Novel Polyvinyl Alcohol (PVA)/Cellulose Nanocrystal (CNC) Supramolecular Composite Hydrogels: Preparation and Application as Soil Conditioners
Abstract
1. Introduction
2. Experimental
2.1. Materials
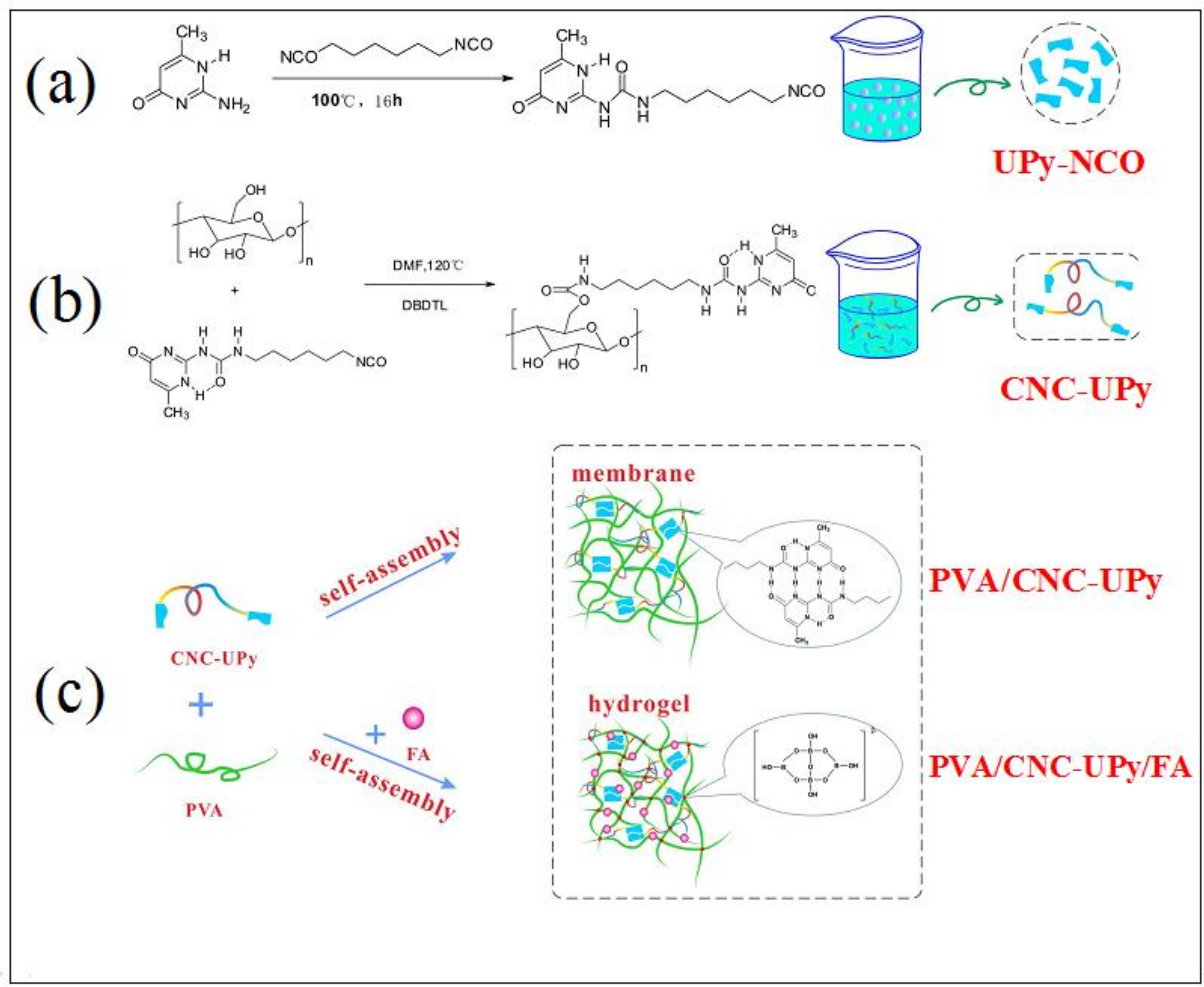
2.2. Preparation of CNC-UPy
2.3. Preparation of PVA Supramolecular Composite Membranes
2.4. Preparation of PVA Supramolecular Composite Hydrogels
2.5. Characterization
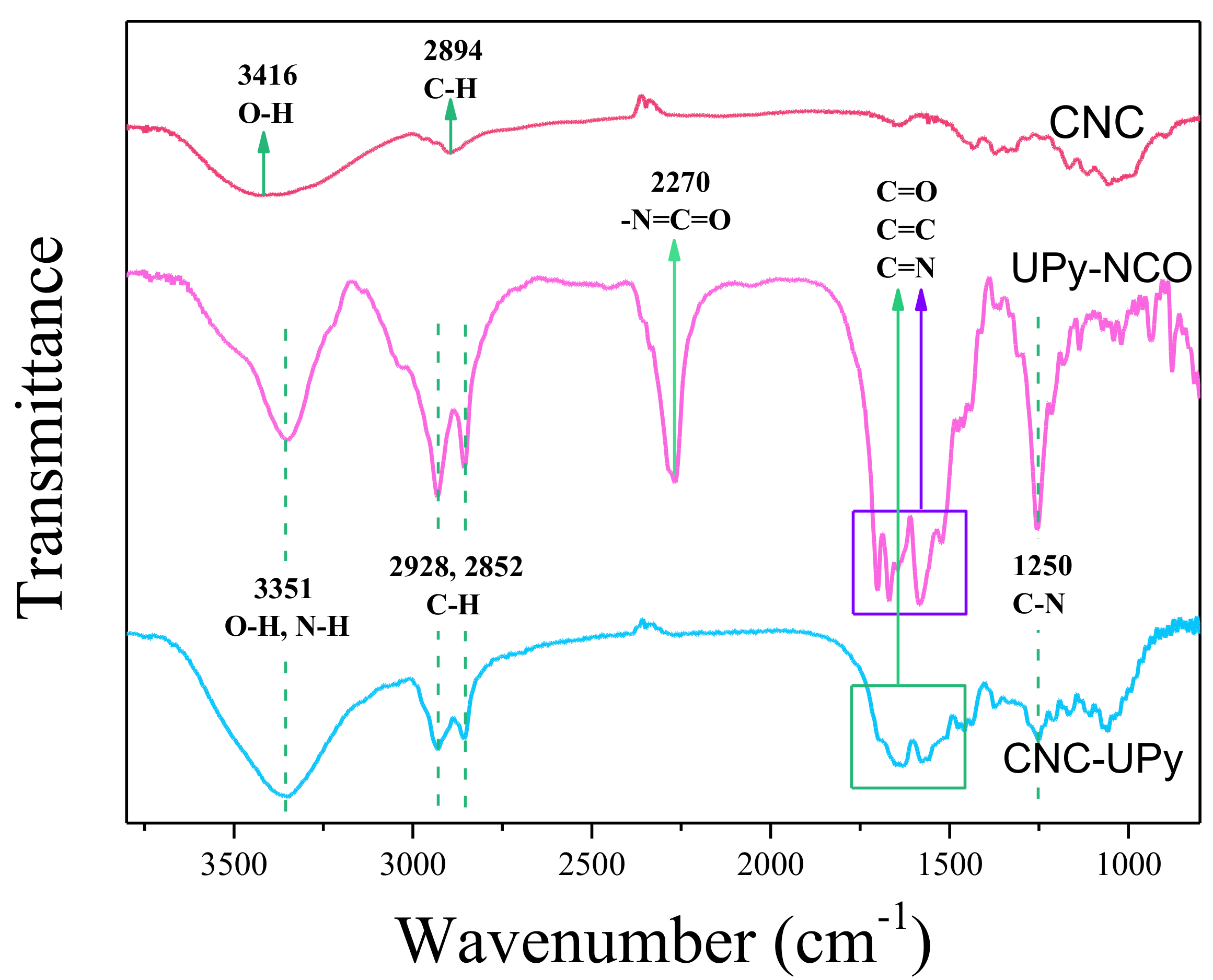
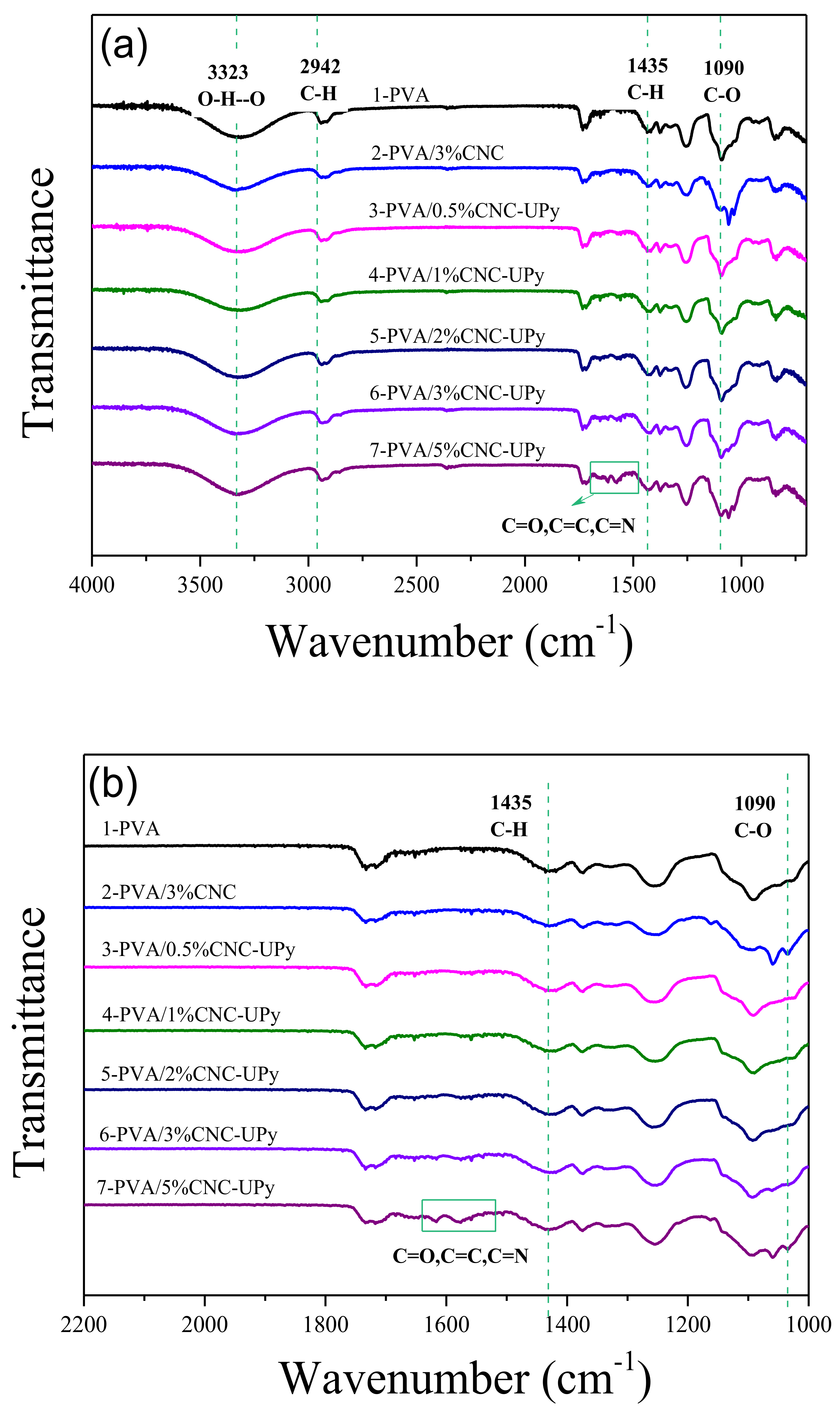
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.5.2. X-ray Diffraction (XRD)
