Fabrication of Ag and Mn Co-Doped Cu2ZnSnS4 Thin Film
Abstract
:1. Introduction
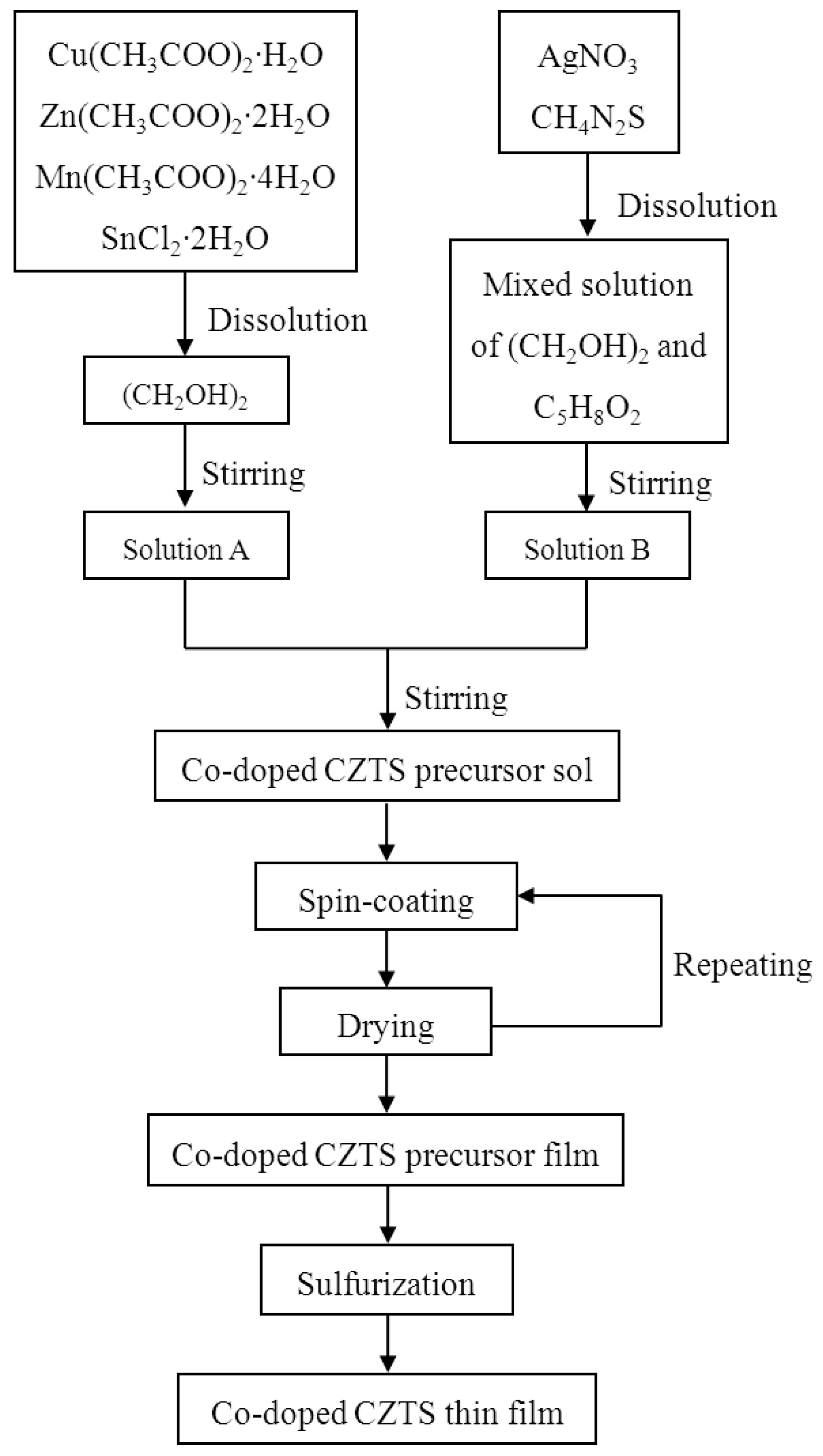
2. Materials and Methods
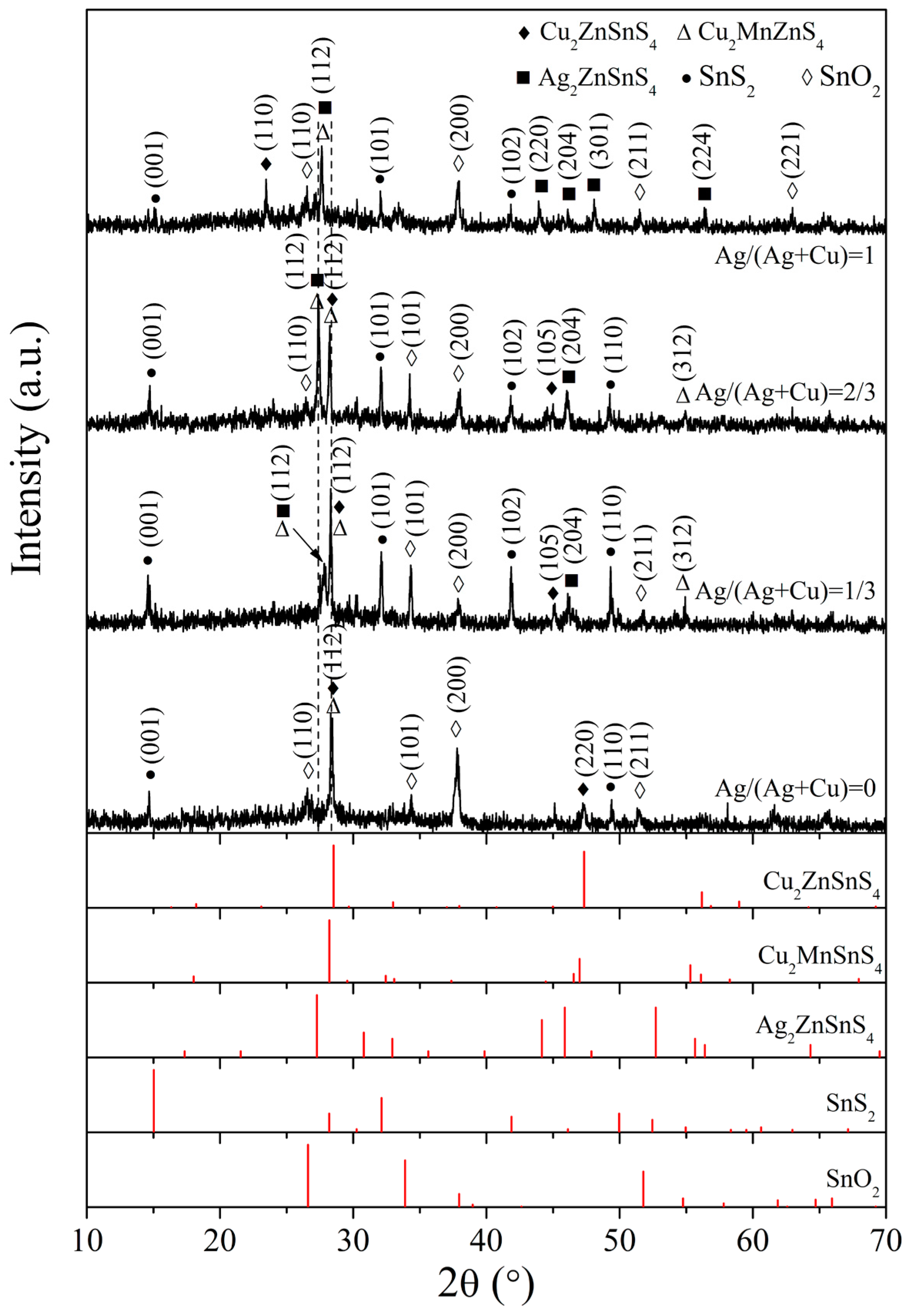
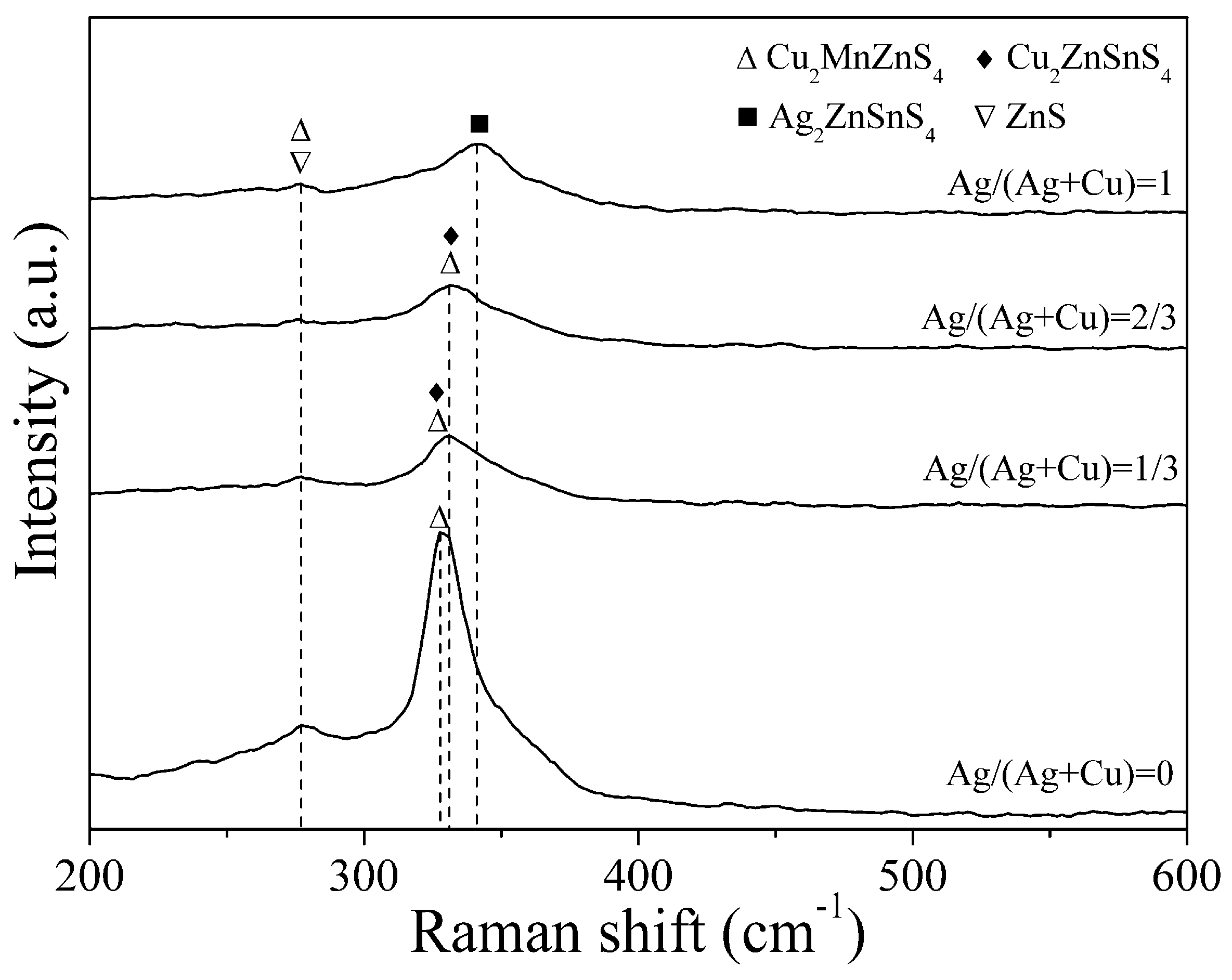
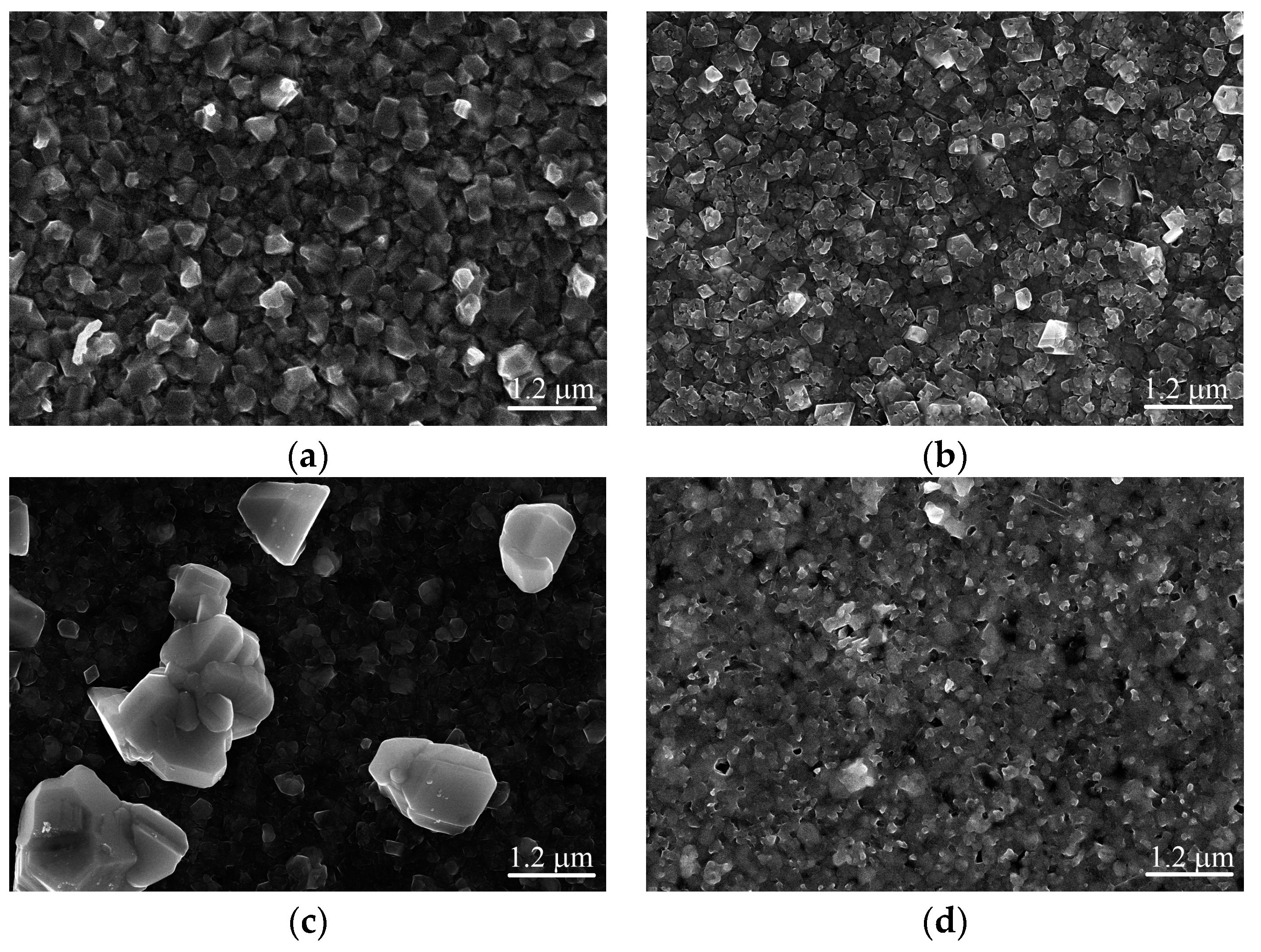
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wibowo, R.A. Powder-to-film approach for fabricating critical raw material-free kesterite Cu2ZnSn(S,Se)4 thin film photovoltaic: A review. Sol. Energy 2018, 176, 157–169. [Google Scholar] [CrossRef]
- Shi, Z.Q.; Attygalle, D.; Jayatissa, A.H. Kesterite-based next generation high performance thin film solar cell: Current progress and future prospects. J. Mater. Sci. Mater. Electron. 2017, 28, 2290–2306. [Google Scholar] [CrossRef]
- Zhang, C.J.; Tao, J.H.; Chu, J.H. An 8.7% efficiency co-electrodeposited Cu2ZnSnS4 photovoltaic device fabricated via a pressurized post-sulfurization process. J. Mater. Chem. C 2018, 6, 13275–13282. [Google Scholar] [CrossRef]
- Jiang, J.C.; Zhang, L.Q.; Wang, W.; Hong, R.J. The role of sulphur in the sulfurization of CZTS layer prepared by DC magnetron sputtering from a single quaternary ceramic target. Ceram. Int. 2018, 44, 11597–11602. [Google Scholar] [CrossRef]
