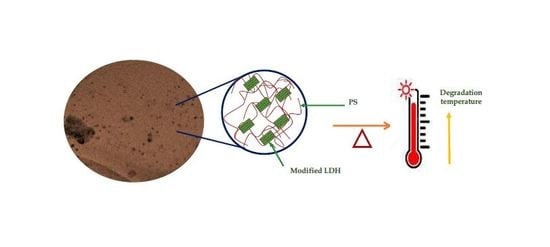
Thermal Stability Evaluation of Polystyrene-Mg/Zn/Al LDH Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LDH Synthesis
2.2.1. Co-Precipitation at Constant pH
2.2.2. Urea Hydrolysis
2.3. Thermal Treatments
2.4. Modification of LDHs with Anionic Surfactants
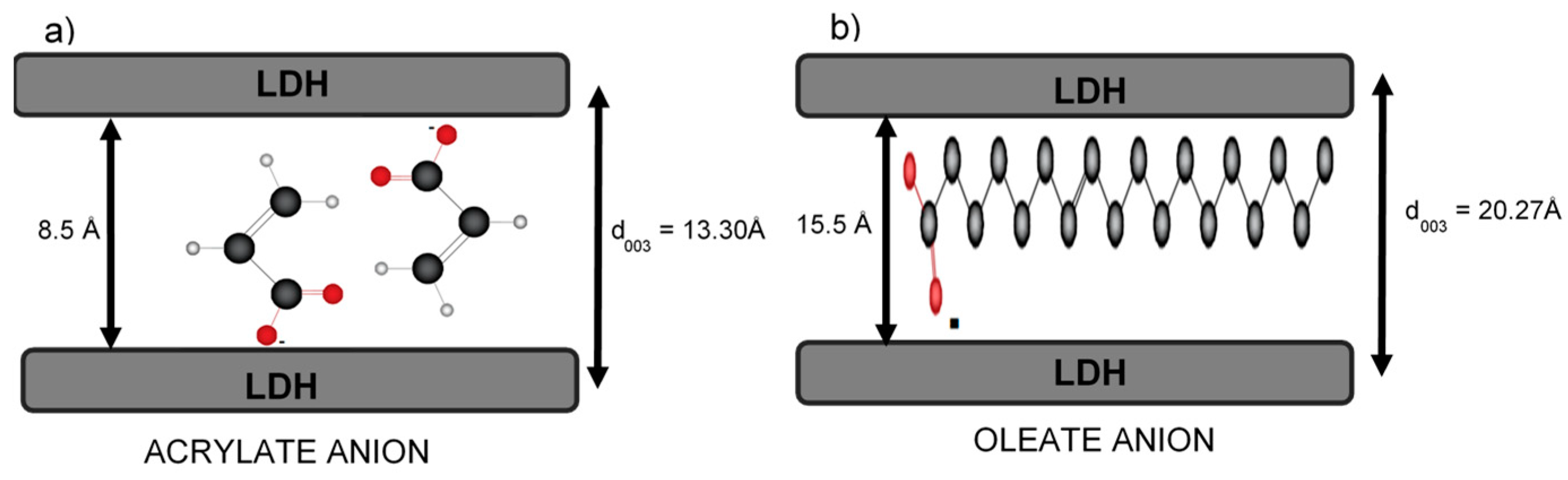
2.4.1. Acrylate Anion
2.4.2. Oleate Anion
2.5. Polystyrene Synthesis
2.6. Nanocomposite Preparation
2.7. Characterization and Measurements
3. Results and Discussion
3.1. XRD Results
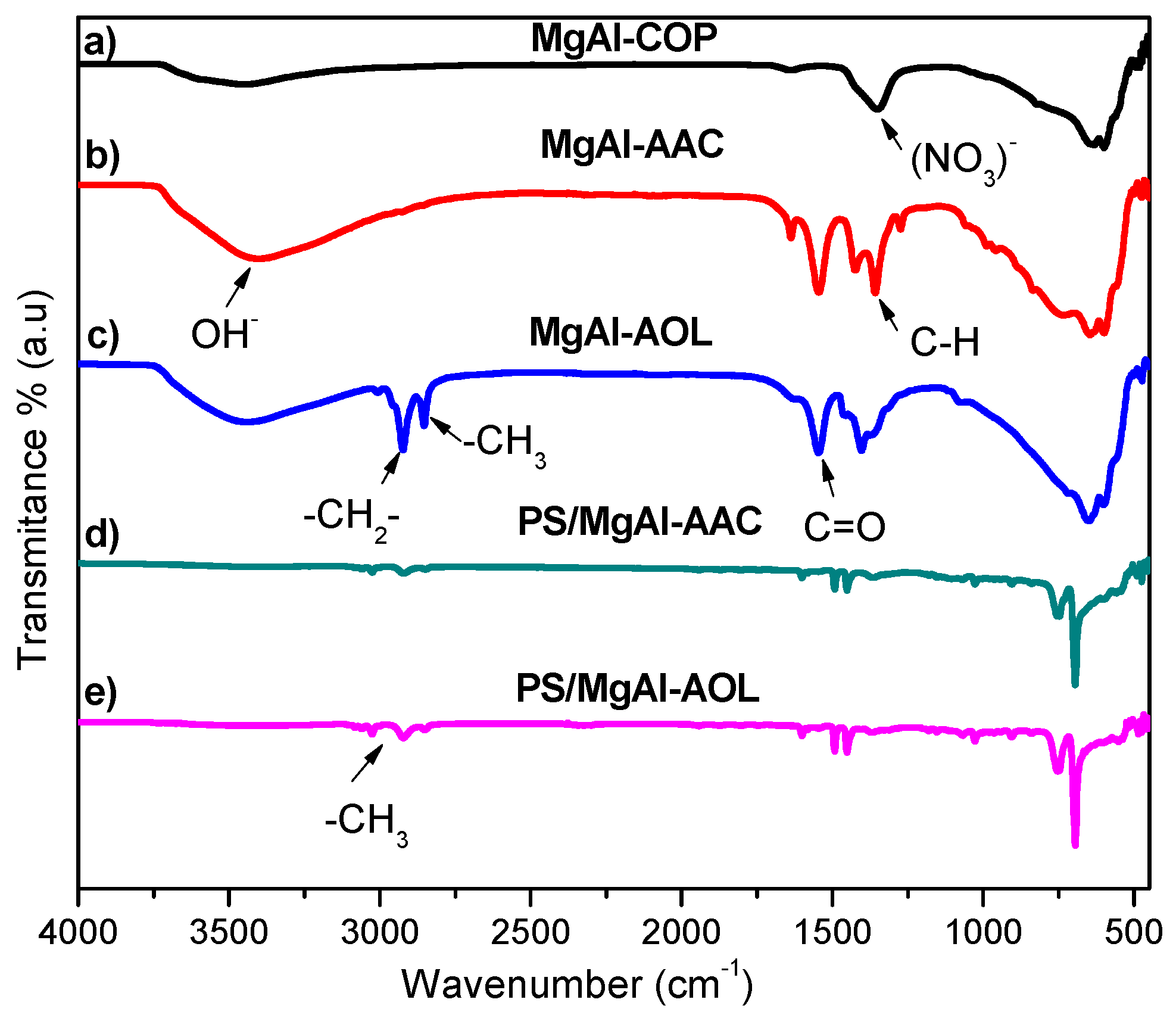
3.2. FTIR Spectroscopy
3.3. MALDI-TOF Mass Spectrometry
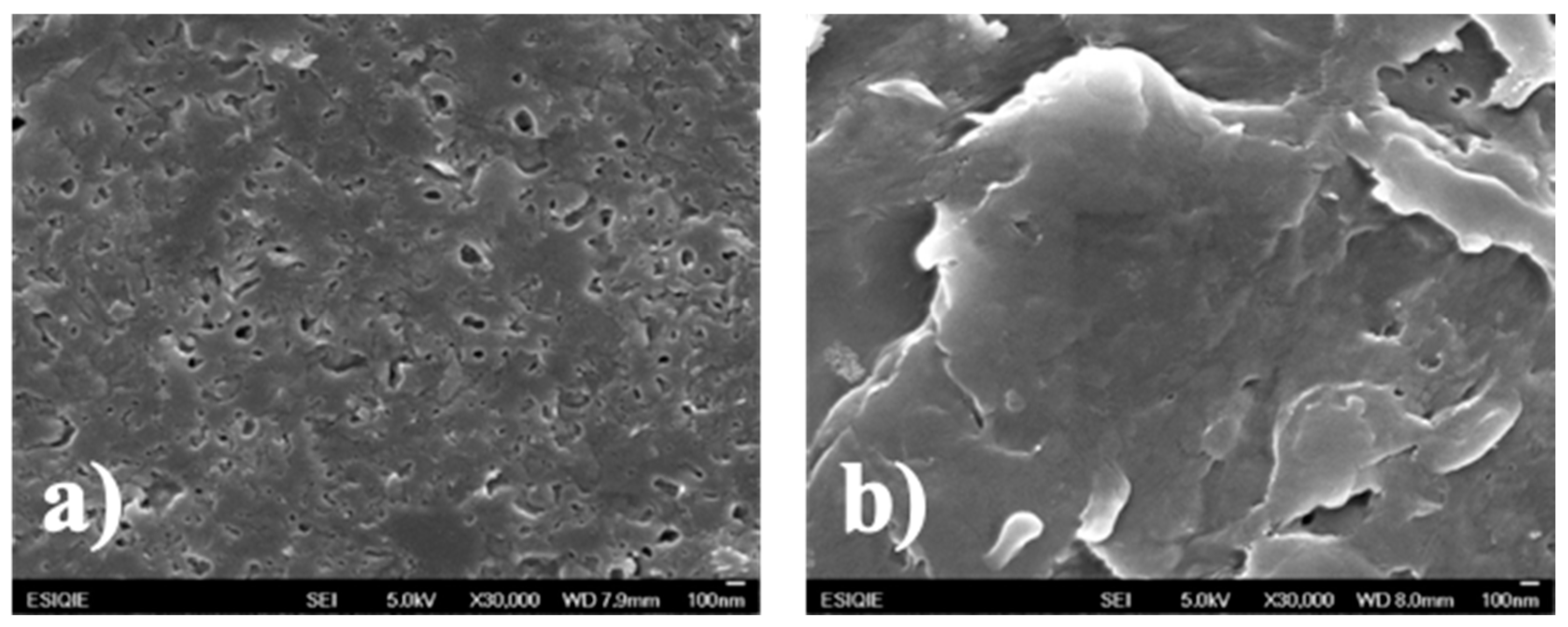
3.4. Scanning Electron Microscopy (SEM)
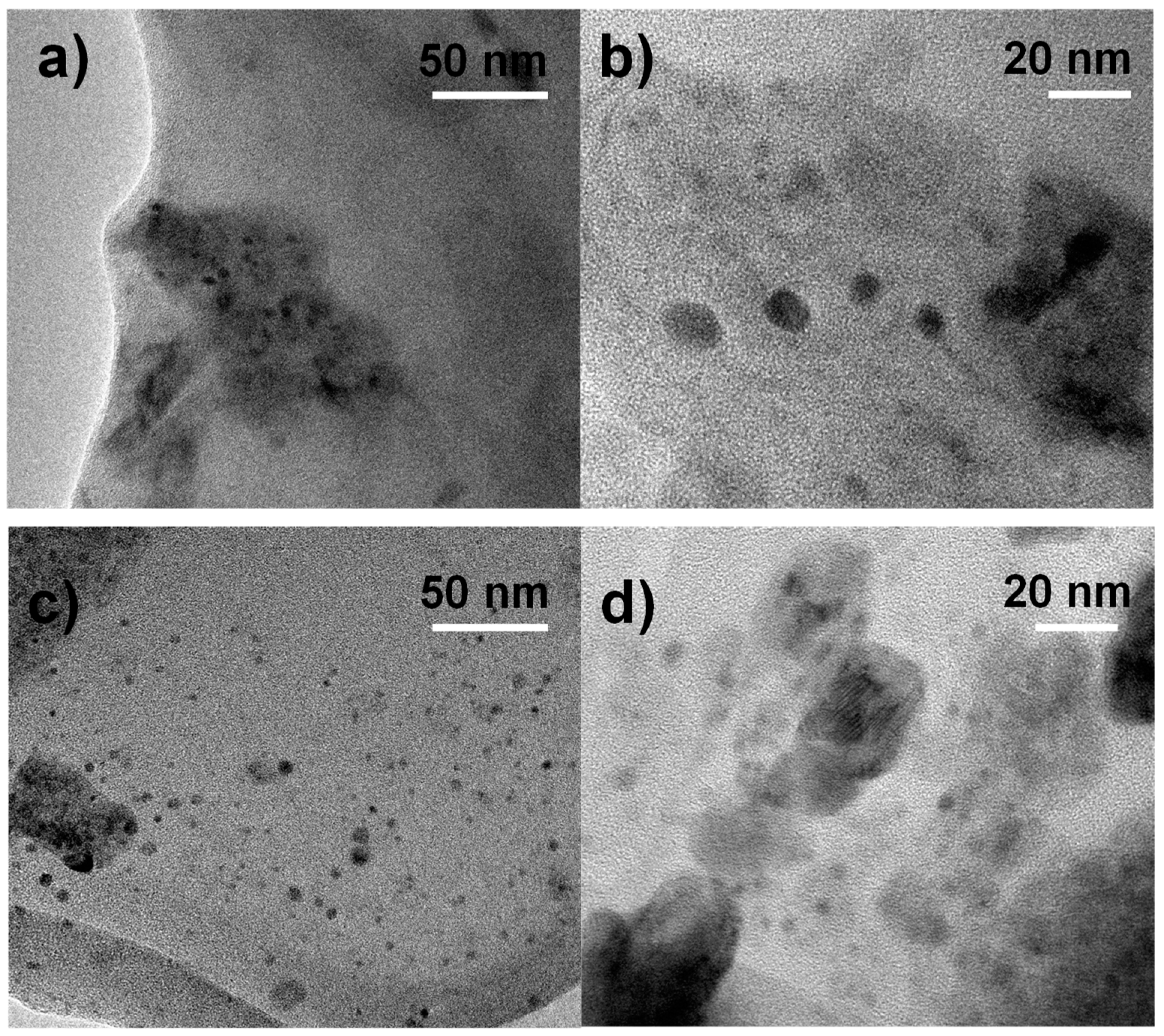
3.5. TEM
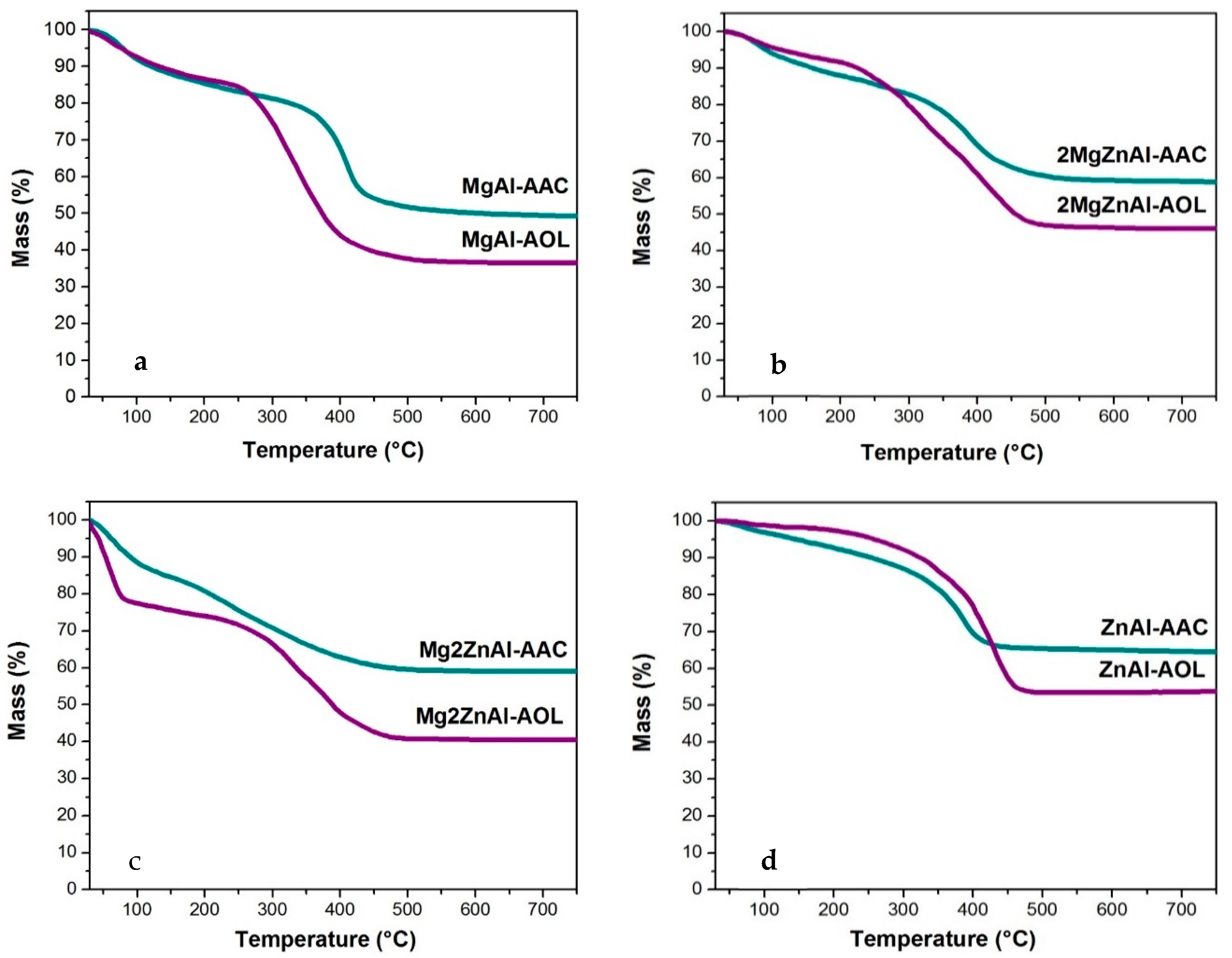
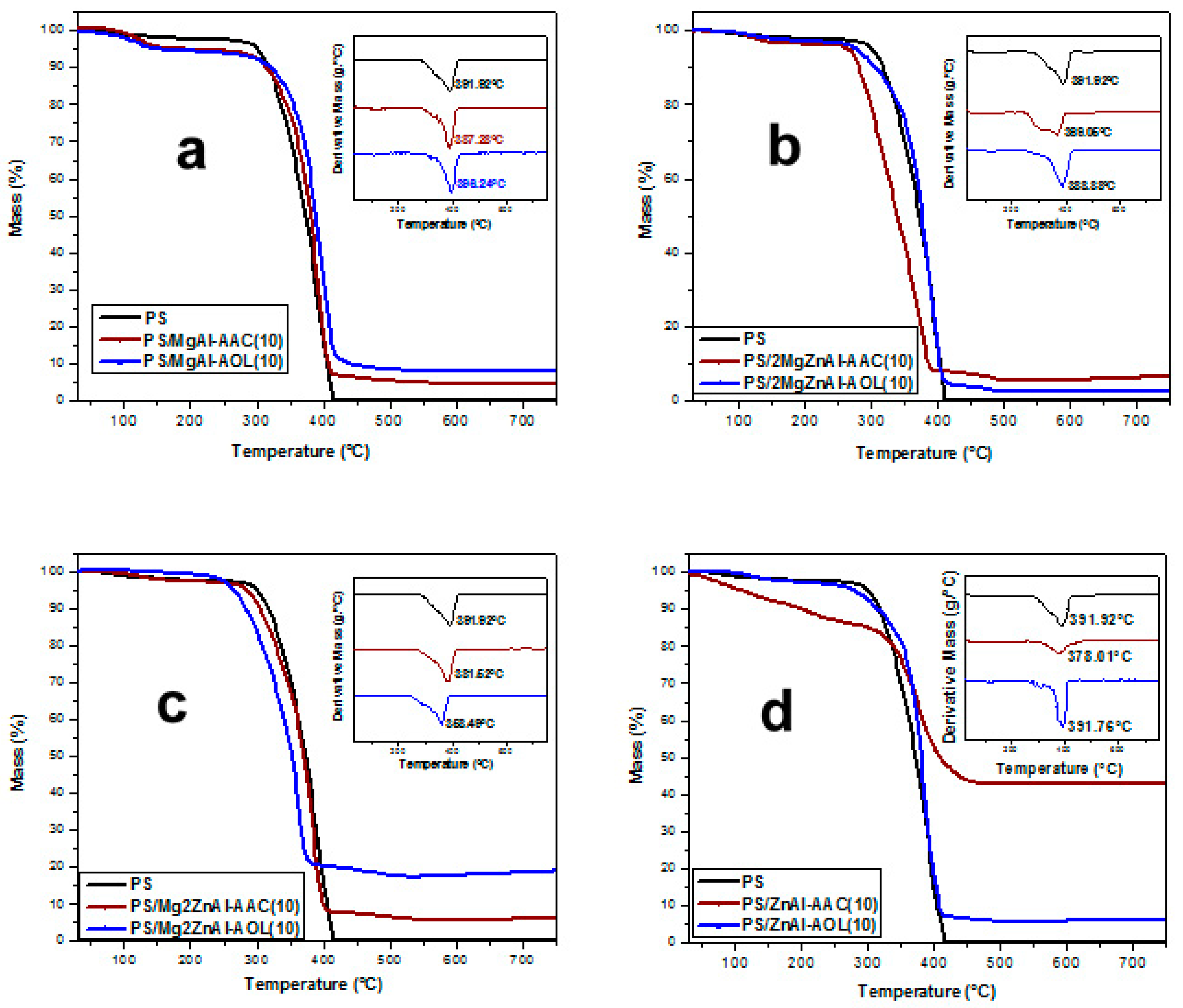
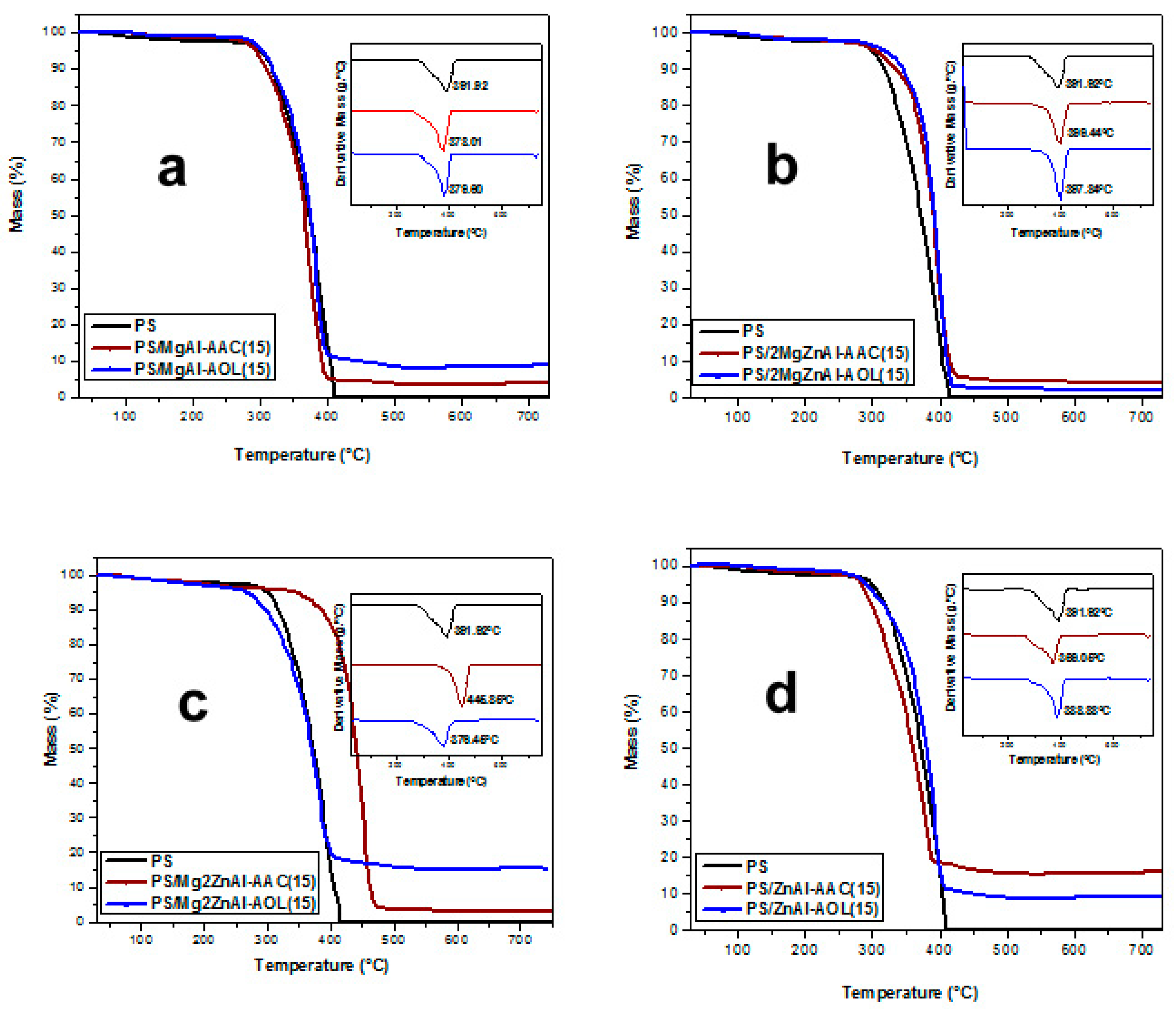
3.6. Thermal Analysis
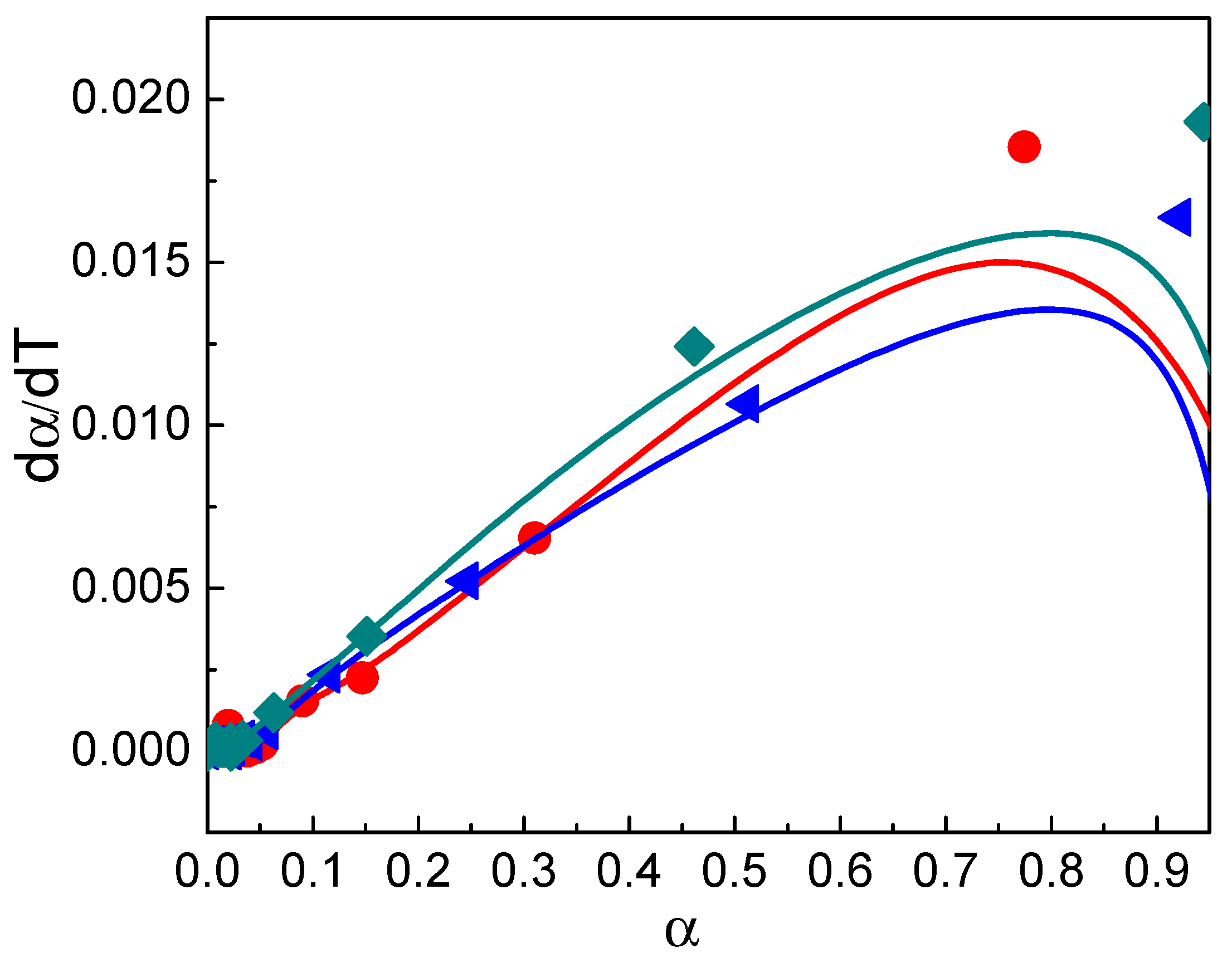
3.7. Decomposition Kinetic Analysis
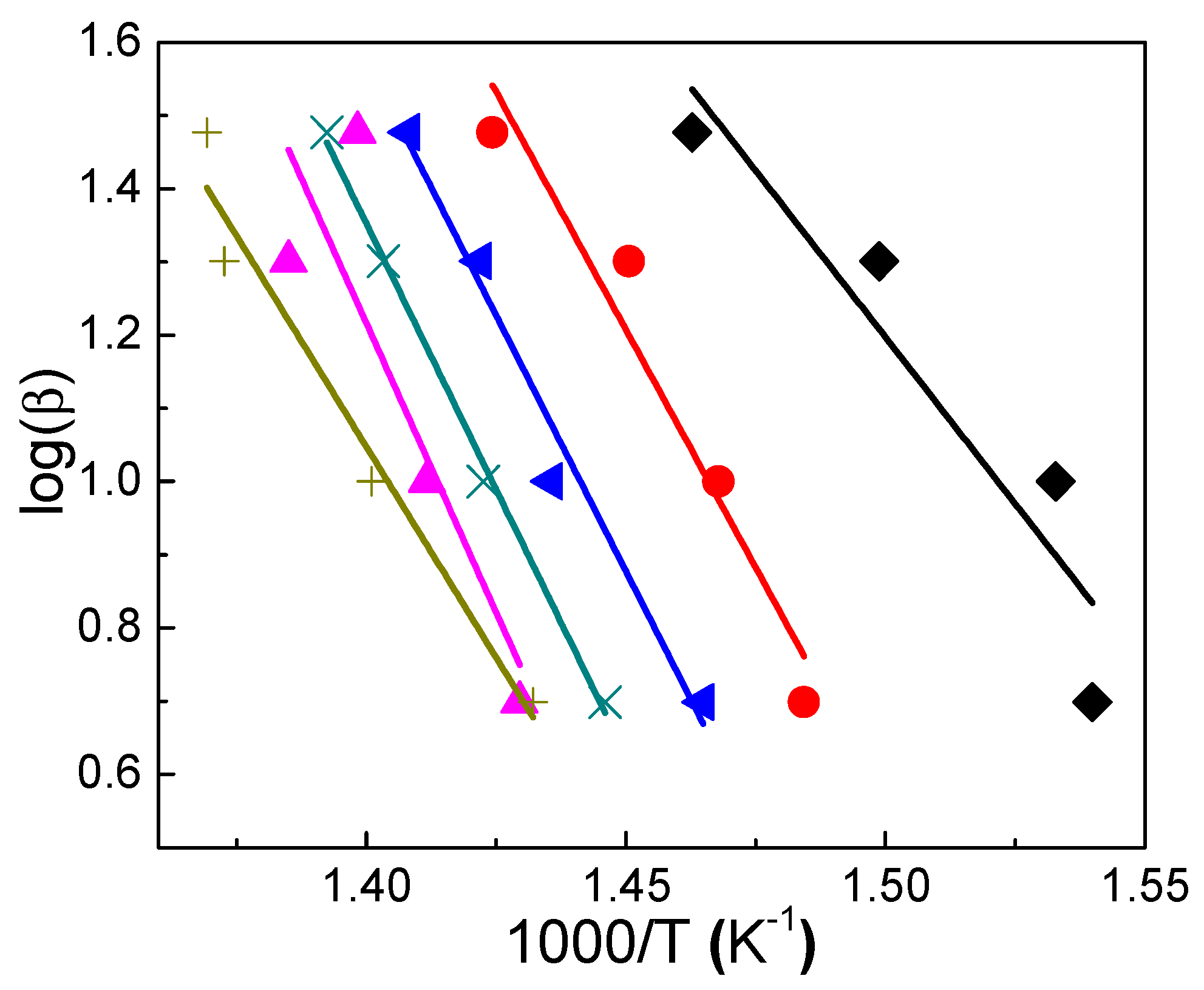
3.7.1. Friedman Method
3.7.2. Flynn–Wall–Ozawa Method
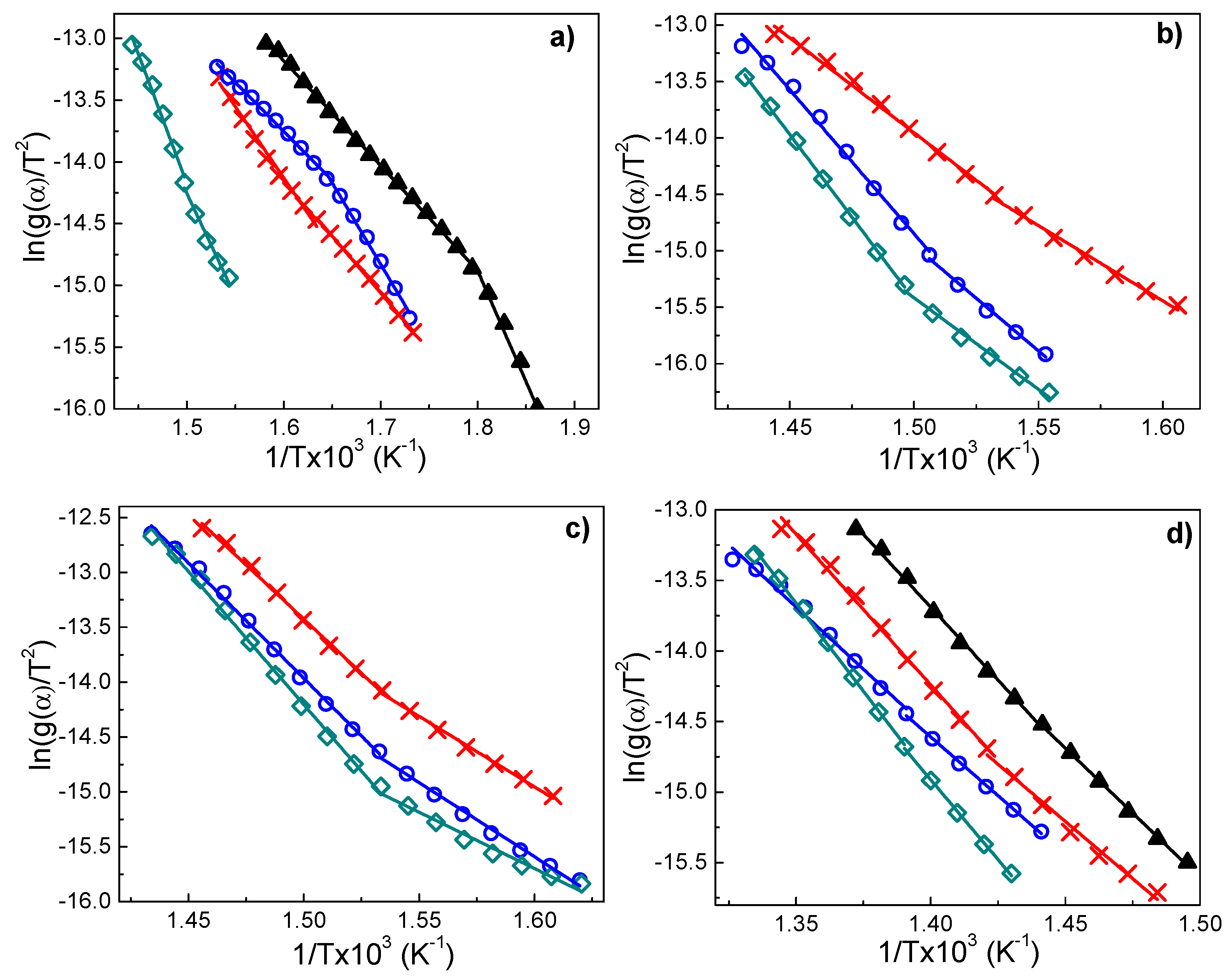
3.7.3. Coats–Redfern Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Edenharter, A.; Feicht, P.; Diar-Bakerly, B.; Beyer, G.; Breu, J. Superior flame retardant by combining high aspect ratio layered double hydroxide and graphene oxide. Polymer 2016, 91, 41–49. [Google Scholar] [CrossRef]
- Fromme, H.; Becher, G.; Hilge, B.; Völkel, W. Brominated flame retardants-Exposure and risk assessment for the general population. Int. J. Hyg. Environ. Health 2016, 219, 1–23. [Google Scholar] [CrossRef]
- Sun, G.; Liu, L.; Wang, J.; Wang, H.; Wang, W.; Han, S. Effects of hydrotalcites and tris (1-chloro-2-propyl) phosphate on thermal stability, cellular structure and fire resistance of isocyanate-based polyimide foams. Polym. Degrad. Stab. 2015, 115, 1–15. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Wang, Q.; Wilkie, C.A.; O’Hare, D. Flame retardant polymer/layered double hydroxide nanocomposites. J. Mater. Chem. A 2014, 2, 10996–11016. [Google Scholar] [CrossRef]
- Wang, D.Y.; Das, A.; Costa, F.R.; Leuteritz, A.; Wang, Y.Z.; Wagenknecht, U.; Heinrich, G. Synthesis of organo cobalt-aluminum layered double hydroxide via a novel single-step self-assembling method and its use as flame retardant nanofiller in PP. Langmuir 2010, 26, 14162–14169. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Hollingbery, L.A.; Hull, T.R. The fire retardant behaviour of huntite and hydromagnesite—A review. Polym. Degrad. Stab. 2010, 95, 2213–2225. [Google Scholar] [CrossRef]
- Suresh, K.; Kumar Vinoth, R.; Pugazhenthi, G. Processing and characterization of polystyrene nanocomposites based on CoAl layered double hydroxide. J. Sci. Adv. Mater. Devices 2016, 1, 351–361. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Su, S.; Chen, D.; Wilkie, CA. Variation of anions in layered double hydroxides: Effects on dispersion and fire properties. Polym. Degrad. Stab. 2009, 94, 770–781. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The thermal degradation of polystyrene nanocomposite. Polymer 2005, 46, 2933–2942. [Google Scholar] [CrossRef]