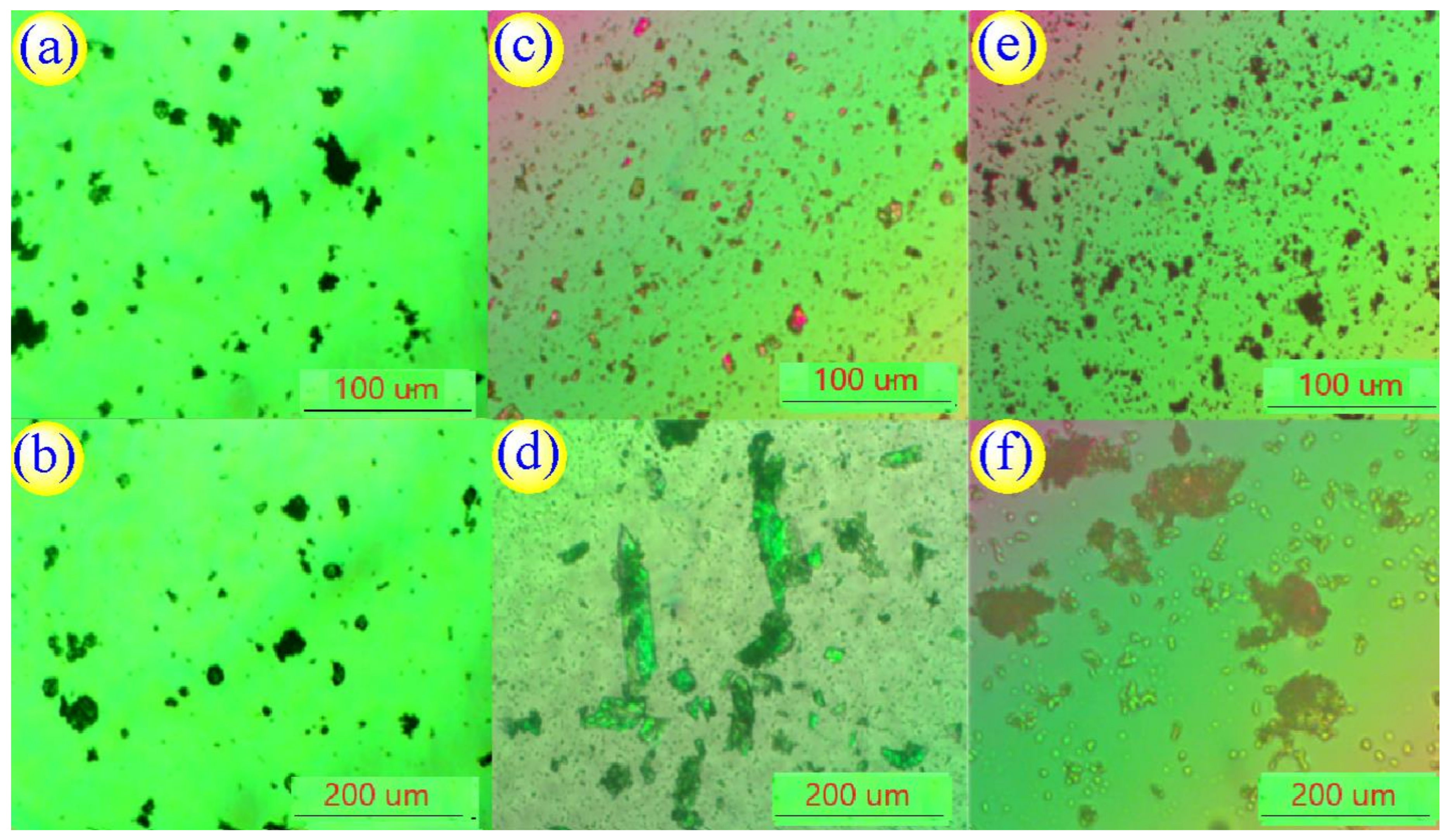
2.5.3. Scanning Electron Microscopy (SEM) and Polarizing Microscope (POM)
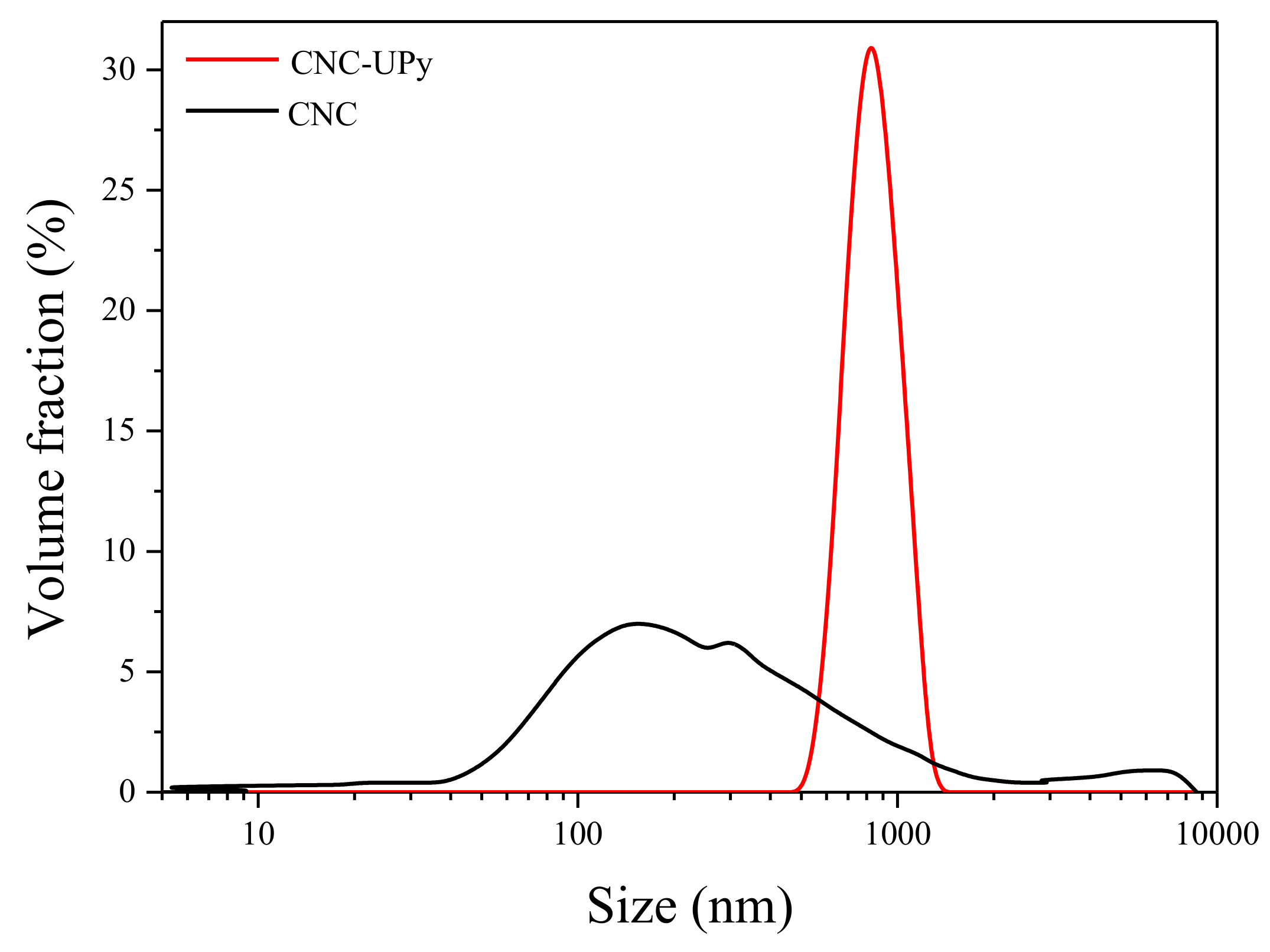
2.5.4. Particle Size Distribution (PSD)
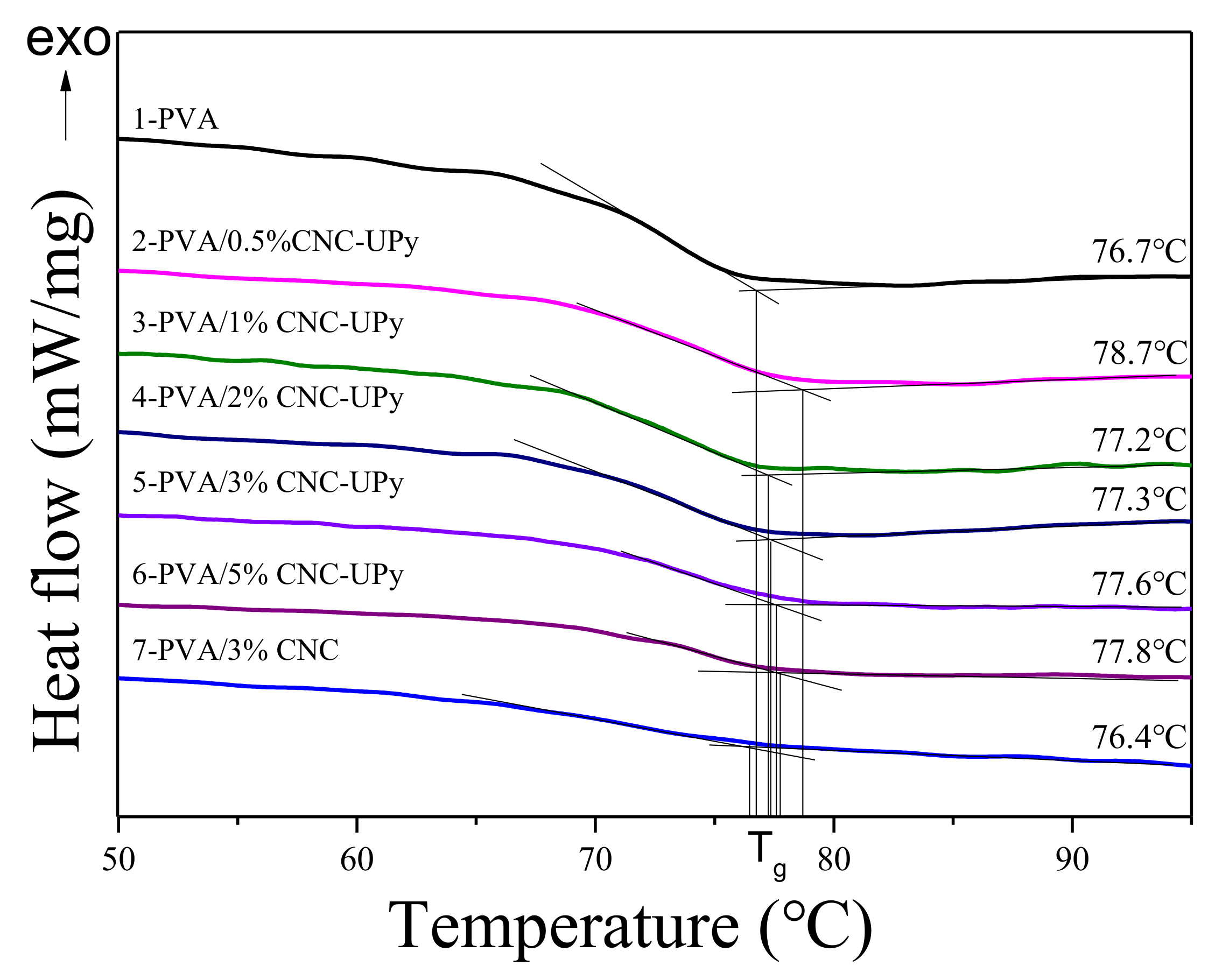
2.5.5. Differential Scanning Calometry (DSC)
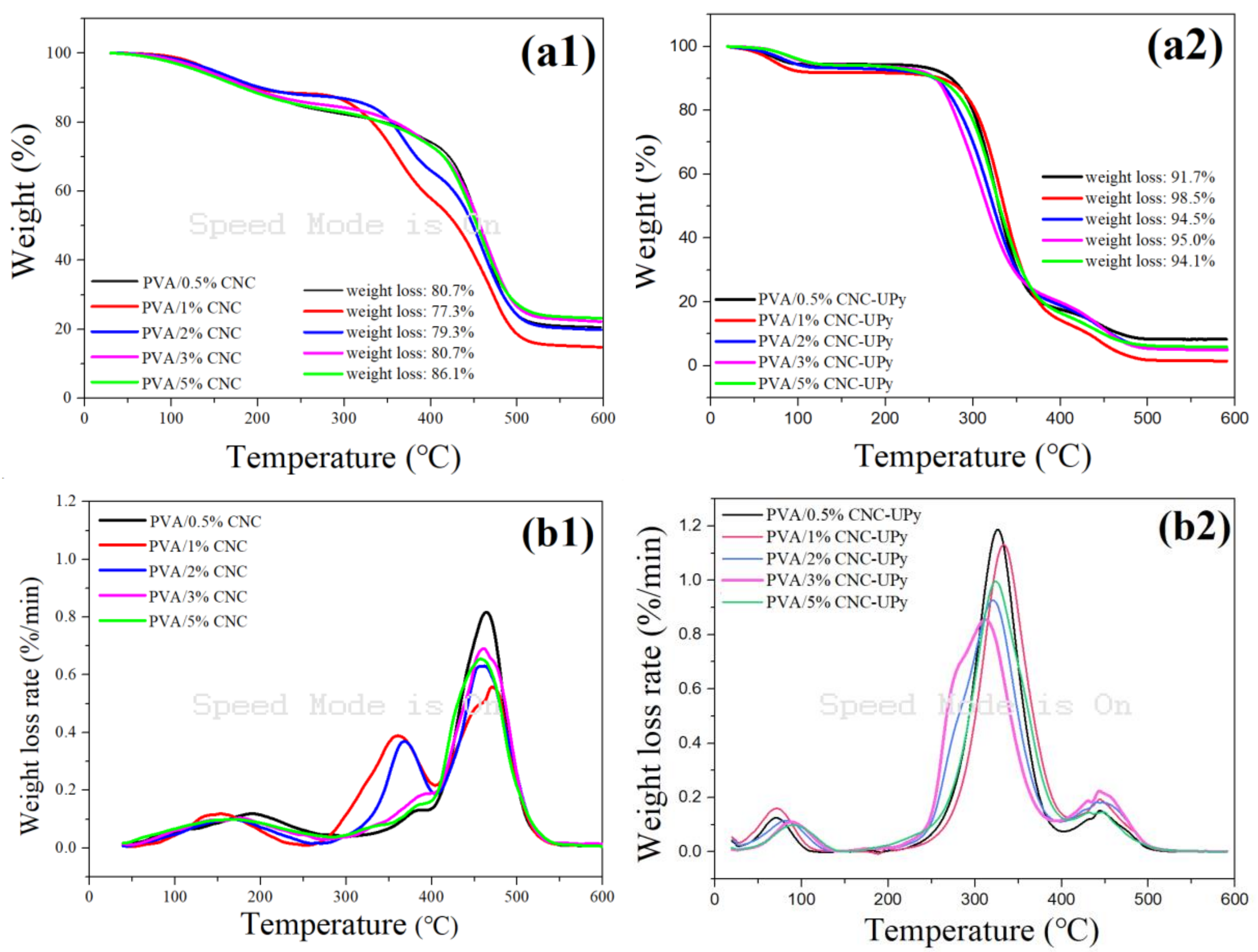
2.5.6. Thermogravimetric Analysis (TGA)
2.5.7. Swelling Behavior Analysis
2.5.8. Sustained Release Behavior Analysis
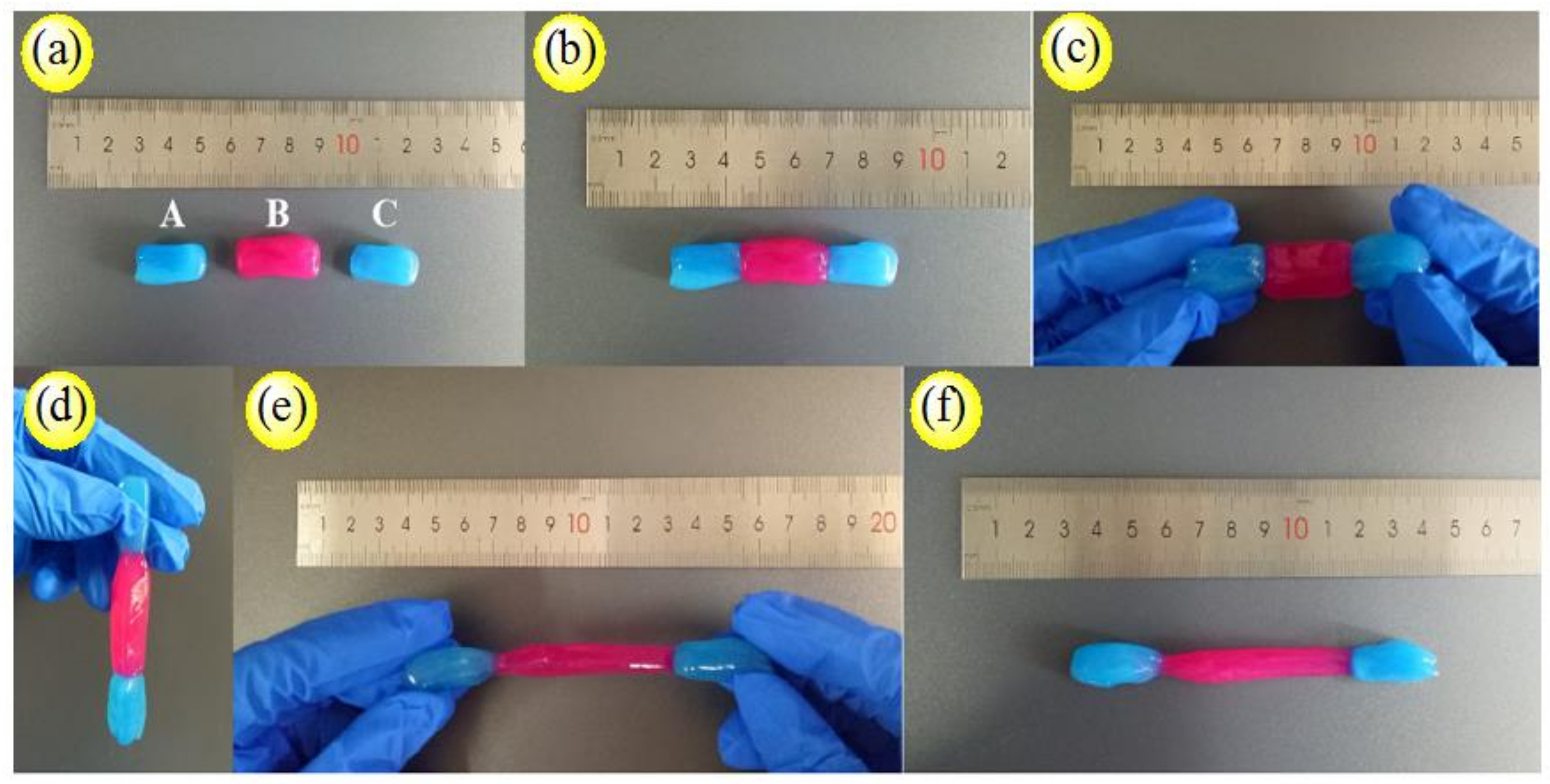
2.5.9. Self-healing Performance Analysis
3. Results and Discussion
3.1. Preparation Mechanism of PVA Supramolecular Composite Hydrogels for Soil Conditioner
3.2. Analysis of CNC-UPy Crystals.
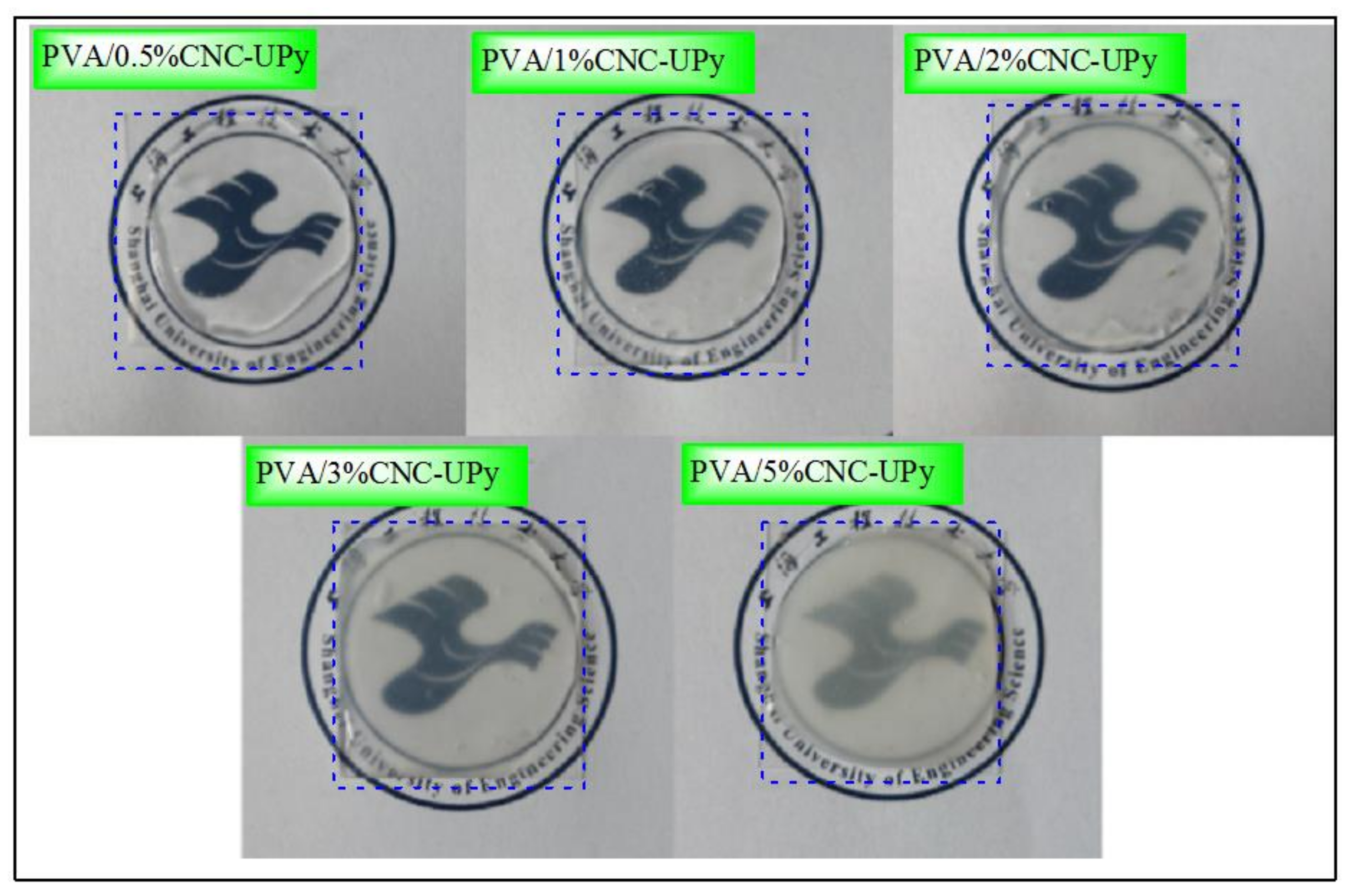
3.3. Analysis of PVA/CNC-UPy Composite Membranes
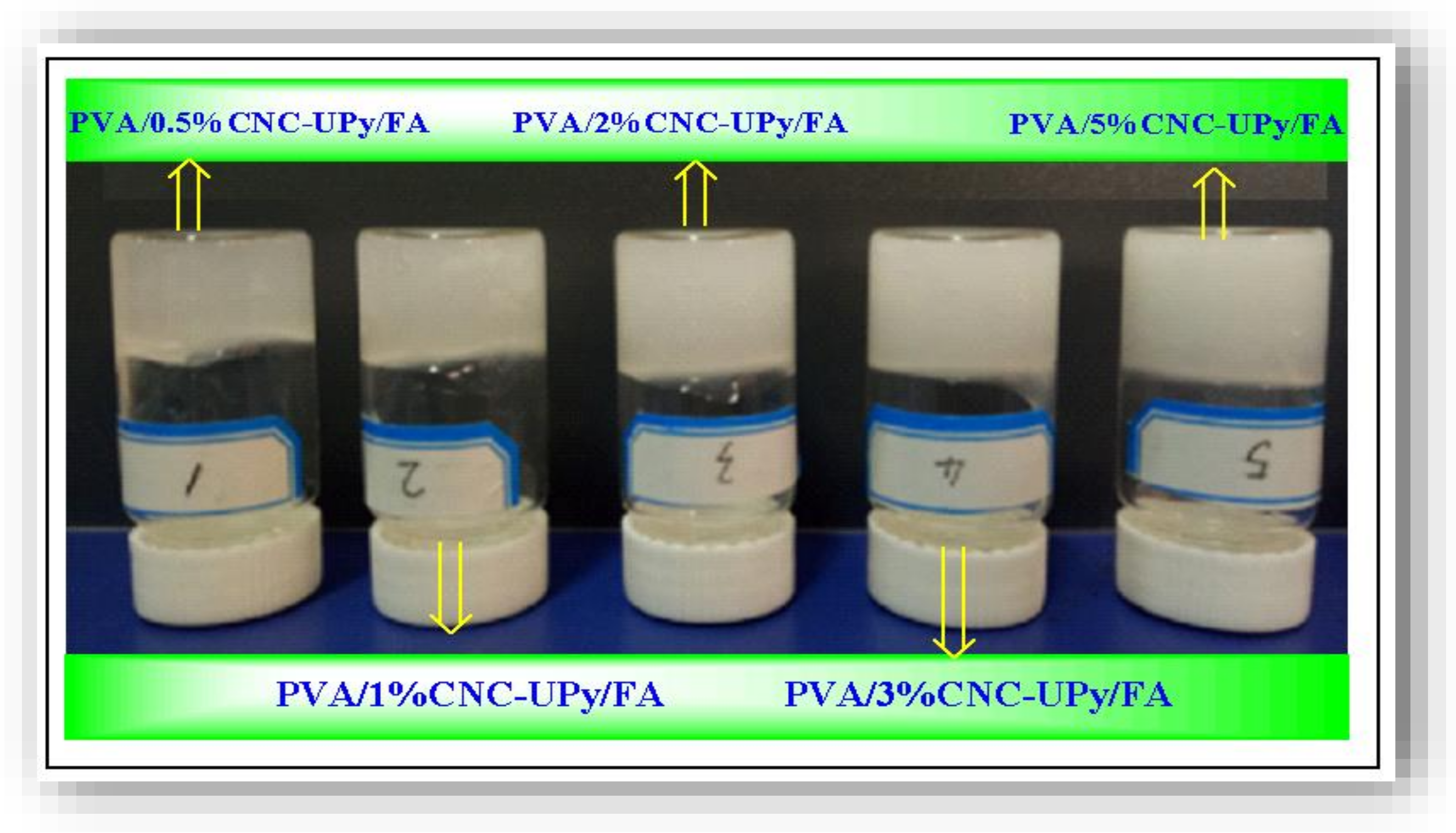
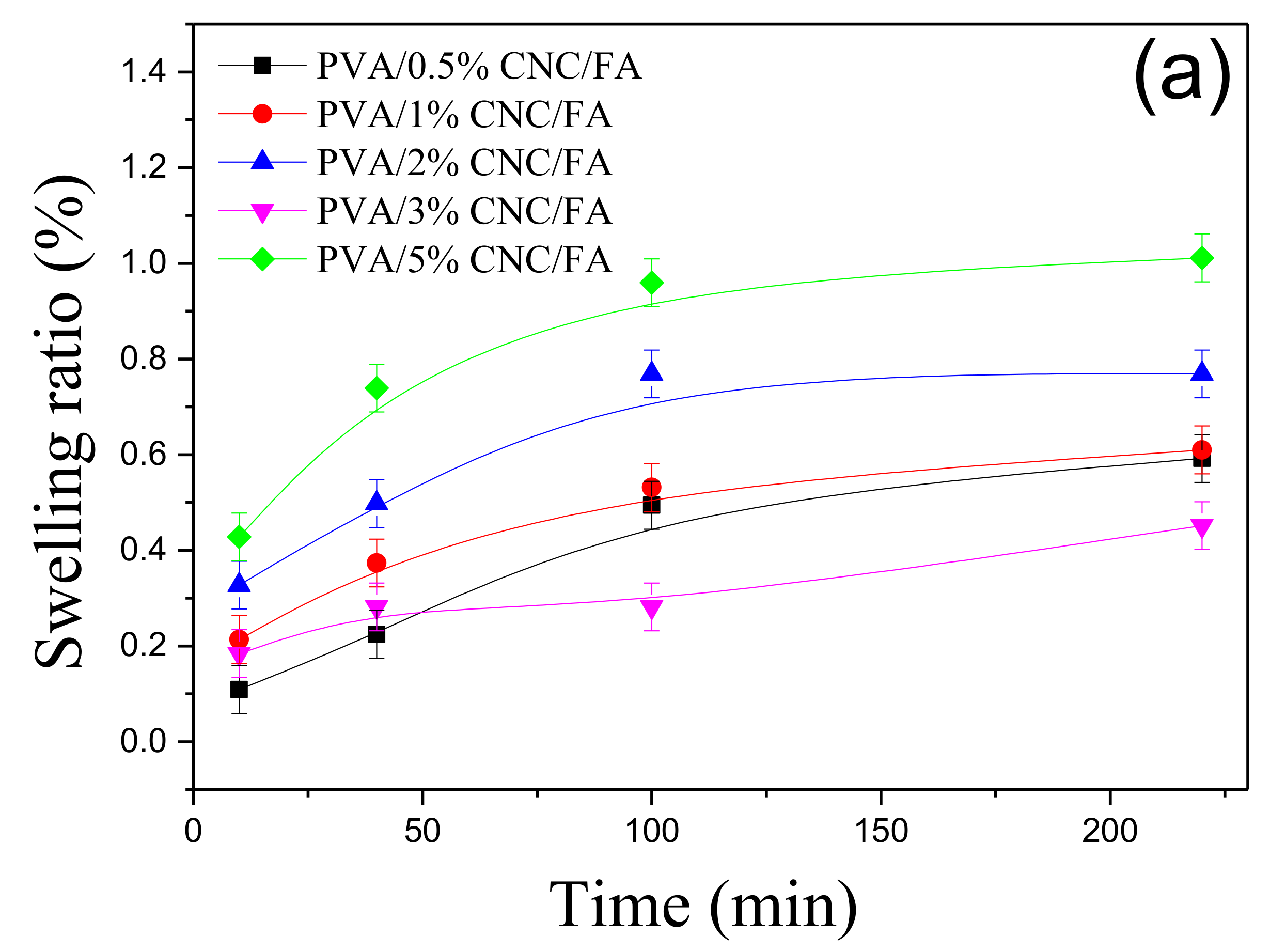
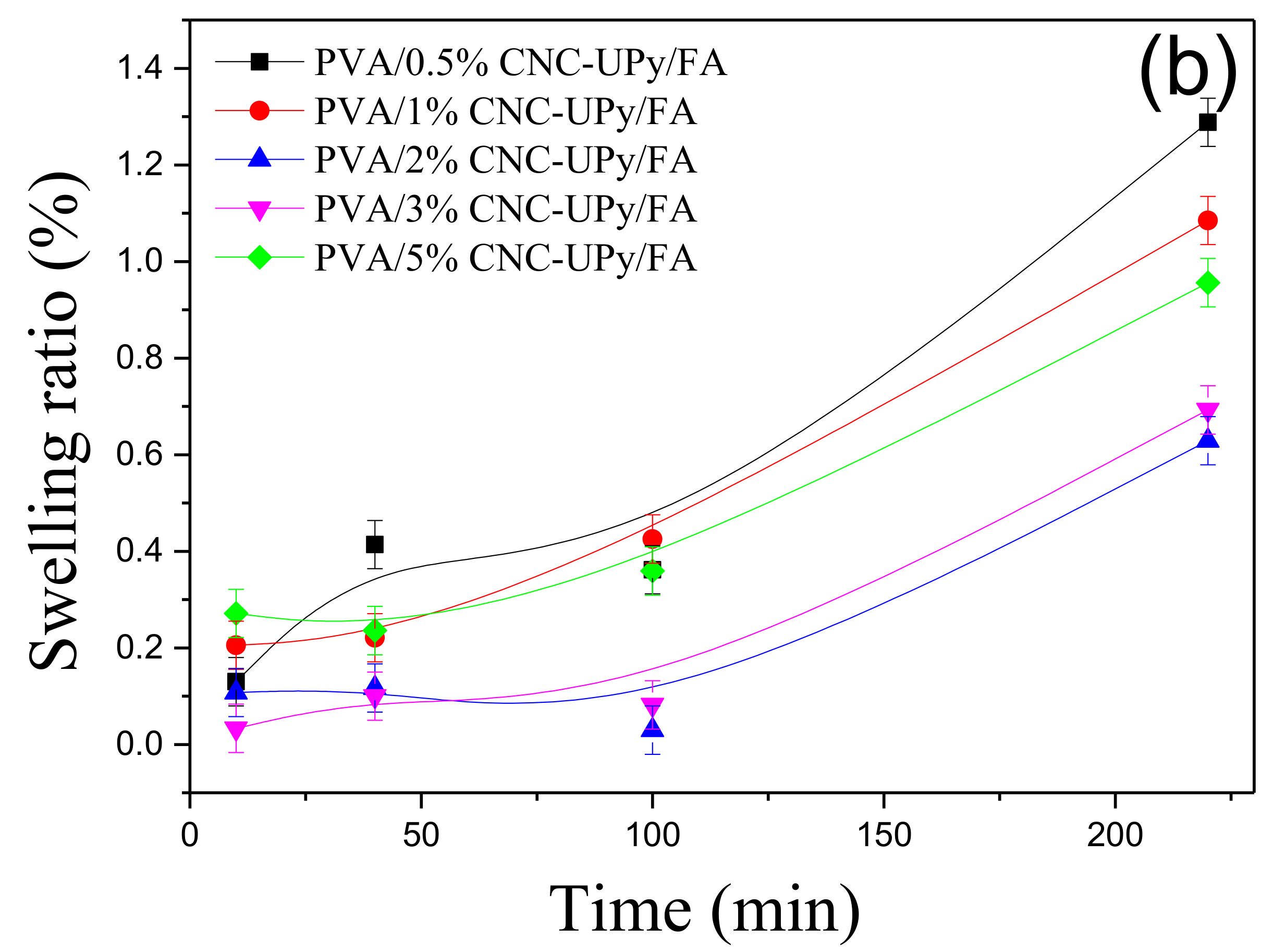
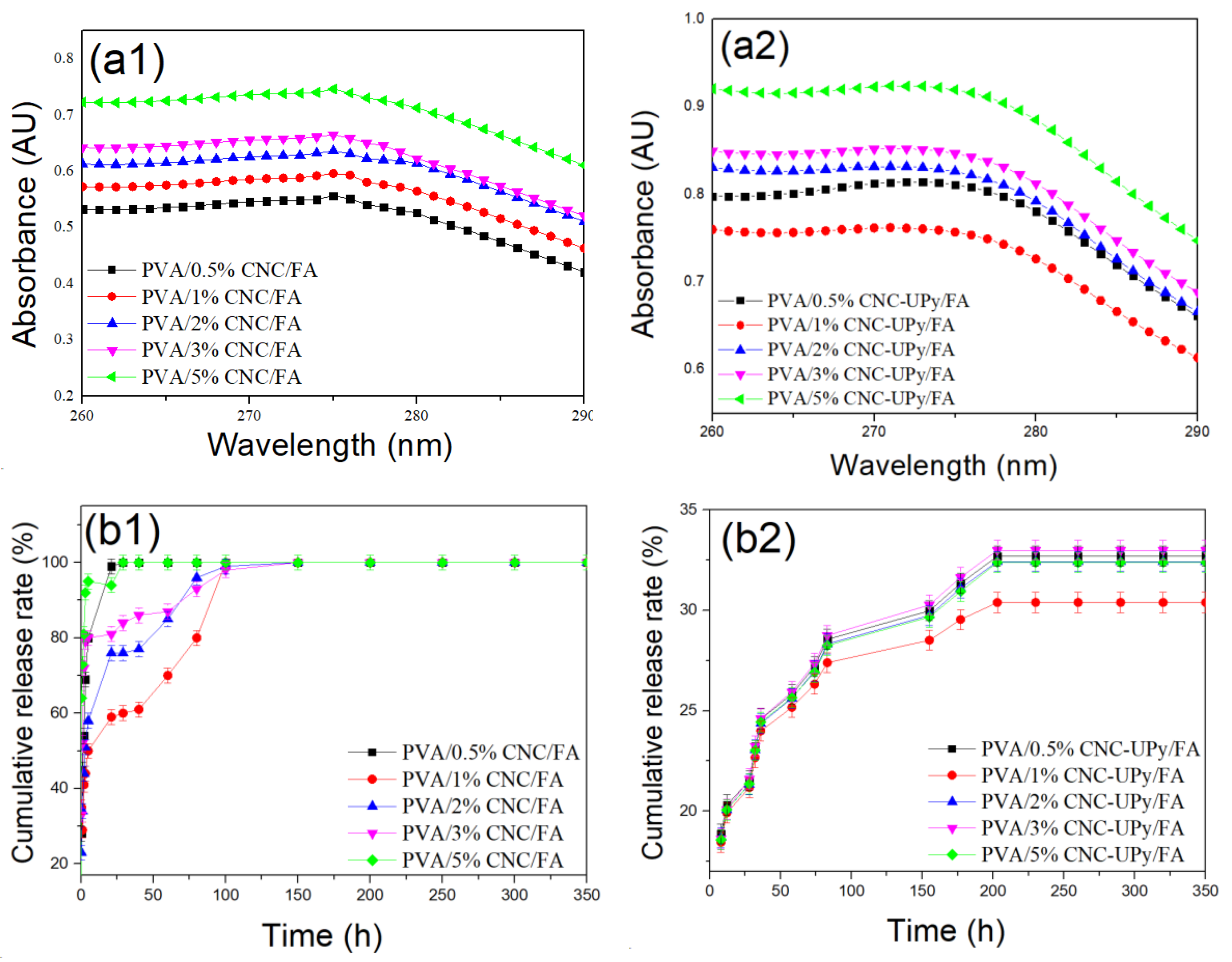
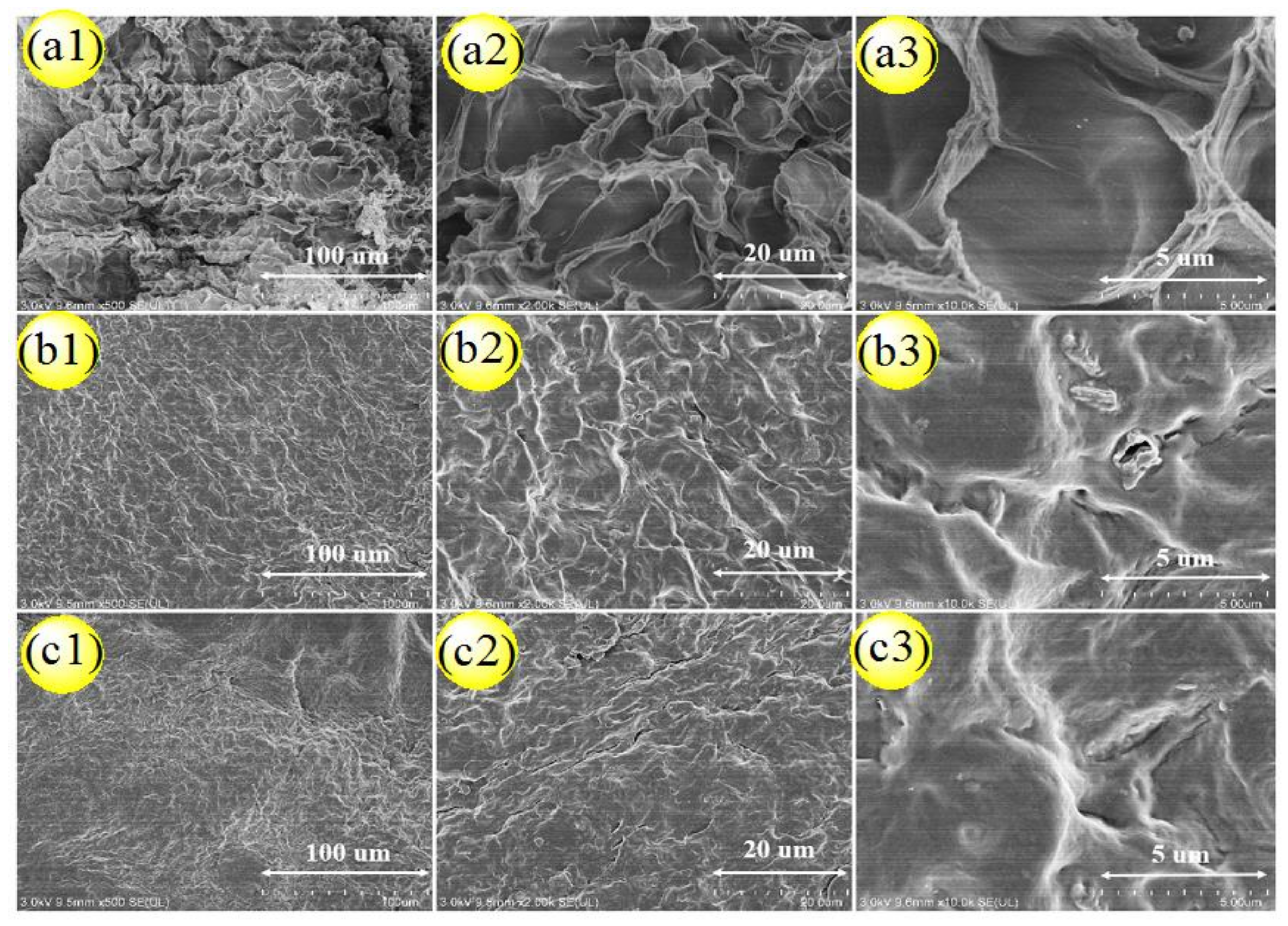
3.4. Analysis of PVA/CNC-UPy Composite Hydrogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hopfenberg, H.B.; Apicella, A.; Saleeby, D.E. Factors affecting water sorption in and solute release from glassy ethylene-vinyl alcohol copolymers. J. Membr. Sci. 1981, 8, 273–282. [Google Scholar] [CrossRef]