- Yan, C.; Huang, J.L.; Sun, K.W.; Johnston, S.; Zhang, Y.F.; Sun, H.; Pu, A.B.; He, M.R.; Liu, F.Y.; Eder, K. Cu2ZnSnS4 solar cells with over 10% power conversion efficiency enabled by heterojunction heat treatment. Nat. Energy 2018, 3, 764–772. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of CZTSSe thin-film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Pal, K.; Singh, P.; Bhaduri, A.; Thapa, K.B. Current challenges and future prospects for a highly efficient (>20%) kesterite CZTS solar cell: A review. Sol. Energy Mater. Sol. Cells. 2019, 196, 138–156. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.L.; Cui, H.T.; Huang, S.J.; Hao, X.J. The role of Ag in (Ag, Cu)2ZnSnS4 thin film for solar cell application. J. Alloys Compd. 2015, 625, 277–283. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, X.X.; Xu, B.; Li, W.; Li, J.; Li, J.J.; Wang, M.; Dong, C.; Ju, P.; Li, J.S. Enhancing open-circuit voltage of solution-processed Cu2ZnSn(S,Se)4 solar cells with Ag substitution. IEEE J. Photovolt. 2017, 7, 874–881. [Google Scholar] [CrossRef]
- Henry, J.; Mohanraj, K.; Sivakumar, G. Vacuum evaporated FTO/(Cu, Ag)2ZnSnSe4 thin films and its electrochemical analysis. Vacuum 2019, 160, 347–354. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kawaguchi, T.; Chantana, J.; Minemoto, T.; Harada, T.; Nakanishi, S.; Ikeda, S. Structural and solar cell properties of a Ag-containing Cu2ZnSnS4 thin film derived from spray pyrolysis. ACS Appl. Mater. Interfaces 2018, 10, 5455–5463. [Google Scholar] [CrossRef]
- Kumar, J.; Ingole, S. Structural and optical properties of (AgxCu1-x)2ZnSnS4 thin films synthesised via solution route. J. Alloys Compd. 2017, 727, 1089–1094. [Google Scholar] [CrossRef]
- Chen, X.Y.; Wang, J.L.; Zhou, W.H.; Chang, Z.X.; Kou, D.X.; Zhou, Z.J.; Tian, Q.W.; Meng, Y.N.; Wu, S.X. Rational synthesis of (Cu1-xAgx)2ZnSnS4 nanocrystals with low defect and tuning band gap. Mater. Lett. 2016, 181, 317–320. [Google Scholar] [CrossRef]
- Kumar, M.S.; Madhusudanan, S.P.; Batabyal, S.K. Substitution of Zn in earth-abundant Cu2ZnSn(S,Se)4 based thin film solar cells—A status review. Sol. Energy Mater. Sol. Cells. 2018, 185, 287–299. [Google Scholar] [CrossRef]
