Reductive and Coordinative Effects of Hydrazine in Structural Transformations of Copper Hydroxide Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cu(OH)2 Precursor Synthesis
2.3. N2H4 Reduction of Cu(OH)2
2.4. Electrochemical Reduction of Cu2O and Cu2O-N2H4
2.5. Nanoparticle Characterization
3. Results and Discussion
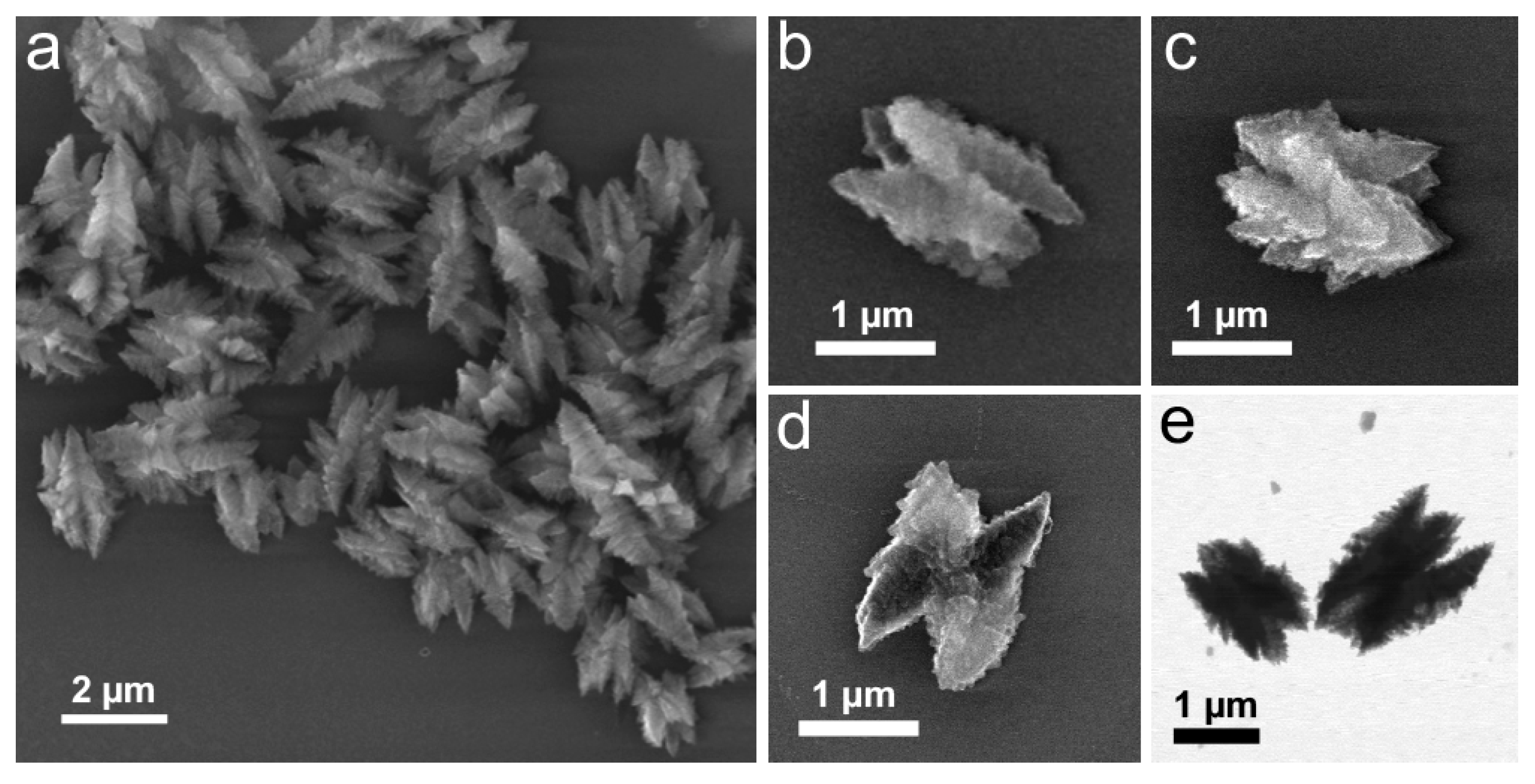
3.1. Synthesis of the Starting Material
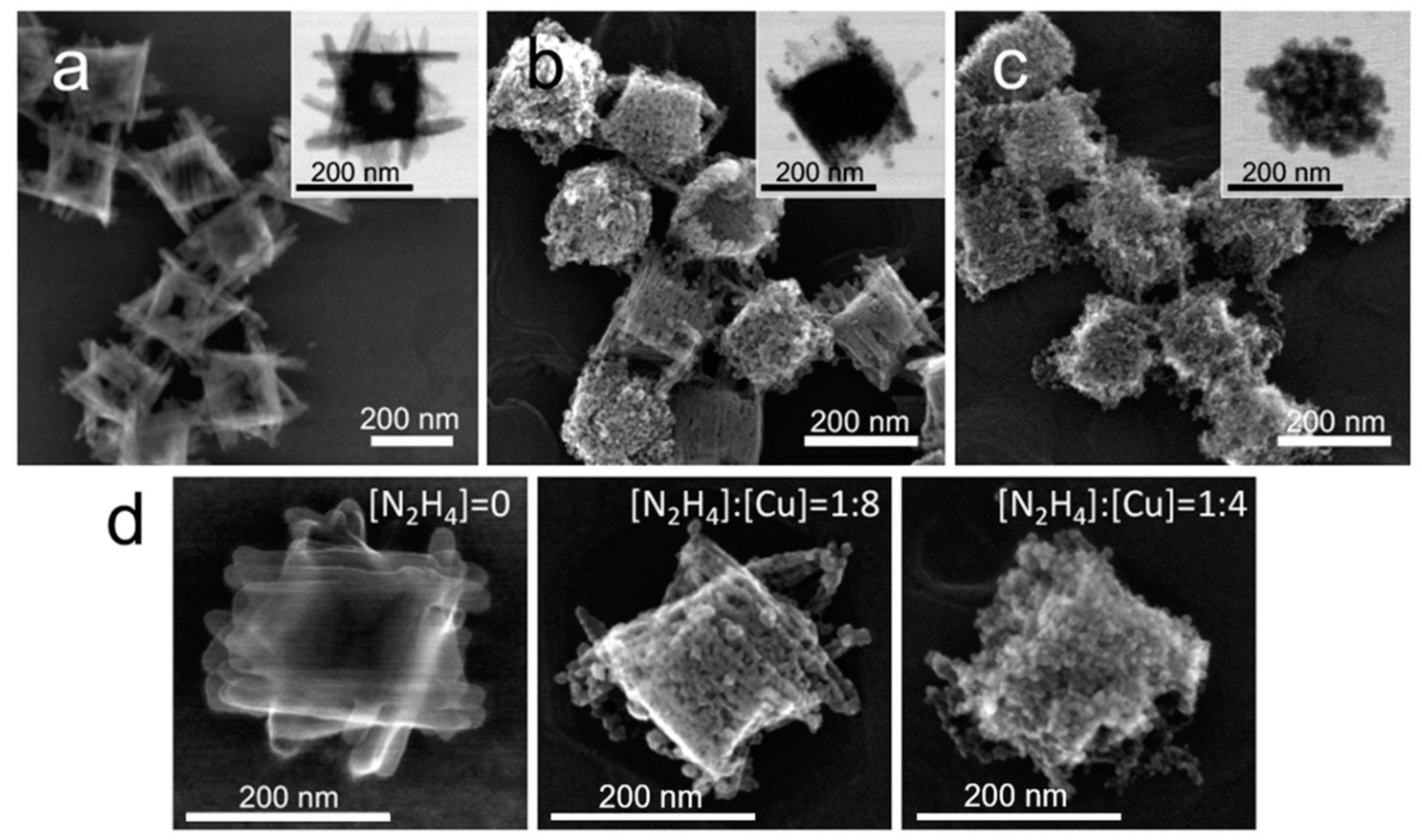
3.2. Structural Evolution at a Stochiometric N2H4 to Cu Ratio
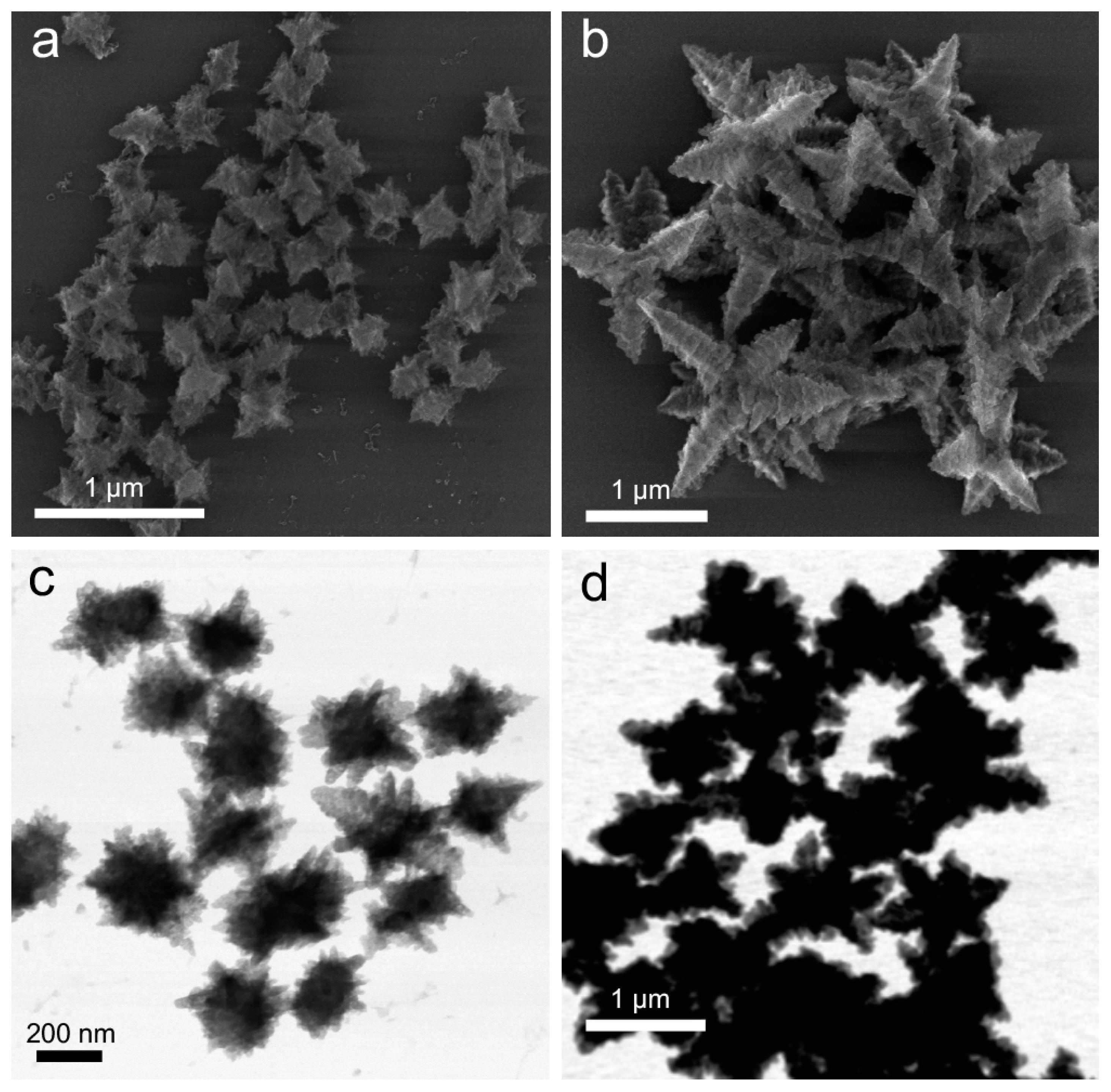
3.3. Structural Evolution in Excess N2H4
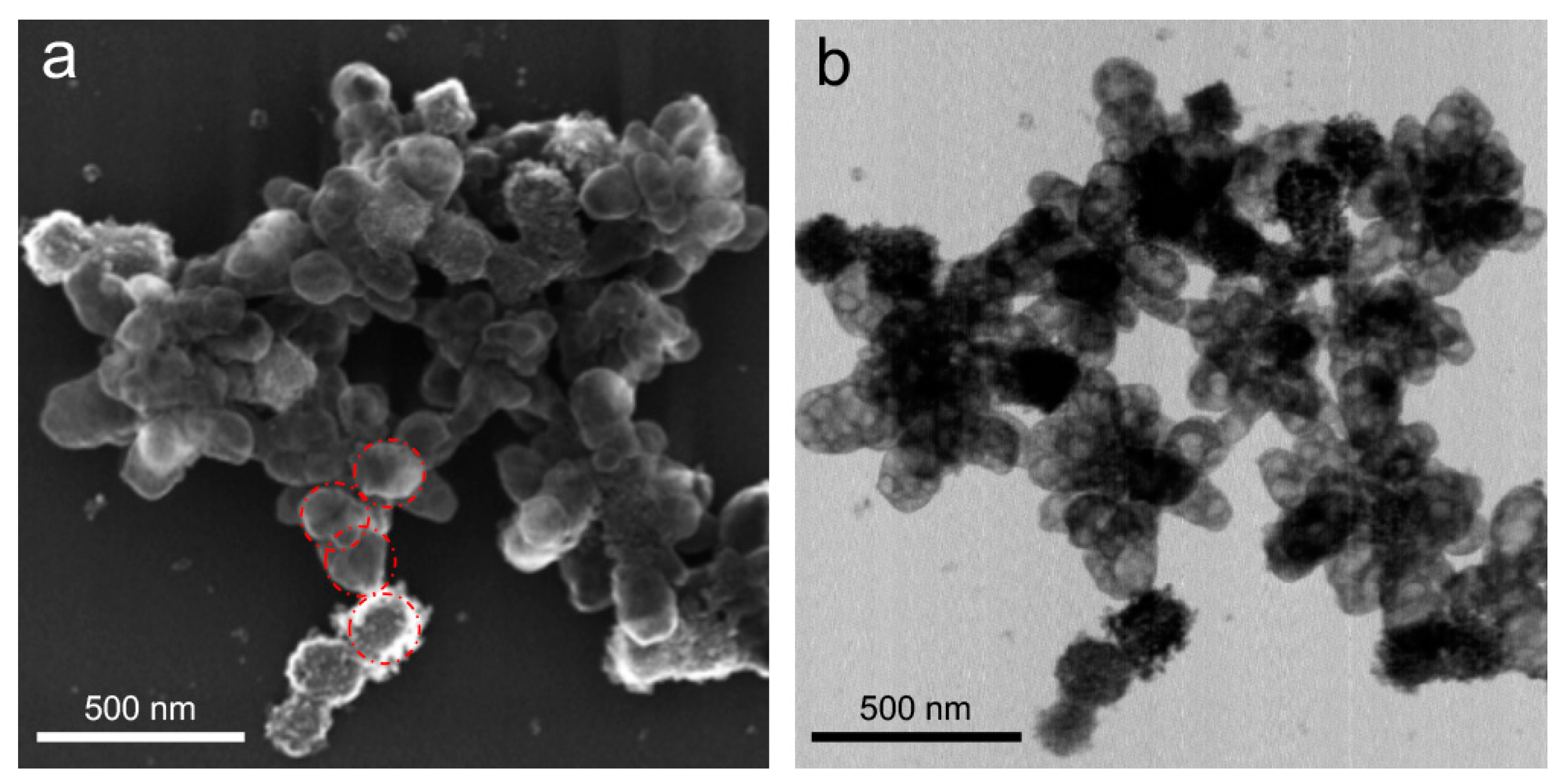
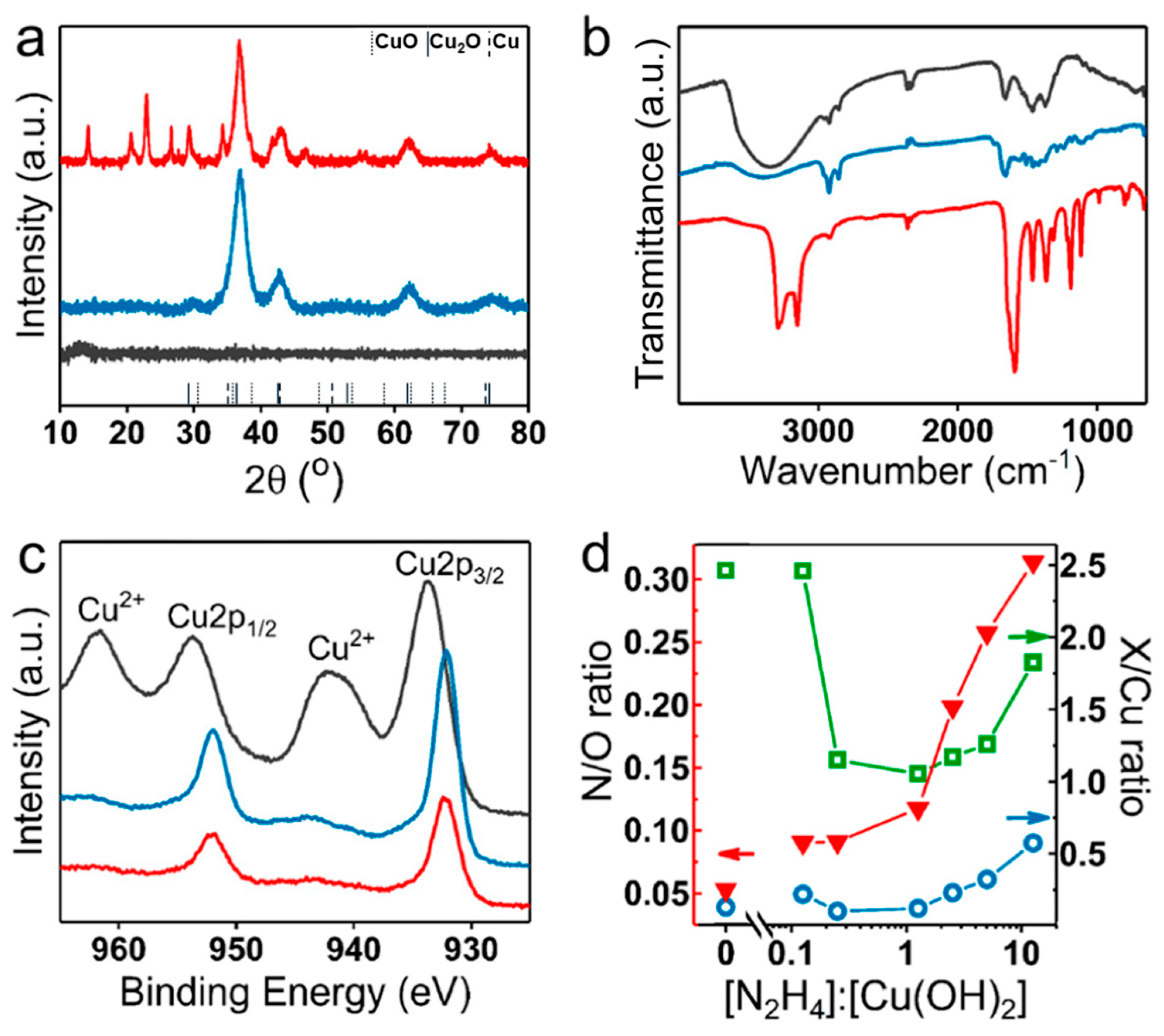
3.4. Composition Evolution
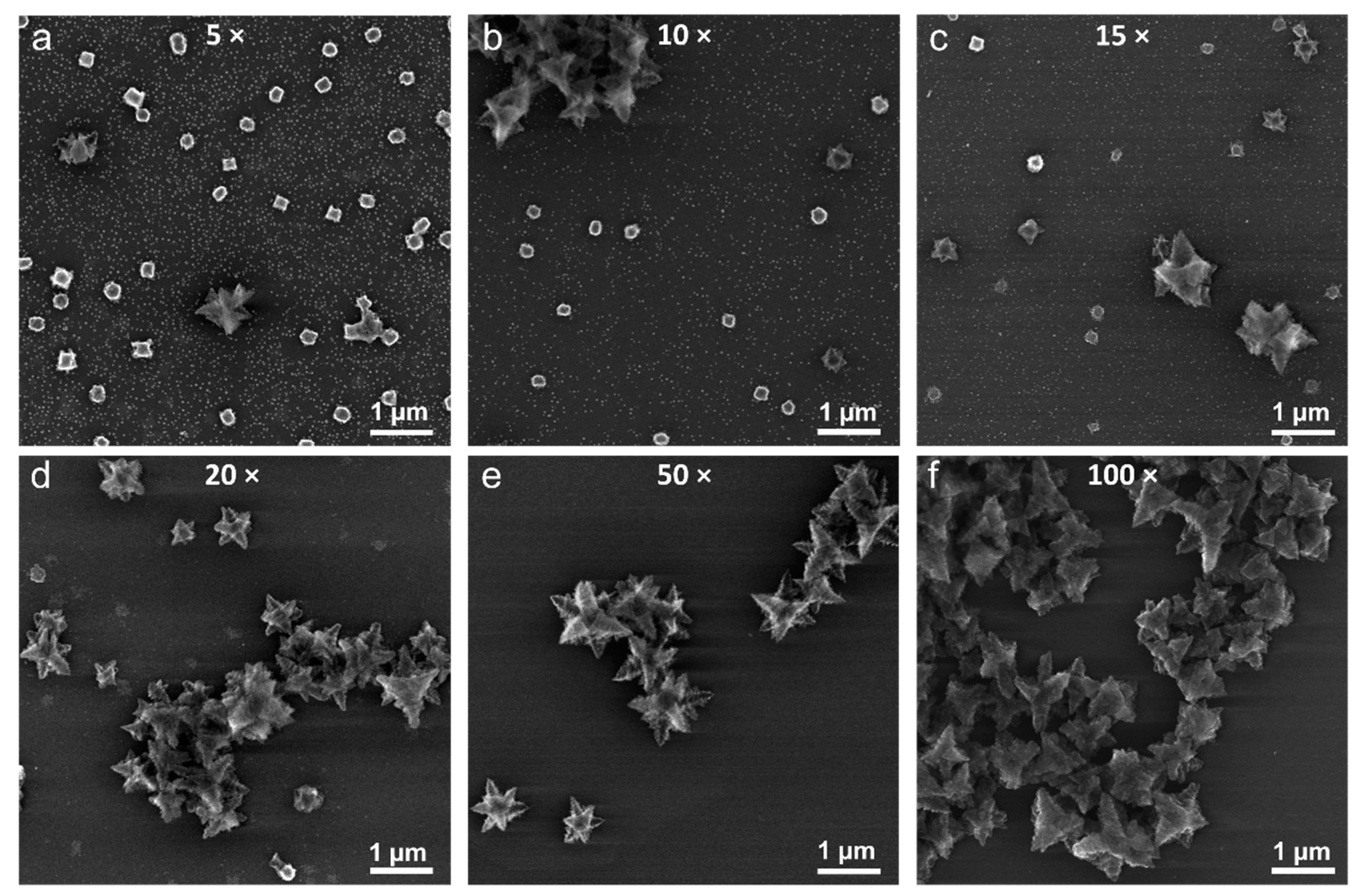
3.5. Solvent Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, M.H. Morphologically controlled synthesis of Cu2O nanocrystals and their properties. Nano Today 2010, 5, 106–116. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.; Kwon, H.; Song, H. Gram-Scale Synthesis of Cu2O Nanocubes and Subsequent Oxidation to CuO Hollow Nanostructures for Lithium-Ion Battery Anode Materials. Adv. Mater. 2009, 21, 803–807. [Google Scholar] [CrossRef]