- Grunlan, J.C.; Grigorian, A.; Hamilton, C.B.; Mehrabi, A.R. Effect of clay concentration on the oxygen permeability and optical properties of a modified poly (vinyl alcohol). J. Appl. Polym. Sci. 2004, 93, 1102–1109. [Google Scholar] [CrossRef]
- Yi, G.; Fan, X.; Quan, X.; Chen, S.; Yu, H. Comparison of CNT-PVA membrane and commercial polymeric membranes in treatment of emulsified oily wastewater. Front. Environ. Sci. Eng. 2019, 13, 23. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, Y.; Fang, J.; Liu, W.; Zhou, Z. Preparation and characterization of PVA/SA/HA composite hydrogels for wound dressing. Int. J. Polym. Anal. Charact. 2019, 24, 132–141. [Google Scholar] [CrossRef]
- Abdel Bary, E.M.; Soliman, Y.A.; Fekri, A.; Harmal, A.N. Aging of novel membranes made of PVA and cellulose nanocrystals extracted from egyptian rice husk manufactured by compression moulding process. Int. J. Environ. Stud. 2018, 75, 750–762. [Google Scholar] [CrossRef]
- Yang, J.M.; Su, W.Y.; Leu, T.L.; Yang, M.C. Evaluation of chitosan/PVA blended hydrogel membranes. J. Membr. Sci. 2004, 236, 39–51. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Hua, R.; Wang, Y.; Liu, Y.; Pang, C.; Wang, Y. Selective adsorption of uranyl ion on ion-imprinted chitosan/PVA cross-linked hydrogel. Hydrometallurgy 2010, 104, 150–155. [Google Scholar] [CrossRef]