- Bera, O.; Pilić, B.; Pavličević, J.; Jovičić, M.; Holló, B.; Szécsényi, K.M.; Mészáros-Szécsényi, K.; Špirkova, M. Preparation and thermal properties of polystyrene/silica nanocomposites. Thermochim. Acta 2011, 515, 1–5. [Google Scholar] [CrossRef]
- Hood, M.A.; Mari, M.; Muñoz-Espí, R. Synthetic strategies in the preparation of polymer/inorganic hybrid nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.M.C.; Herrero, M.; Labajos, F.M.; Marques, A.T.; Rives, V. Preparation and properties of new flame retardant unsaturated polyester nanocomposites based on layered double hydroxides. Polym. Degrad. Stab. 2009, 94, 939–946. [Google Scholar] [CrossRef]
- Jiao, C.; Wang, Z.; Chen, X.; Yu, B.; Hu, Y. Irradiation crosslinking and halogen-free flame retardation of EVA using hydrotalcite and red phosphorus. Radiat. Phys. Chem. 2006, 75, 557–563. [Google Scholar] [CrossRef]
- Montañez, M.K.; Molina, R.; Moreno, S. Nickel catalysts obtained from hydrotalcites by coprecipitation and urea hydrolysis for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 8225–8237. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Qu, W.; Wang, B.; Tian, Z.; Ma, H.; Xu, R. The application of Zr incorporated Zn-Al dehydrated hydrotalcites as solid base in transesterification. Catal. Today 2014, 234, 161–166. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inorganica Chim. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Berber, M.R.; Hafez, I.H.; Minagawa, K.; Katoh, M.; Mori, T.; Tanaka, M. Uniform nanoparticles of hydrotalcite-like materials and their textural properties at optimized conditions of urea hydrothermal treatment. J. Mol. Struct. 2013, 1033, 104–112. [Google Scholar] [CrossRef]
- Pilch-Pitera, B.; Kędzierski, M.; Olejnik, E.; Zapotoczny, S. Structure and properties of polyurethane-based powder clear coatings systems modified with hydrotalcites. Prog. Org. Coat. 2016, 95, 120–126. [Google Scholar] [CrossRef]
- Soori, M.M.; Ghahramani, E.; Kazemian, H.; Al-Musawi, T.J.; Zarrabi, M. Intercalation of tetracycline in nano sheet layered double hydroxide: An insight into UV/VIS spectra analysis. J. Taiwan Inst. Chem. Eng. 2016, 63, 271–285. [Google Scholar] [CrossRef]
- Peng, H.; Tjiu, W.C.; Shen, L.; Huang, S.; He, C.; Liu, T. Preparation and mechanical properties of exfoliated CoAl layered double hydroxide (LDH)/polyamide 6 nanocomposites by in situ polymerization. Compos. Sci. Technol. 2009, 69, 991–996. [Google Scholar] [CrossRef]
- Mostafa, M.S.; Bakr, A.A.; Naggar, A.M.A.; Sultan, E.A. Water decontamination via the removal of Pb (II) using a new generation of highly energetic surface nano-material: Co+2 Mo+6 LDH. J. Colloid Interface Sci. 2016, 461, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.W.; Redfern, J.P. Thermogravimetric Analysis. Analyst 1963, 88, 906–924. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Gilbert, E.P.; Scholten, E. Organogel formation via supramolecular assembly of oleic acid and sodium oleate. RSC Adv. 2015, 5, 47466–47475. [Google Scholar] [CrossRef]
- Vaysse, C.; Guerlou-Demourgues, L.; Duguet, E.; Delmas, C. Acrylate intercalation and in situ polymerization in iron-, cobalt-, or manganese-substituted nickel hydroxides. Inorg Chem. 2003, 42, 4559–4567. [Google Scholar] [CrossRef]