- Paolella, A.; Brescia, R.; Prato, M.; Povia, M.; Marras, S.; Trizio, L.D.; Falqui, A.; Manna, L.; George, C. Colloidal Synthesis of Cuprite (Cu2O) Octahedral Nanocrystals and Their Electrochemical Lithiation. ACS Appl. Mater. Interfaces 2013, 5, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Paracchino, A.; Laporte, V.; Sivula, K.; Gratzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bornoz, P.; Abdi, F.F.; Tilley, S.D.; Dam, B.; van de Krol, R.; Graetzel, M.; Sivula, K. A Bismuth Vanadate–Cuprous Oxide Tandem Cell for Overall Solar Water Splitting. J. Phys. Chem. C 2014, 118, 16959–16966. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, R.; Xu, B.; Li, Y. Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 2006, 17, 3939–3943. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Q.; Flytzani-Stephanopoulos, M. Preferential oxidation of CO in H2 over CuO-CeO2 catalysts. Catal. Today 2004, 93, 241–246. [Google Scholar] [CrossRef]
- Xu, L.; Sithambaram, S.; Zhang, Y.; Chen, C.-H.; Jin, L.; Joesten, R.; Suib, S.L. Novel Urchin-like CuO Synthesized by a Facile Reflux Method with Efficient Olefin Epoxidation Catalytic Performance. Chem. Mater. 2009, 21, 1253–1259. [Google Scholar] [CrossRef]
- Xu, H.-J.; Zhao, X.-Y.; Deng, J.; Fu, Y.; Feng, Y.-S. Efficient C–S cross coupling catalyzed by Cu2O. Tetrahedron Lett. 2009, 50, 434–437. [Google Scholar] [CrossRef]