- Salmawi, E.; Kariman, M. Application of polyvinyl alcohol (PVA)/carboxymethyl cellulose (CMC) hydrogel produced by conventional crosslinking or by freezing and thawing. J. Macromol. Sci. Part A 2007, 44, 619–624. [Google Scholar] [CrossRef]
- Sato, A.; Kabusaki, D.; Okumura, H.; Nakatani, T.; Yano, H. Surface modification of cellulose nanofibers with alkenyl succinic anhydride for high-density polyethylene reinforcement. Compos. Part A Appl. Sci. Manuf. 2015, 83, 72–79. [Google Scholar] [CrossRef]
- Lee, K.Y.; Quero, F.; Blaker, J.J.; Hill, C.A.S.; Eichhorn, S.J.; Bismarck, A. Surface only modification of bacterial cellulose nanofibres with organic acids. Cellulose 2011, 18, 595–605. [Google Scholar] [CrossRef]
- Neng, W.; Enyong, D.; Rongshi, C. Surface modification of cellulose nanocrystals. Front. Chem. Eng. China 2007, 1, 228–232. [Google Scholar]
- Huang, P.; Wu, M.; Kuga, S.; Wang, D.; Wu, D.; Huang, Y. One-step dispersion of cellulose nanofibers by mechanochemical esterification in an organic solvent. ChemSusChem 2012, 5, 2319–2322. [Google Scholar] [CrossRef] [PubMed]
- Berlioz, S.; Molina-Boisseau, S.; Nishiyama, Y.; Heux, L. Gas-phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 2009, 10, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Maeda, H.; Nakajima, M.; Hagiwara, T.; Sawaguchi, T. Preparation and mechanical properties of bacterial cellulose nanocomposites loaded with silica nanoparticles. Cellulose 2008, 15, 111–120. [Google Scholar] [CrossRef]