- Su, Z.H.; Tan, J.M.R.; Li, X.L.; Zeng, X.; Batabyal, S.K.; Wong, L.H. Cation substitution of solution-processed Cu2ZnSnS4 thin film solar cell with over 9% efficiency. Adv. Energy Mater. 2015, 5, 1500682. [Google Scholar] [CrossRef]
- Yan, C.; Sun, K.W.; Huang, J.L.; Johnston, S.; Liu, F.Y.; Puthen, B.; Veettil, B.P.; Sun, K.L.; Pu, A.B.; Zhou, F.Z.; et al. Beyond 11% efficient sulfide Kesterite Cu2ZnxCd1-xSnS4 solar cell: Effects of cadmium alloying. ACS Energy Lett. 2017, 2, 930–936. [Google Scholar] [CrossRef]
- Ananthoju, B.; Mohapatra, J.; Jangid, M.K.; Bahadur, D.; Medhekar, N.V.; Aslam, M. Cation/anion substitution in Cu2ZnSnS4 for improved photovoltaic performance. Sci. Rep. 2016, 6, 35369. [Google Scholar] [CrossRef]
- Huang, K.L.; Huang, C.H.; Lin, W.T.; Fu, Y.S.; Guo, T.F. Solvothermal synthesis and tunable bandgap of Cu2(Zn1-xCox)SnS4 and Cu2(Fe1-xCox)SnS4 nanocrystals. J. Alloys Compd. 2015, 646, 1015–1022. [Google Scholar] [CrossRef]