- Rout, L.; Sen, T.K.; Punniyamurthy, T. Efficient CuO-Nanoparticle-Catalyzed C–S Cross-Coupling of Thiols with Iodobenzene. Angew. Chem. Int. Ed. 2007, 46, 5583–5586. [Google Scholar] [CrossRef]
- Li, C.W.; Ciston, J.; Kanan, M.W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508, 504–507. [Google Scholar] [CrossRef]
- Eilert, A.; Cavalca, F.; Roberts, F.S.; Osterwalder, J.; Liu, C.; Favaro, M.; Crumlin, E.J.; Ogasawara, H.; Friebel, D.; Pettersson, L.G.M.; et al. Subsurface Oxygen in Oxide-Derived Copper Electrocatalysts for Carbon Dioxide Reduction. J. Phys. Chem. Lett. 2017, 8, 285–290. [Google Scholar] [CrossRef]
- Kas, R.; Kortlever, R.; Milbrat, A.; Koper, M.T.M.; Mul, G.; Baltrusaitis, J. Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: Controlling the catalytic selectivity of hydrocarbons. Phys. Chem. Chem. Phys. 2014, 16, 12194–12201. [Google Scholar] [CrossRef]
- Lignier, P.; Bellabarba, R.; Tooze, R.P. Scalable strategies for the synthesis of well-defined copper metal and oxide nanocrystals. Chem. Soc. Rev. 2012, 41, 1708–1720. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, M.H. Facile Synthesis of Cu2O Nanocrystals with Systematic Shape Evolution from Cubic to Octahedral Structures. J. Phys. Chem. C 2008, 112, 18355–18360. [Google Scholar] [CrossRef]