- Goffin, A.L.; Habibi, Y.; Raquez, J.M.; Dubois, P. Polyester-grafted cellulose nanowhiskers: A new approach for tuning the microstructure of immiscible polyester blends. ACS Appl. Mater. Interfaces 2012, 4, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Kloser, E.; Gray, D.G. Surface grafting of cellulose nanocrystals with poly(ethylene oxide) in aqueous media. Langmuir 2010, 26, 13450–13456. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaille, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Favier, V.; Cavaille, J.Y.; Canova, G.R.; Shrivastava, S.C. Mechanical percolation in cellulose whisker nanocomposites. Polym. Eng. Sci. 2010, 37, 1732–1739. [Google Scholar] [CrossRef]
- Li, Z.; Bai, H.; Zhang, S.; Wang, W. DN strategy constructed photo-crosslinked PVA/CNC/ P(NIPPAm-co-AA) hydrogels with temperature-sensitive and pH-sensitive properties. New J. Chem. 2018, 42, 13453–13460. [Google Scholar] [CrossRef]
- Popescu, M. Structure and sorption properties of cnc reinforced pva films. Int. J. Biol. Macromol. 2017, 101, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Tanpichai, S.; Oksman, K. Cross-linked nanocomposite hydrogels based on cellulose nanocrystals and pva: Mechanical properties and creep recovery. Compos. Part A Appl. Sci. Manuf. 2016, 88, 226–233. [Google Scholar] [CrossRef]