- Hadke, S.H.; Levcenko, S.; Lie, S.; Hages, C.J.; Marquez, J.A.; Unold, T.; Wong, L.H. Synergistic effects of double cation substitution in solution-processed CZTS solar cells with over 10% efficiency. Adv. Energy Mater. 2018, 8, 1802540. [Google Scholar] [CrossRef]
- Wang, W.H.; Chen, B.W.; Chen, G.L.; Cai, H.L.; Dong, J.B.; Liao, Y.B.; Chen, S.Y.; Huang, Z.G. A general oxide-based preparation strategy for Cu2MSnS4 (M: Zn, Mn, Cd) thin films and relevant solar cells. Mater. Lett. 2018, 214, 170–173. [Google Scholar] [CrossRef]
- Yu, J.J.; Deng, H.M.; Tao, J.H.; Chen, L.L.; Cao, H.Y.; Sun, L.; Yang, P.X.; Chu, J.H. Synthesis of Cu2MnSnS4 thin film deposited on seeded fluorine doped tin oxide substrate via a green and low-cost electrodeposition method. Mater. Lett. 2017, 191, 186–188. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Yao, B.; Li, Y.F.; Ding, Z.H.; Luan, H.M.; Jia, J.H.; Li, Y.; Shi, K.; Sui, Y.R.; Zhang, B.Y. Structure, optical and electrical properties of (Cu1-xAgx)2ZnSn(S,Se)4 alloy thin films for photovoltaic application. Mater. Sci. Semicond. Process. 2018, 81, 54–59. [Google Scholar] [CrossRef]