- Ho, J.-Y.; Huang, M.H. Synthesis of Submicrometer-Sized Cu2O Crystals with Morphological Evolution from Cubic to Hexapod Structures and Their Comparative Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 14159–14164. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Zhu, W. Shape Evolution and Size-Controllable Synthesis of Cu2O Octahedra and Their Morphology-Dependent Photocatalytic Properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, G.; Murugan, R. Synthesis of Cu2O microcrystals with morphological evolution from octahedral to microrod through a simple surfactant-free chemical route. CrystEngComm 2012, 14, 8338–8341. [Google Scholar] [CrossRef]
- Periasamy, A.P.; Liu, J.; Lin, H.-M.; Chang, H.-T. Synthesis of copper nanowire decorated reduced graphene oxide for electro-oxidation of methanol. J. Mater. Chem. A 2013, 1, 5973–5981. [Google Scholar] [CrossRef]
- Zhang, W.; Wen, X.; Yang, S.; Berta, Y.; Wang, Z.L. Single-Crystalline Scroll-Type Nanotube Arrays of Copper Hydroxide Synthesized at Room Temperature. Adv. Mater. 2003, 15, 822–825. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, M.H. Fabrication of Truncated Rhombic Dodecahedral Cu2O Nanocages and Nanoframes by Particle Aggregation and Acidic Etching. J. Am. Chem. Soc. 2008, 130, 12815–12820. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Z.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Nørskov, J.K.; Jaramillo, T.F. Combining Theory and Experiment in Electrocatalysis: Insights into Materials Design. Science 2017, 355, eaad4998. [Google Scholar]
- Klinkova, A.; De Luna, P.; Dinh, C.T.; Voznyy, O.; Larin, E.M.; Kumacheva, E.; Sargent, E.H. Rational Design of Efficient Palladium Catalysts for Electroreduction of Carbon Dioxide to Formate. ACS Catal. 2016, 6, 8115–8120. [Google Scholar] [CrossRef]
- Hwee, C.; Ng, B.; Fan, W.Y. Shape Evolution of Cu2O Nanostructures via Kinetic and Thermodynamic Controlled Growth. J. Phys. Chem. B 2006, 110, 20801–20807. [Google Scholar]
- Basiratnia, A.; Rempel, J.; Li, F.; Pogodaev, A.; Zienchuk, T.A.; Klinkova, A. Cu(II)-nanoparticle-derived structures under CO2 reduction conditions: A matter of shape. Phys. Chem. Chem. Phys. 2019, 21, 5894–5897. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by Particle Attachment in Synthetic, Biogenic, and Geologic Environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A. Morphological Study and Potential Applications of Nano Matal-Organic Coordination Polymers. RSC Adv. 2013, 3, 19191–19218. [Google Scholar] [CrossRef]