- Crespo, I.; Barriga, C.; Ulibarri, M.A.; Gonza, G.; Malet, P.; Rives, V. An X-ray Diffraction and Absorption Study of the Phases Formed upon Calcination of Zn–Al–Fe Hydrotalcites. Chem. Mater. 2001, 13, 1518–1527. [Google Scholar] [CrossRef]
- Bezen, M.C.I.; Breitkopf, C.; Lercher, J.A. On the acid-base properties of Zn-Mg-Al mixed oxides. Appl. Catal. A Gen. 2011, 399, 93–99. [Google Scholar] [CrossRef]
- Smoláková, L.; Frolich, K.; Kocík, J.; Kikhtyanin, O.; Čapek, L. Surface properties of hydrotalcite-based Zn(Mg)Al oxides and their catalytic activity in aldol condensation of furfural with acetone. Ind. Eng. Chem. Res. 2017, 56, 4638–4648. [Google Scholar] [CrossRef]
- De La Rosa-Guzmán, M.A.; Martínez-Ortiz, M.J.; Castillo, N.; Ramírez-Salazar, C.A.; Méndez-Ceja, A.; Canseco-Morales, J.E. Spectroscopic and structural characterization of Pd nanoparticles supported on hydrotalcite-like materials. In Materials Characterization; Pérez-Campos, R., Contreras-Cuevas, A., Esparza-Muñoz, R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; p. 51. [Google Scholar]
- Inayat, A.; Klumpp, M.; Schwieger, W. The urea method for the direct synthesis of ZnAl layered double hydroxides with nitrate as the interlayer anion. Appl. Clay Sci. 2011, 51, 452–459. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Chimentão, R.J.; Barrabés, N.; Föttinger, K.; Gispert-Guirado, F.; Kleymenov, E.; Tichit, D.; Medina, F. Structure evolution of layered double hydroxides activated by ultrasound induced reconstruction. Appl. Clay Sci. 2013, 83–84, 1–11. [Google Scholar] [CrossRef]
- Kunde, L.B.; Gade, S.M.; Kalyani, V.S.; Gupte, S.P. Catalytic synthesis of chalcone and flavanone using Zn-Al hydrotalcite adhere ionic liquid. Catal. Commun. 2009, 10, 1881–1888. [Google Scholar] [CrossRef]
- Xu, S.; Liao, M.C.; Zeng, H.Y.; Zhang, Z.Q.; Liu, X.J.; Zhu, P.H. Ultrafine hydrotalcite particles prepared with novel technology to improve the flame retardancy of polypropylene. Appl. Clay Sci. 2015, 108, 215–221. [Google Scholar] [CrossRef]
- Kowalik, P.; Konkol, M.; Kondracka, M.; Próchniak, W.; Bicki, R.; Wiercioch, P. Memory effect of the CuZnAl-LDH derived catalyst precursor—In situ XRD studies. Appl. Catal. A Gen. 2013, 464–465, 339–347. [Google Scholar] [CrossRef]
- Miranda, L.D.L.; Bellato, C.R.; Fontes, M.P.F.; de Almeida, M.F.; Milagres, J.L.; Minim, L.A. Preparation and evaluation of hydrotalcite-iron oxide magnetic organocomposite intercalated with surfactants for cationic methylene blue dye removal. Chem. Eng. J. 2014, 254, 88–97. [Google Scholar] [CrossRef]
- Heredia, A.C.; Oliva, M.I.; Zandalazini, C.I.; Aguú, U.A.; Eimer, G.A.; Casuscelli, S.G.; Herrero, E.R.; Pérez, C.F.; Crivello, M.E. Synthesis, Characterization, and Catalytic Behavior of Mg–Al–Zn–Fe Mixed Oxides from Precursors Layered Double Hydroxide. Ind. Eng. Chem. Res. 2011, 50, 6695–6703. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Korneeva, E.V.; Bondareva, V.M.; Glazneva, T.S. Gas-phase dehydration of glycerol over calcined tungsten-modified Zn-Al-O hydrotalcite-type catalysts. J. Mol. Catal. A Chem. 2015, 408, 98–106. [Google Scholar] [CrossRef]