- Le Donne, A.; Marchionna, S.; Acciarri, M.; Cernuschi, F.; Binetti, S. Relevant efficiency enhancement of emerging Cu2MnSnS4 thin film solar cells by low temperature annealing. Sol. Energy 2017, 149, 125–131. [Google Scholar] [CrossRef]
- Ma, C.H.; Guo, H.F.; Zhang, K.Z.; Yuan, N.Y.; Ding, J.N. Fabrication of p-type kesterite Ag2ZnSnS4 thin films with a high hole mobility. Mater. Lett. 2017, 186, 390–393. [Google Scholar] [CrossRef]
- Serrano, J.; Cantarero, A.; Cardona, M.; Garro, N.; Lauck, R.; Tallman, R.E.; Ritter, T.M.; Weinstein, B.A. Raman scattering in β-ZnS. Phys. Rev. B 2004, 69, 014301. [Google Scholar] [CrossRef]
- Price, L.S.; Parkin, I.P.; Hardy, A.M.E.; Clark, R.J.H.; Hibbert, T.G.; Molloy, K.C. Atmospheric pressure chemical vapor deposition of tin sulfides (SnS,Sn2S3, and SnS2) on glass. Chem. Mater. 1999, 11, 1792–1799. [Google Scholar] [CrossRef]
- Kuzyutkina, Y.S.; Romanova, E.A.; Kochubei, V.I.; Shiryaev, V.S. Specific features of linear and nonlinear optical responses of chalcogenide glasses in the As-S-Se and As-Se-Te systems. Opt. Spectrosc. 2014, 117, 53–59. [Google Scholar] [CrossRef]