- Liu, K.; Shen, Z.-R.; Li, Y.; Han, S.-D.; Hu, T.-L.; Zhang, D.-S.; Bu, X.-H.; Ruan, W.-J. Solvent Induced Rapid Modulation of Micro/Nano Structures of Metal Carboxylates Coordination Polymers: Mechanism and Morphology Dependent Magnetism. Sci. Rep. 2014, 4, 6023. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Littrell, D.M.; Bowers, D.H.; Tatarchuk, B.J. Hydrazine reduction of transition-metal oxides. J. Chem. Soc. Faraday Trans. 1 1987, 83, 3271–3282. [Google Scholar] [CrossRef]
- Djokić, S.S. Electroless Deposition of Cobalt Using Hydrazine as a Reducing Agent. J. Electrochem. Soc. 1997, 144, 2358–2363. [Google Scholar] [CrossRef]
- Eluri, R.; Paul, B. Synthesis of nickel nanoparticles by hydrazine reduction: Mechanistic study and continuous flow synthesis. J. Nanopart. Res. 2012, 14, 800. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Sinatra, L.; Elshewy, A.; Huang, K.W.; Katsiev, K.; Kirmani, A.R.; Amassian, A.; Anjum, D.H.; Bakr, O.M. Synthesis of Copper Hydroxide Branched Nanocages and Their Transformation to Copper Oxide. J. Phys. Chem. C 2014, 118, 19374–19379. [Google Scholar] [CrossRef]