- Carriazo, D.; Del Arco, M.; García-López, E.; Marc, G.; Martín, C.; Palmisano, L. Zn,Al hydrotalcites calcined at different temperatures: Preparation, characterization and photocatalytic activity in gas-solid regime. J. Mol. Catal. A Chem. 2011, 342–343, 83–90. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Tanaka, M.; Park, I.Y.; Kuroda, K.; Kato, C. Formation of Hydrotalcite-Acrylate Compounds Their Heat-Treated Products. Bull. Chem. Soc. Jpn. 1989, 62, 3442–3445. [Google Scholar] [CrossRef]
- Aguzzi, A.; Ambrogi, V.; Costantino, U.; Marmottini, F. Intercalation of acrylate anions into the galleries of Zn-Al layered double hydroxide. J. Phys. Chem. Solids 2007, 68, 808–812. [Google Scholar] [CrossRef]
- Viéville, J.; Tanty, M.; Delsuc, M.A. Polydispersity index of polymers revealed by DOSY NMR. J. Magn. Reson. 2011, 212, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Lynd, N.A.; Meuler, A.J.; Hillmyer, M.A. Polydispersity and block copolymer self-assembly. Prog. Polym. Sci. 2008, 33, 875–893. [Google Scholar] [CrossRef]
- Li, L. Maldi Mass Spectrometry for Synthetic Polymer Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 175, pp. 1–9. [Google Scholar]
- Rogosic, M.; Mencer, H.J.; Gomzi, Z. Polydispersity index and molecular weight distributions of polymers. Eur. Polym. J. 1996, 32, 1337–1344. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Yahyaoui, R.; Sánchez-Jimenez, P.E.; Pérez-Maqueda, L.A.; Nahdi, K.; Criado-Luque, J.M. Synthesis, characterization and combined kinetic analysis of thermal decomposition of hydrotalcite (Mg6Al2(OH)16CO3·4H2O). Thermochim. Acta 2018, 667, 177–184. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Abelló, S. Thermal decomposition of hydrotalcite-like compounds studied by a novel tapered element oscillating microbalance (TEOM). Comparison with TGA and DTA. Thermochim. Acta 2006, 444, 75–82. [Google Scholar] [CrossRef]
- Reverón, H.; Gutiérrez-Campos, D.; Rodríguez, R.M.; Bonassin, J.C.; Yahyaoui, R.; Sánchez-Jimenez, P.E.; Pérez-Maqueda, L.A.; Nahdi, K.; Criado-Luque, J.M. Chemical synthesis and thermal evolution of MgAl2O4 spinel precursor prepared from industrial gibbsite and magnesia powder. Thermochim. Acta 2006, 667, 75–82. [Google Scholar] [CrossRef]
- Oliveira-Corrêa, C.L.; Licea, Y.E.; Palacio, L.A.; Zanon-Zotin, F.M. Effect of the composition and thermal treatment in catalysts derived from Cu-Al hydrotalcites-like compounds in the NO reduction by CO. Catal. Today 2016, 289, 1–10. [Google Scholar] [CrossRef]
- Aristizábal, A.; Kolafa, M.; Contreras, S.; Domínguez, M.; Llorca, J.; Barrabés, N.; Tichit, D.; Medina, F. Catalytic activity and characterization of Pt/calcined CuZnAl hydrotalcites in nitrate reduction reaction in water. Catal. Today 2011, 175, 370–379. [Google Scholar] [CrossRef]
- Yei, D.R.; Fu, H.K.; Chang, Y.H.; Kuo, S.W.; Huang, J.M.; Chang, F.C. Thermal properties of polystyrene nanocomposites formed from rigid intercalation agent-treated montmorillonite. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 1781–1787. [Google Scholar] [CrossRef]
- Li, S.; Bhushan, B. Lubrication performance and mechanisms of Mg/Al-, Zn/Al-, and Zn/Mg/Al-layered double hydroxide nanoparticles as lubricant additives. Appl. Surf. Sci. 2016, 378, 308–319. [Google Scholar] [CrossRef]