- Lie, S.; Sandi, M.I.; Tay, Y.F.; Li, W.J.; Tan, J.M.R.; Bishop, D.M.; Gunawan, O.; Wong, L.H. Improving the charge separation and collection at the buffer/absorber interface by double-layered Mn-substituted CZTS. Sol. Energy Mater. Sol. Cells. 2018, 185, 351–358. [Google Scholar] [CrossRef]
- Chen, L.L.; Deng, H.M.; Cui, J.Y.; Tao, J.H.; Zhou, W.L.; Cao, H.Y.; Sun, L.; Yang, P.X.; Chu, J.H. Composition dependence of the structure and optical properties of Cu2MnxZn1-xSnS4 thin films. J. Alloys Compd. 2015, 627, 388–392. [Google Scholar] [CrossRef]
- Guan, H.; Shen, H.; Li, J. Synthesis and optical properties of Cu2(Zn1-XMnX)SnS4 thin films with tunable band gap. Chalcogenide Lett. 2017, 14, 217–221. [Google Scholar]
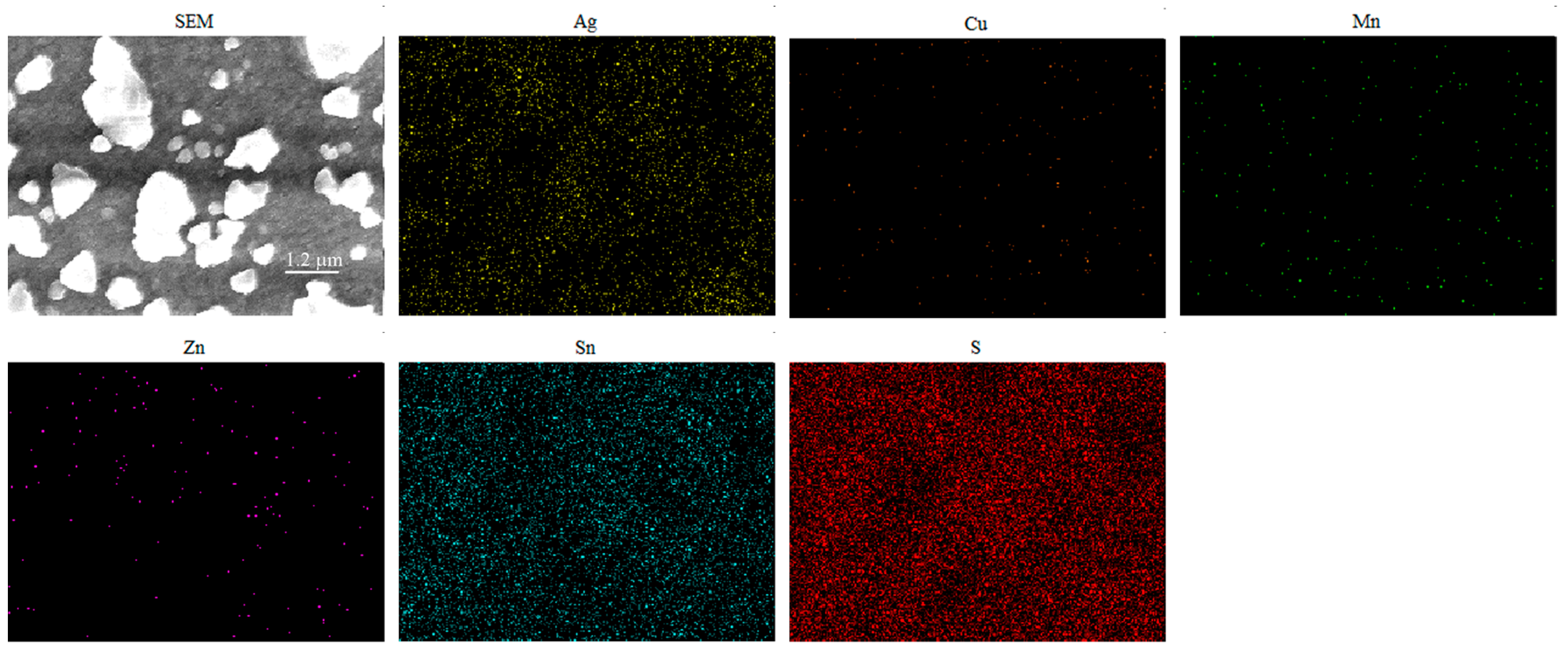
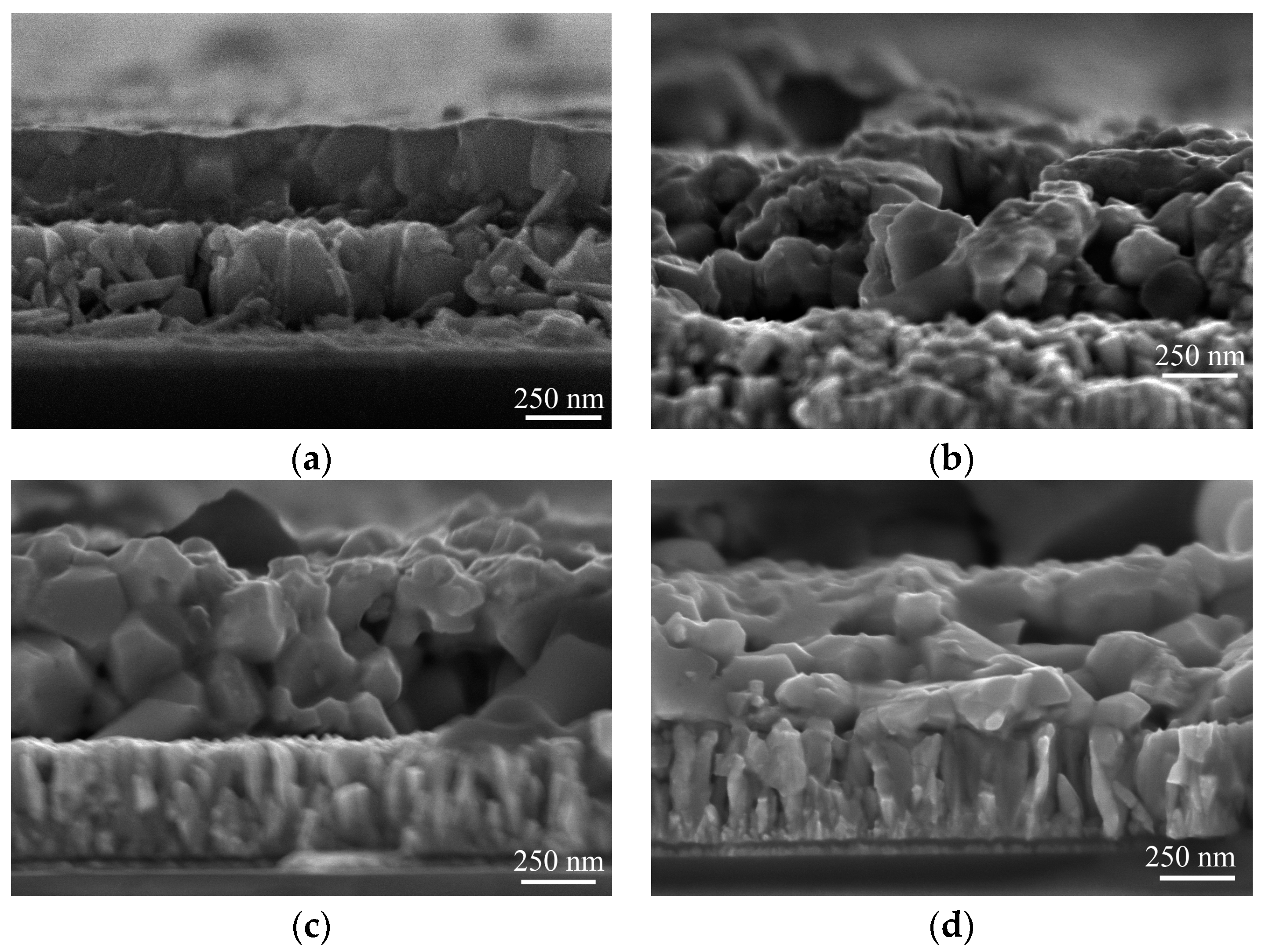
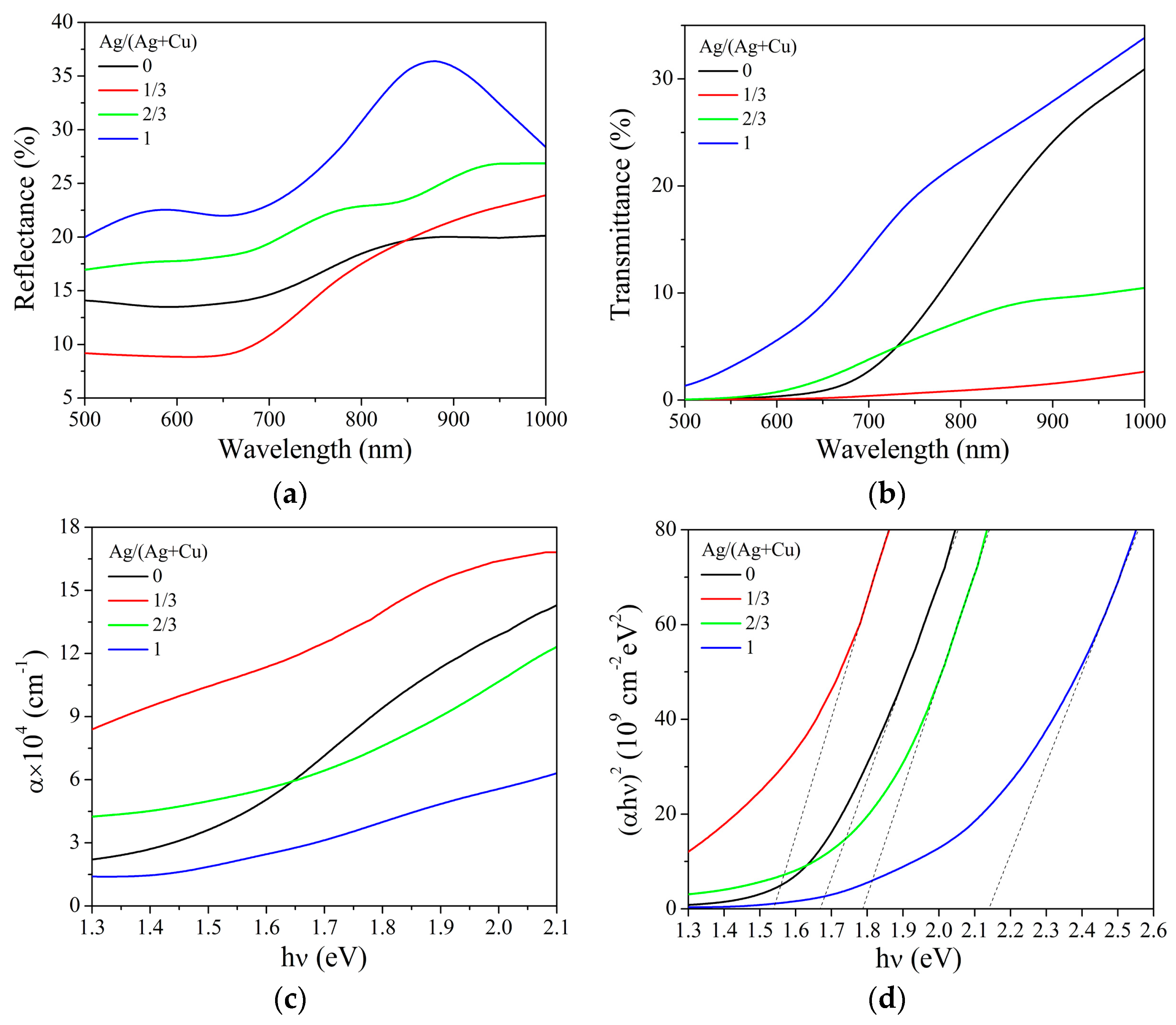
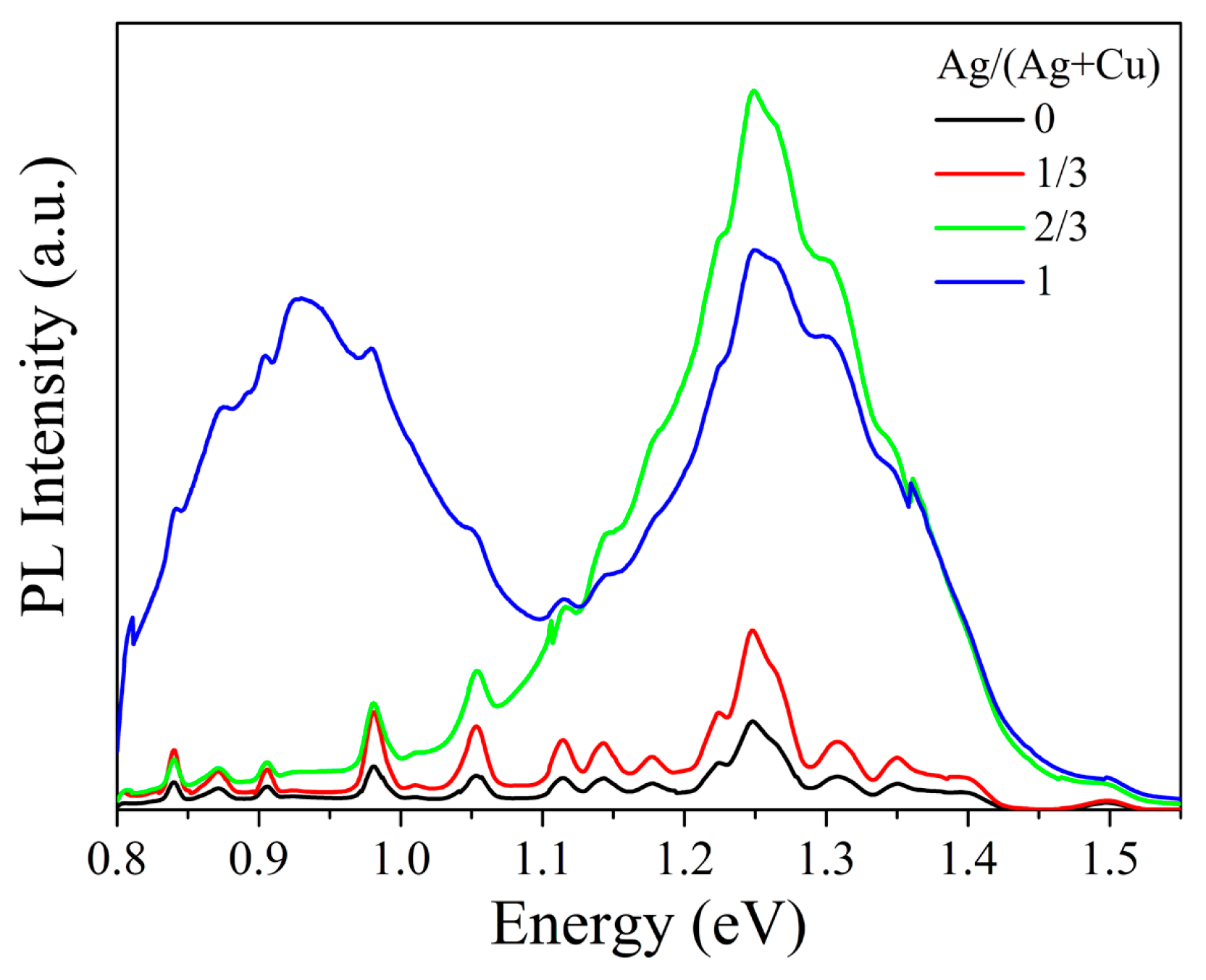
| Composition | Ag/(Ag + Cu) in Sol | |||
|---|---|---|---|---|
| 0 | 1/3 | 2/3 | 1 | |
| Ag (at.%) | 0 | 5.88 ± 0.03 | 11.93 ± 0.44 | 21.09 ± 0.82 |
| Cu (at.%) | 17.46 ± 0.13 | 9.37 ± 0.15 | 6.07 ± 0.44 | 0.33 ± 0.03 |
| Mn (at.%) | 2.29 ± 0.15 | 2.55 ± 0.07 | 2.37 ± 0.10 | 2.04 ± 0.16 |