- Cai, R.; Yang, D.; Peng, S.; Chen, X.; Huang, Y.; Liu, Y.; Hou, W.; Yang, S.; Liu, Z.; Tan, W. Single Nanoparticle to 3D Supercage: Framing for an Artificial Enzyme System. J. Am. Chem. Soc. 2015, 137, 13957–13963. [Google Scholar] [CrossRef]
- Heaton, B.T.; Jacob, C.; Page, P. Transition metal complexes containing hydrazine and substituted hydrazines. Coord. Chem. Rev. 1996, 154, 193–229. [Google Scholar] [CrossRef]
- Brown, D.B.; Donner, J.A.; Hall, J.W.; Wilson, S.R.; Wilson, R.B.; Hodgson, D.J.; Hatfield, W.E. Interaction of hydrazine with copper(II) chloride in acidic solutions. Formation, spectral and magnetic properties, and structures of copper(II), copper(I), and mixed-valence species. Inorg. Chem. 1979, 18, 2635–2641. [Google Scholar] [CrossRef]
- Nicholls, D.; Swlndells, R. Hydrazine complexes of copper(I) chloride. J. Inorg. Nucl. Chem. 1969, 31, 3313–3315. [Google Scholar] [CrossRef]
- Sui, Y.; Fu, W.; Yang, H.; Zeng, Y.; Zhang, Y.; Zhao, Q.; Li, Y.; Zhou, X.; Leng, Y.; Li, M.; et al. Low Temperature Synthesis of Cu2O Crystals: Shape Evolution and Growth Mechanism. Cryst. Growth Des. 2010, 10, 99–108. [Google Scholar] [CrossRef]