- Kang, J.; Miyajima, D.; Mori, T.; Inoue, Y.; Itoh, Y.; Aida, T. A rational strategy for the realization of chain-growth supramolecular polymerization. Science 2015, 347, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Huang, W.; Zhu, X.; Zhou, Y.; Yan, D. Cheminform abstract: Biocompatible or biodegradable hyperbranched polymers: From self-assembly to cytomimetic applications. ChemInform 2012, 43, 5986–5997. [Google Scholar] [CrossRef]
- Kushner, A.M.; Vossler, J.D.; Williams, G.A.; Guan, Z. A biomimetic modular polymer with tough and adaptive properties. J. Am. Chem. Soc. 2009, 131, 8766–8768. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.J.; Stoddart, J.F. Supramolecular polymers: Molecular machines muscle up. Nat. Nanotechnol. 2012, 8, 9–10. [Google Scholar] [CrossRef]
- Ge, Z.; Hu, J.; Huang, F.; Liu, S. Responsive supramolecular gels constructed by crown ether based molecular recognition. Angew. Chem. 2010, 48, 1798–1802. [Google Scholar] [CrossRef] [PubMed]
- Domjan, A.; Manek, E.; Geissler, E.; Lászloó, K. Host-guest interactions in poly (N-isopropylacrylamide) hydrogel seen by one- and two-dimensional 1H CRAMPS Solid-State NMR Spectroscopy. Macromolecules 2013, 46, 3118–3124. [Google Scholar] [CrossRef]
- Auletta, J.T.; Ledonne, G.J.; Gronborg, K.C.; Ladd, C.D.; Liu, H.; Clark, W.W.; Meyer, T.Y. Stimuli-responsive iron-cross-linked hydrogels that undergo redox-driven switching between hard and soft states. Macromolecules 2015, 48, 1736–1747. [Google Scholar] [CrossRef]