- Zejiang, Z.; Chenghua, X.; Fali, Q.; Xiujuan, M.; Bin, L.; Shuosheng, Z. Study on fire-retardant nanocrystalline Mg-Al layered double hydroxides synthesized by micro-wave-crystallization method. Sci. China Ser. B 2004, 47, 488–498. [Google Scholar]
- Ahmed, L.; Zhang, B.; Hawkins, S.; Sam-Mannan, M.; Cheng, Z. Study of thermal and mechanical behaviors of flame retardant polystyrene-based nanocomposites prepared via in-situ polymerization method. J. Loss Prev. Process Ind. 2017, 49, 228–239. [Google Scholar] [CrossRef]
- Zonglin, Z.; Yongqin, H.; Tingxi, L.; Xiaoxiao, W.; Qing, L.; Liang, C. Polyaniline/montmorillonite nanocomposites as an effective flame retardant and smoke suppressant for polystyrene. Synth. Met. 2016, 221, 28–38. [Google Scholar]
- Chen, G.; He, S.; Cheng, Z.; Guan, Y.; Yan, B.; Ma, W.; Leung, D.Y.C. Comparison of kinetic analysis methods in thermal decomposition of cattle manure by themogravimetric analysis. Bioresour. Technol. 2017, 243, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Criado, J.M.; Otega, A.; Rouquerol, J.; Rouquerol, F. Un nuevo método de análisis térmico: El analisis térmico a velocidad de transformación controlada. Bol. Soc. Española Cerámica Vidr. 1986, 26, 3–11. [Google Scholar]
- Singh, A.; Soni, P.K.; Singh, M.; Srivastava, A. Thermal degradation, kinetic and correlation models of poly(vinylidene fluoride-chlorotrifluoroetheylene) copolymers. Thermochim. Acta 2012, 548, 88–92. [Google Scholar] [CrossRef]
- Ebrahimi-Kahrizsangi, R.; Abbasi, M.H. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans. Nonferrous Met. Soc. China 2008, 18, 217–221. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Parres, F. Aplicación de modelos matemáticos para el estudio de degradación térmica de polímeros. Model. Sci. Educ. Learn. 2013, 6, 119–130. [Google Scholar] [CrossRef]
- Majoni, S.; Chaparadza, A. Thermal degradation degradation kinetic study of polystyrene/organophosphate composite. Thermochim. Acta 2018, 662, 8–15. [Google Scholar] [CrossRef]
- Costantino, U.; Gallipoli, A.; Nocchetti, M.; Camino, G.; Bellucci, F.; Frache, A. New nanocomposites constituted of polyethylene and organically modified ZnAl-hydrotalcites. Polym. Degrad. Stab. 2005, 90, 586–590. [Google Scholar] [CrossRef]
- Font, R.; Garrido, M.A. Friedman and n-reaction order methods applied to pine needles and polyurethane thermal decompositions. Thermochim. Acta 2018, 660, 124–133. [Google Scholar] [CrossRef]
- Venkatesh, M.; Ravi, P.; Tewari, S.P. Isoconversional kinetic analysis of decomposition of nitroimidazoles: Friedman method vs Flynn-Wall-Ozawa method. J. Phys. Chem. A 2013, 117, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Bercic, G. The universality of Friedman’s isoconversional analysis results in a model-less prediction of thermodegradation profiles. Thermochim. Acta 2017, 650, 1–7. [Google Scholar] [CrossRef]
- Huidobro, J.A.; Iglesias, I.; Alfonso, B.F.; Espina, A.; Trobajo, C.; Garcia, J.R. Reducing the effects of noise in the calculation of activation energy by the Friedman method. Chemom. Intell. Lab. Syst. 2016, 151, 146–152. [Google Scholar] [CrossRef]