- Su, D.S.; Zhang, B.; Schlögl, R. Electron Microscopy of Solid Catalysts—Transforming from a Challenge to a Toolbox. Chem. Rev. 2015, 115, 2818–2882. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jirgensons, B. Solubility and fractionation of polyvinylpyrrolidone. J. Polym. Sci. 1952, 8, 519–527. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedeva, X.; Vidyakina, A.; Li, F.; Mereshchenko, A.; Klinkova, A. Reductive and Coordinative Effects of Hydrazine in Structural Transformations of Copper Hydroxide Nanoparticles. Nanomaterials 2019, 9, 1445. https://doi.org/10.3390/nano9101445
Medvedeva X, Vidyakina A, Li F, Mereshchenko A, Klinkova A. Reductive and Coordinative Effects of Hydrazine in Structural Transformations of Copper Hydroxide Nanoparticles. Nanomaterials. 2019; 9(10):1445. https://doi.org/10.3390/nano9101445
Chicago/Turabian StyleMedvedeva, Xenia, Aleksandra Vidyakina, Feng Li, Andrey Mereshchenko, and Anna Klinkova. 2019. "Reductive and Coordinative Effects of Hydrazine in Structural Transformations of Copper Hydroxide Nanoparticles" Nanomaterials 9, no. 10: 1445. https://doi.org/10.3390/nano9101445
APA StyleMedvedeva, X., Vidyakina, A., Li, F., Mereshchenko, A., & Klinkova, A. (2019). Reductive and Coordinative Effects of Hydrazine in Structural Transformations of Copper Hydroxide Nanoparticles. Nanomaterials, 9(10), 1445. https://doi.org/10.3390/nano9101445