- Girouard, N.M.; Xu, S.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Site-selective modification of cellulose nanocrystals with isophoronediisocyanate and formation of polyurethane-cnc composites. ACS Appl. Mater. Interfaces 2016, 8, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Kam, D.; Nevo, Y.; Slattegard, R.; Rivkin, A.; Lapidot, S.; Shoseyov, O. Highly modified cellulose nanocrystals and formation of epoxy-cncnanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 28086–28095. [Google Scholar] [CrossRef] [PubMed]
- Khanjanzadeh, H.; Behrooz, R.; Bahramifar, N.; Gindl-Altmutter, W.; Griesser, T. Surface chemical functionalization of cellulose nanocrystals by 3-aminopropyltriethoxysilane. Int. J. Biol. Macromol. 2017, 106, 1288–1296. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, L.; Lin, F.; Wang, S.; Chen, Y.; Chen, X.; Huang, B. Preparation and characterization of cellulose nanocrystals via ultrasonication-assisted FeCl3-catalyzed hydrolysis. Cellulose 2014, 21, 3497–3506. [Google Scholar] [CrossRef]
- Zhang, M.; Fives, C.; Waldron, K.C.; Zhu, X.X. Self-assembly of a bile acid dimer in aqueous solutions: From nanofibers to nematic hydrogels. Langmuir 2017, 33, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Robertson, D.D.; Van