- Muhamad-Bisyrul.Hafi, O.; Zulkifli, A.; Hasnan, O.; Mohd-Firdaus, O.; Hazizan, A.; Muhamad-Bisyrul. Thermal degradation behavior of a flame retardant melamine derivative hyperbranched polyimide with different terminal groups. RSC Adv. 2015, 5, 92664–92676. [Google Scholar]
- Hu, J.; Chen, M.; Tian, H.; Deng, W. Preparation and pyrolysis characteristics of PNIPAM-grafted SiO2 hollow spheres loading vitamin C. RSC Adv. 2015, 5, 81134–81141. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Luo, L.; Zhang, H.; Jiang, X. Pyrolysis of superfine pulverized coal. Part 5. Thermogravimetric analysis. Energy Convers. Manag. 2017, 154, 491–502. [Google Scholar] [CrossRef]
- Nyambo, C.; Wilkie, C.A. Layered double hydroxides intercalated with borate anions: Fire and thermal properties in ethylene vinyl acetate copolymer. Polym. Degrad. Stab. 2009, 94, 506–512. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Lin, Y.; Li, R.; Zhang, F. Layered double hydroxides used as flame retardant for engineering plastic acrylonitrile–butadiene–styrene (ABS). J. Phys. Chem. Solids 2012, 73, 1514–1517. [Google Scholar] [CrossRef]
- Kaspersma, J.; Doumen, C.; Munro, S.; Prins, A.M. Fire retardant mechanism of aliphatic bromine compounds in polystyrene and polypropylene. Polym. Degrad. Stab. 2002, 77, 325–331. [Google Scholar] [CrossRef]
- Fernandez, A.; Mazza, G.; Rodriguez, R. Thermal decomposition under oxidative atmosphere of lignocellulosic wastes: Different kinetic methods application. J. Environ. Chem. Eng. 2018, 6, 404–415. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Hao, J.; Qiao, Y.; Tian, Y. Thermal degradation of typical plastics under high heating rate conditions by TG-FTIR: Pyrolysis behaviors and kinetic analysis. Energy. Convers. Manag. 2018, 171, 1106–1115. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Q.; Chen, T.; Lu, T. Preparation and thermal decomposition of PS/Ni microspheres. Mater. Chem. Phys. 2009, 113, 428–434. [Google Scholar] [CrossRef]
- Ge, J.; Wang, R.Q.; Liu, L. Study on the Thermal Degradation Kinetics of the Common Wooden Boards. Procedia Eng. 2016, 135, 72–82. [Google Scholar] [CrossRef]
- Nyambo, C.; Chen, D.; Su, S.; Wilkie, C.A. Does organic modification of layered double hydroxides improve the fire performance of PMMA? Polym. Degrad. Stab. 2009, 94, 1298–1306. [Google Scholar] [CrossRef]
- Azizul-Haque, M.; Paliwal, L.J. Synthesis, spectral characterization and thermal aspects of coordination polymers of some transition metal ions with adipoyl bis(isonicotinoylhydrazone). J. Mol. Struct. 2017, 1134, 278–291. [Google Scholar] [CrossRef]
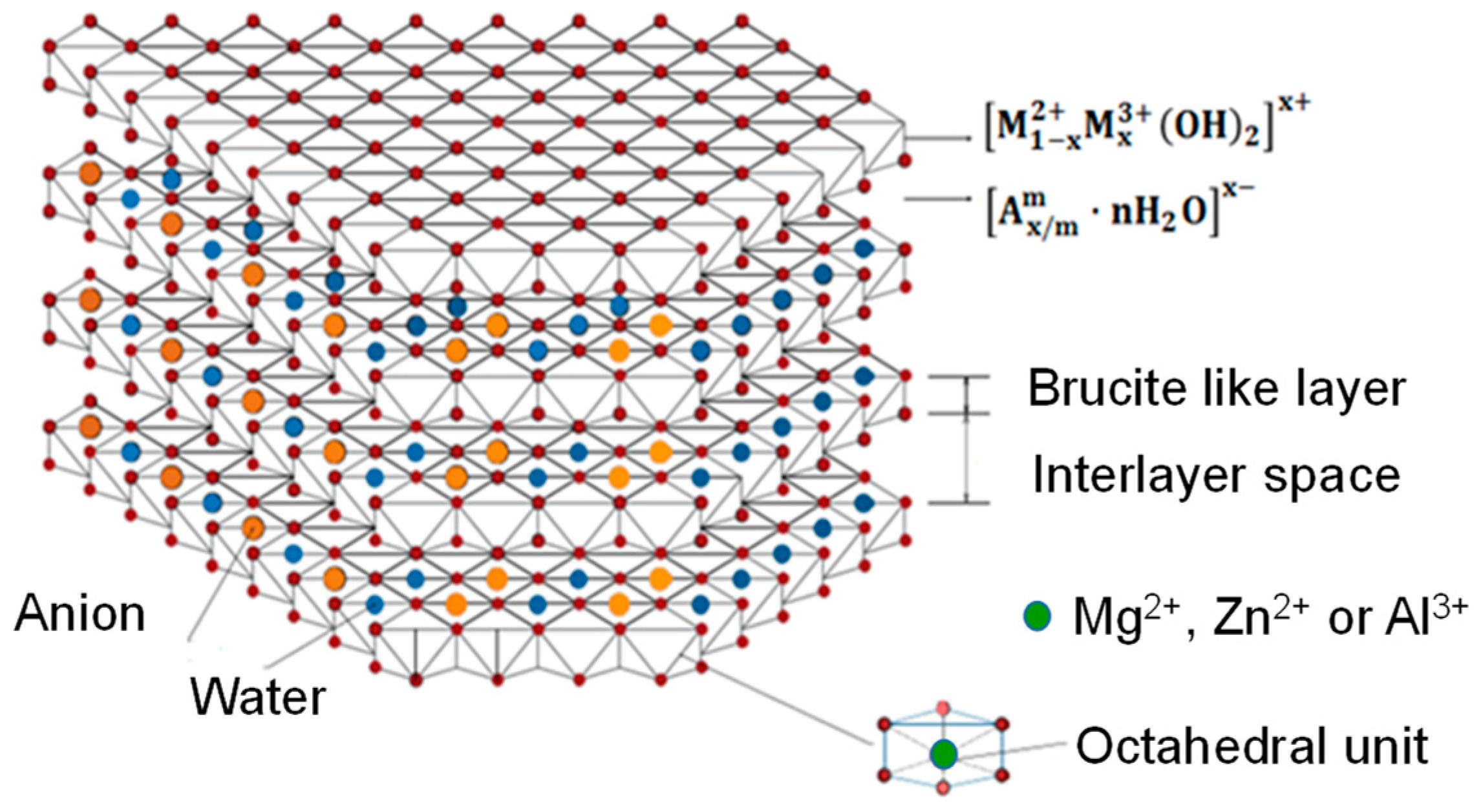
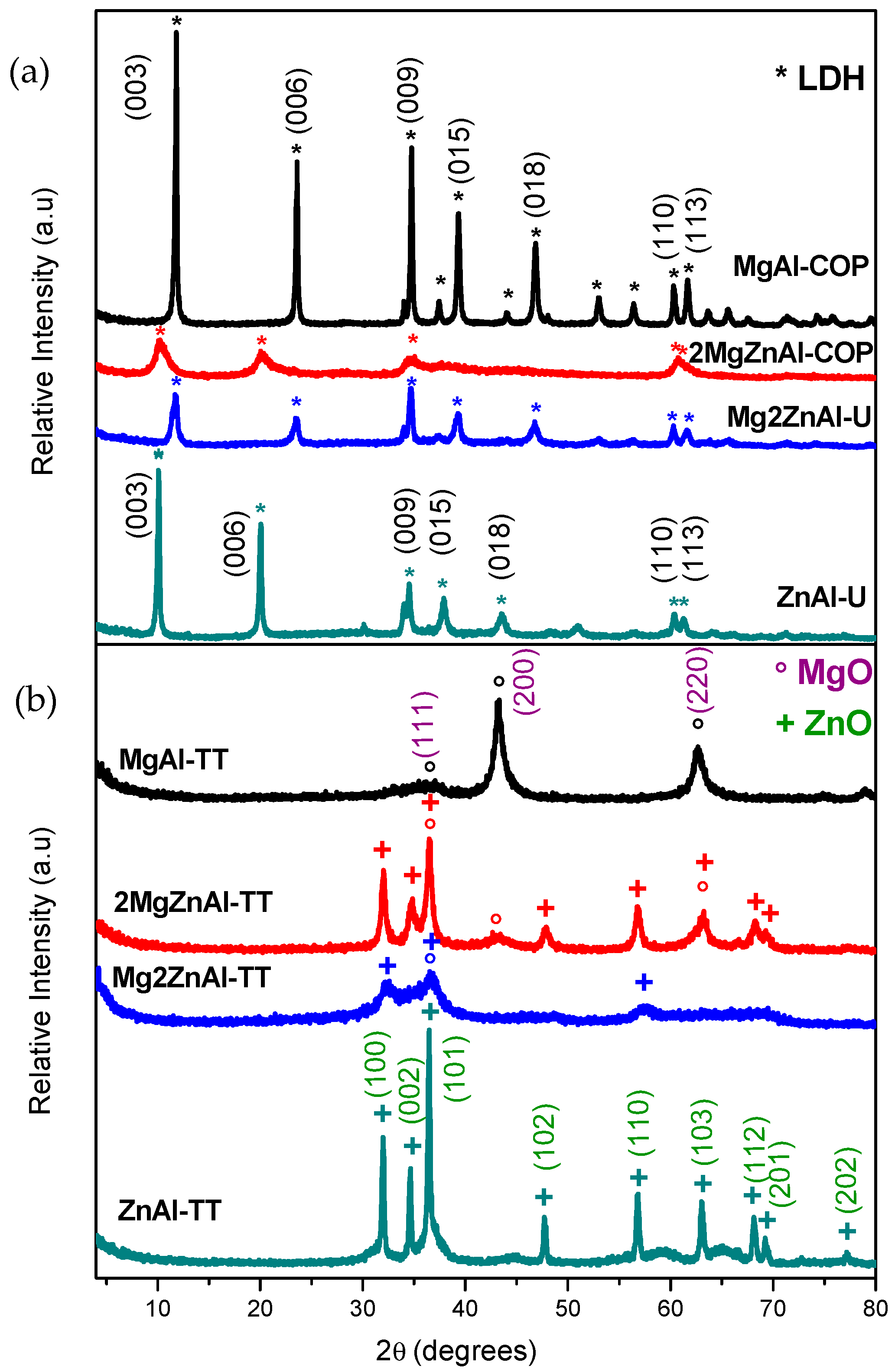
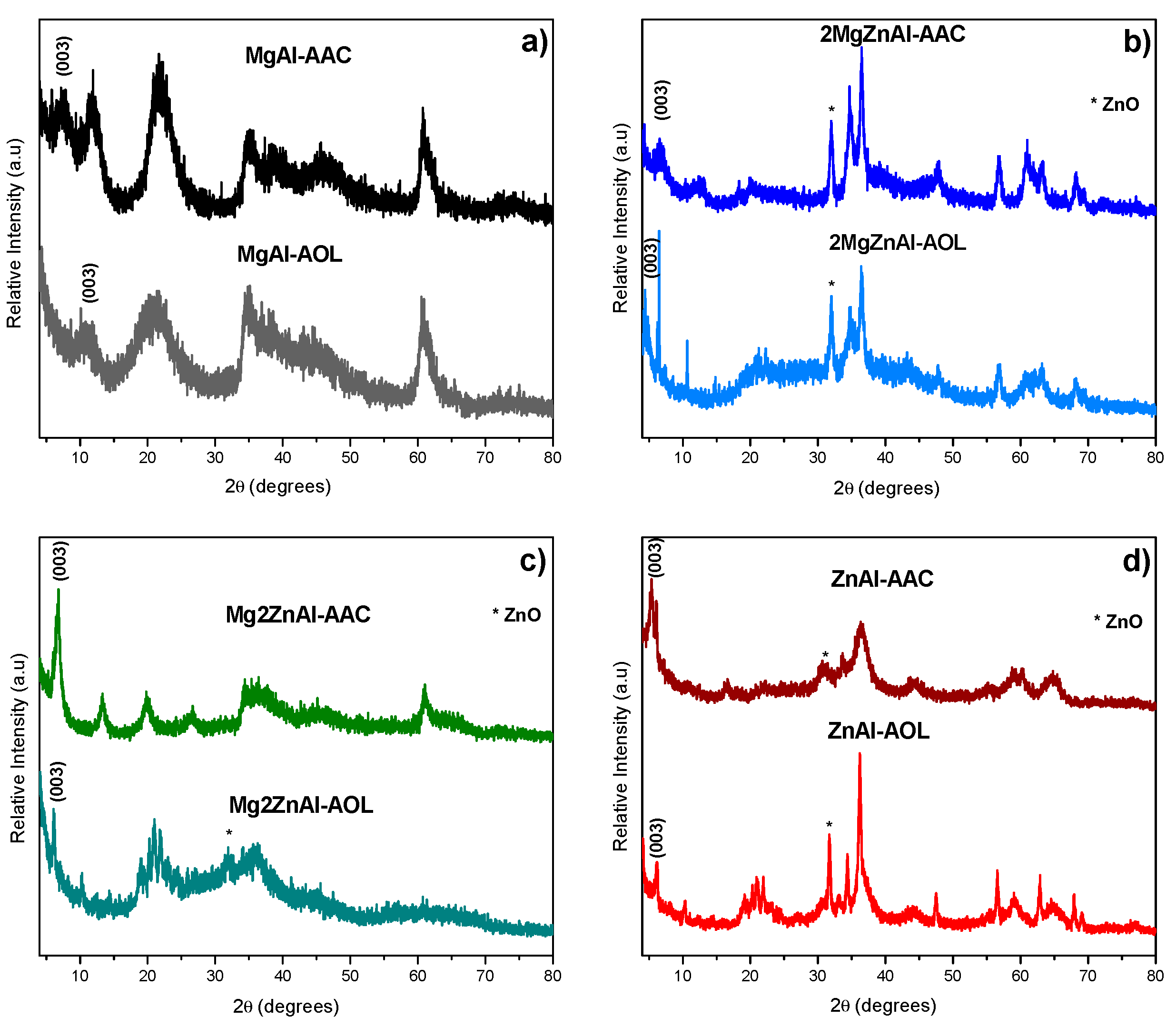

| LDH | Mg/Zn | Formula | Synthesis Method | AEC (mmol·g−1) |
|---|---|---|---|---|
| MgAl-COP | 2/0 | Mg6Al2(OH)16(NO3)2·4H2O | Co-precipitation | 3.00 |
| 2MgZnAl-COP | 2/1 | Mg4Zn2Al2(OH)16(NO3)2·4H2O | Co-precipitation | 2.67 |
| Mg2ZnAl-U | 1/2 | Mg2Zn4Al2(OH)16(NO3)2·4H2O | Urea | 2.40 |
| ZnAl-U | 0/2 | Zn6Al2(OH)16(NO3)2·4H2O | Urea | 2.19 |
| Reagents | Reactor (g) | Addition Tank (g) |
|---|---|---|
| Surfactant sol. 2.66 wt.% | 3 | - |
| Surfactant sol. 6.5 wt.% | - | 30 |
| Styrene | - | 120 |