| Zn (at.%) | 7.06 ± 0.21 | 6.07 ± 0.45 | 5.58 ± 0.61 | 5.71 ± 0.30 |
| Sn (at.%) | 25.89 ± 0.30 | 29.65 ± 0.68 | 26.29 ± 0.87 | 21.90 ± 0.41 |
| S (at.%) | 47.31 ± 0.52 | 46.50 ± 0.44 | 47.76 ± 1.04 | 48.93 ± 1.44 |
| Ag/(Ag + Cu) | 0 | 0.39 | 0.66 | 0.98 |
| Mn/(Mn + Zn) | 0.24 | 0.30 | 0.30 | 0.26 |
| (Cu + Ag)/(Mn + Zn) | 1.87 | 1.77 | 2.26 | 2.76 |
| S/(Ag + Cu) | 2.71 | 3.05 | 2.65 | 2.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Xu, J.; Tian, X. Fabrication of Ag and Mn Co-Doped Cu2ZnSnS4 Thin Film. Nanomaterials 2019, 9, 1520. https://doi.org/10.3390/nano9111520
Qiu L, Xu J, Tian X. Fabrication of Ag and Mn Co-Doped Cu2ZnSnS4 Thin Film. Nanomaterials. 2019; 9(11):1520. https://doi.org/10.3390/nano9111520
Chicago/Turabian StyleQiu, Lei, Jiaxiong Xu, and Xiao Tian. 2019. "Fabrication of Ag and Mn Co-Doped Cu2ZnSnS4 Thin Film" Nanomaterials 9, no. 11: 1520. https://doi.org/10.3390/nano9111520
APA StyleQiu, L., Xu, J., & Tian, X. (2019). Fabrication of Ag and Mn Co-Doped Cu2ZnSnS4 Thin Film. Nanomaterials, 9(11), 1520. https://doi.org/10.3390/nano9111520