| Na2S2O8 sol. 3.86 wt.% | 1 | 10 |
| Distilled water | 37 | - |
| Sample | d003 (Å) | c (Å) | a (Å) |
|---|---|---|---|
| MgAl-COP | 7.43 | 22.29 | 3.07 |
| MgAl-AAC | 11.62 | 34.85 | 3.05 |
| MgAl-AOL | 8.72 | 26.17 | 3.05 |
| 2MgZnAl-COP | 8.73 | 26.17 | 3.05 |
| 2MgZnAl-AAC | 13.30 | 39.91 | 3.03 |
| 2MgZnAl-AOL | 20.27 | 60.81 | 3.05 |
| Mg2ZnAl-U | 7.58 | 22.75 | 3.06 |
| Mg2ZnAl-AAC | 12.73 | 38.20 | 3.03 |
| Mg2ZnAl-AOL | 14.40 | 43.90 | 3.05 |
| ZnAl-U | 8.73 | 26.18 | 3.07 |
| ZnAl-AAC | 15.98 | 47.94 | 2.87 |
| ZnAl-AOL | 14.40 | 43.19 | 2.95 |
| PDI | ||
|---|---|---|
| 1505.8 ± 12 | 1526.9 ± 13 | 1.01 |
| Material | Decomposition Temperature (°C) | |
|---|---|---|
| 10 wt.% LDH | 15 wt.% LDH | |
| PS | 391.9 | |
| PS/MgAl-AAC | 387.3 | 378.0 |
| PS/MgAl-AOL | 396.2 | 379.6 |
| PS/2MgZnAl-AAC | 369.1 | 399.4 |
| PS/2MgZnAl-AOL | 389.9 | 397.8 |
| PS/Mg2ZnAl-AAC | 381.5 | 445.9 |
| PS/Mg2ZnAl-AOL | 358.5 | 376.5 |
| PS/ZnAl-AAC | 378.0 | 369.1 |
| PS/ZnAl-AOL | 391.8 | 388.9 |
| Material | Activation Energy (Ea-F) (kJ·mol−1) | |||
|---|---|---|---|---|
| Heating Rate (°C/min) | Average | |||
| 10 | 20 | 30 | ||
| PS | 50.2 | 50.2 | ||
| PS/MgAl-AAC (10 wt.%) | 103.6 | 86.9 | 102.4 | 97.6 ± 9 |
| PS/MgAl-AAC (15 wt.%) | 78.0 | 103.8 | 107.6 | 96.5 ± 16 |
| PS/MgAl-AOL (10 wt.%) | 53.9 | 169.4 | 104.1 | 109.1 ± 58 |
| PS/MgAl-AOL (15 wt.%) | 78.4 | 109.0 | 118.0 | 101.8 ± 21 |
| PS/2MgZnAl-AAC (10 wt.%) | 92.4 | 55.5 | 151.3 | 99.8 ± 48 |
| PS/2MgZnAl-AAC (15 wt.%) | 134.8 | 196.4 | 211.4 | 180.9 ± 41 |
| PS/2MgZnAl-AOL (10 wt.%) | 92.2 | 68.3 | 139.5 | 100.0 ± 36 |
| PS/2MgZnAl-AOL (15 wt.%) | 126.8 | 186.1 | 198.4 | 170.5 ± 38 |
| PS/Mg2ZnAl-AAC (10 wt.%) | 80.7 | 118.5 | 110.8 | 103.3 ± 20 |
| PS/Mg2ZnAl-AAC (15 wt.%) | 170.6 | 122.6 | 180.8 | 158.0 ± 31 |
| PS/Mg2ZnAl-AOL (10 wt.%) | 51.1 | 157.8 | 181.0 | 130.0 ± 69 |
| PS/Mg2ZnAl-AOL (15 wt.%) | 93.6 | 61.0 | 163.8 | 106.2 ± 52 |
| PS/ZnAl-AAC (10 wt.%) | 119.9 | 100.1 | 133.9 | 118.0 ± 17 |
| PS/ZnAl-AAC (15 wt.%) | 57.8 | 69.9 | 70.9 | 66.2 ± 7 |
| PS/ZnAl-AOL (10wt.%) | 105.1 | 107.2 | 109.4 | 107.2 ± 2 |
| PS/ZnAl-AOL (15wt.%) | 67.4 | 101.5 | 125.4 | 98.1 ± 29 |
| Material | Activation Energy (Ea-FWO) (kJ·mol−1) | ||||||
|---|---|---|---|---|---|---|---|
| Conversion (α) | Average | ||||||
| 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | ||
| PS | 50.2 | 50.2 | |||||
| PS/MgAl-AAC (10 wt.%) | 83.9 | 64.2 | 74.8 | 118.8 | 113.2 | 120.3 | 95.8 ± 22 |
| PS/MgAl-AAC (15 wt.%) | 110.3 | 99.5 | 113.2 | 123.4 | 136.8 | 132.4 | 119.3 ± 13 |
| PS/MgAl-AOL (10 wt.%) | 71.4 | 62.3 | 60.8 | 56.7 | 57.7 | 67.9 | 62.8 ± 5 |
| PS/MgAl-AOL (15 wt.%) | 107.0 | 139.5 | 161.2 | 172.6 | 174.7 | 170.2 | 154.2 ± 24 |
| PS/2MgZnAl-AAC (10 wt.%) | 71.9 | 87.9 | 115.9 | 134.5 | 138.5 | 146.4 | 115.8 ± 27 |
| PS/2MgZnAl-AAC (15 wt.%) | 71.0 | 117.3 | 121.0 | 137.2 | 149.0 | 163.9 | 126.6 ± 29 |
| PS/2MgZnAl-AOL (10 wt.%) | 78.0 | 93.9 | 118.5 | 135.0 | 139.4 | 146.4 | 118.5 ± 25 |
| PS/2MgZnAl-AOL (15 wt.%) | 133.5 | 179.6 | 259.2 | 215.8 | 207.7 | 222.0 | 203.0 ± 39 |
| PS/Mg2ZnAl-AAC (10 wt.%) | 80.2 | 85.0 | 100.3 | 115.6 | 137.3 | 138.0 | 109.4 ± 23 |
| PS/Mg2ZnAl-AAC (15 wt.%) | 165.6 | 236.5 | 254.3 | 264.3 | 287.7 | 209.5 | 236.3 ± 40 |
| PS/Mg2ZnAl-AOL (10 wt.%) | 50.3 | 48.8 | 59.2 | 64.8 | 71.7 | 87.0 | 63.6 ± 13 |
| PS/Mg2ZnAl-AOL (15 wt.%) | 23.5 | 28.9 | 34.9 | 41.3 | 46.4 | 48.8 | 37.3 ± 9 |
| PS/ZnAl-AAC (10 wt.%) | 35.1 | 67.7 | 77.2 | 90.1 | 101.0 | 111.0 | 80.3 ± 25 |
| PS/ZnAl-AAC (15 wt.%) | 84.6 | 89.3 | 105.7 | 119.9 | 127.2 | 137.5 | 110.7 ± 19 |
| PS/ZnAl-AOL (10 wt.%) | 122.8 | 123.0 | 114.9 | 102.1 | 90.8 | 74.4 | 104.7 ± 17 |
| PS/ZnAl-AOL (15 wt.%) | 75.3 | 112.4 | 117.0 | 119.9 | 125.9 | 131.4 | 113.6 ± 18 |
| Material | Activation Energy (kJ·mol−1) | ||
|---|---|---|---|
| Ea-F | Ea-FWO | Ea-M | |
| PS | - | - | 50.2 |
| PS/MgAl-AAC (10 wt.%) | 97.6 | 95.8 | 96.7 ± 1 |
| PS/MgAl-AAC (15 wt.%) | 96.5 | 119.3 | 107.9 ± 11 |
| PS/MgAl-AOL (10 wt.%) | 109.1 | 63.3 | 86.2 ± 23 |
| PS/MgAl-AOL (15 wt.%) | 101.8 | 154.2 | 128.0 ± 26 |
| PS/2MgZnAl-AAC (10 wt.%) | 99.8 | 115.8 | 107.8 ± 8 |
| PS/2MgZnAl-AAC (15 wt.%) | 180.9 | 126.6 | 153.7 ± 27 |
| PS/2MgZnAl-AOL (10 wt.%) | 100.0 | 118.5 | 109.3 ± 9 |
| PS/2MgZnAl-AOL (15 wt.%) | 170.5 | 203.0 | 186.7 ± 16 |
| PS/Mg2ZnAl-AAC (10 wt.%) | 103.3 | 109.4 | 106.4 ± 3 |
| PS/Mg2ZnAl-AAC (15 wt.%) | 158.0 | 236.3 | 197.2 ± 39 |
| PS/Mg2ZnAl-AOL (10 wt.%) | 130.0 | 63.6 | 96.8 ± 33 |
| PS/Mg2ZnAl-AOL (15 wt.%) | 106.2 | 37.3 | 71.7 ± 34 |
| PS/ZnAl-AAC (10 wt.%) | 118.0 | 80.3 | 99.2 ± 19 |
| PS/ZnAl-AAC (15 wt.%) | 66.2 | 110.7 | 88.4 ± 22 |
| PS/ZnAl-AOL (10 wt.%) | 107.2 | 104.7 | 105.9 ± 1 |
| PS/ZnAl-AOL (15 wt.%) | 98.1 | 113.6 | 105.9 ± 8 |
| Sample | n | Pyrolysis Process | |||||
|---|---|---|---|---|---|---|---|
| First Stage | Second Stage | ||||||
| Ea (kJ·mol−1) | A (s−1) | R2 | Ea (kJ·mol−1) | A (s−1) | R2 | ||
| PS/MgAl-AOL 10 wt.% | 1/3 | 101.8 | 8.4 × 1011 | 0.9926 | 118.7 | 8.5 × 1011 | 0.9919 |
| PS/2MgZnAl-AAC 15 wt.% | 1/3 | 198.4 | 2.1 × 1017 | 0.9995 | 133.5 | 1.3 × 1010 | 0.9915 |
| PS/2MgZnAl-AOL 15 wt.% | 1/3 | 180.2 | 1.8 × 1014 | 0.9866 | 101.5 | 1.5 × 107 | 0.9810 |
| PS/Mg2ZnAl-AAC 15 wt.% | 1/3 | 175.8 | 1.5 × 1013 | 0.9900 | 153.6 | 5.7 × 1011 | 0.9375 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Rosa-Guzmán, M.Á.; Guzmán-Vargas, A.; Cayetano-Castro, N.; Del Río, J.M.; Corea, M.; Martínez-Ortiz, M.d.J. Thermal Stability Evaluation of Polystyrene-Mg/Zn/Al LDH Nanocomposites. Nanomaterials 2019, 9, 1528. https://doi.org/10.3390/nano9111528
De la Rosa-Guzmán MÁ, Guzmán-Vargas A, Cayetano-Castro N, Del Río JM, Corea M, Martínez-Ortiz MdJ. Thermal Stability Evaluation of Polystyrene-Mg/Zn/Al LDH Nanocomposites. Nanomaterials. 2019; 9(11):1528. https://doi.org/10.3390/nano9111528
Chicago/Turabian StyleDe la Rosa-Guzmán, Miguel Ángel, Ariel Guzmán-Vargas, Nicolás Cayetano-Castro, José Manuel Del Río, Mónica Corea, and María de Jesús Martínez-Ortiz. 2019. "Thermal Stability Evaluation of Polystyrene-Mg/Zn/Al LDH Nanocomposites" Nanomaterials 9, no. 11: 1528. https://doi.org/10.3390/nano9111528
APA StyleDe la Rosa-Guzmán, M. Á., Guzmán-Vargas, A., Cayetano-Castro, N., Del Río, J. M., Corea, M., & Martínez-Ortiz, M. d. J. (2019). Thermal Stability Evaluation of Polystyrene-Mg/Zn/Al LDH Nanocomposites. Nanomaterials, 9(11), 1528. https://doi.org/10.3390/nano9111528