Reenen, A.J.; Dicks, L.M.T. Surfactin-loaded polyvinyl alcohol (PVA) nanofibers alters adhesion of, listeria monocytogenes, to polystyrene. Mater. Sci. Eng. C 2017, 77, 27–33. [Google Scholar] [CrossRef]
- Basha, S.K.S.; Rao, M.C. Spectroscopic and discharge studies on graphene oxide doped pva/pvp blend nanocomposite polymer films. Polym. Sci. Ser. A 2018, 60, 359–372. [Google Scholar] [CrossRef]
- Gómez, I.; Otazo, E.M.; Hernández, H.; Rubio, E.; Varela, J.; Ramírez, M.; Barajasa, I.; Gordilloa, A.J. Thermal degradation study of pva derivative with pendant phenylthionecarbamate groups by DSC/TGA and GC/MS. Polym. Degrad. Stab. 2015, 112, 132–136. [Google Scholar] [CrossRef]
- De-Cai, R.; Peng, L.; Lei, Q.; Pei-Feng, H.E.; Yu-Hong, D.U.; Rui, X. Effect of mixture of plasticizer on properties of PVA films. J. Mater. Eng. 2012, 2, 86–90. [Google Scholar]
- Otsuka, E.; Komiya, S.; Sasaki, S.; Xing, J.; Bando, Y.; Hirashima, Y.; Sugiyama, M.; Suzuki, A. Effects of preparation temperature on swelling and mechanical properties of pva cast gels. Soft Matter 2012, 8, 8129. [Google Scholar] [CrossRef]
- Lu, B.; Lin, F.; Jiang, X.; Cheng, J.; Lu, Q.; Song, J.; Chen, C.; Huang, B. One-pot assembly of microfibrillated cellulose reinforced PVA-borax hydrogels with self-healing and PH responsive properties. ACS Sustain. Chem. Eng. 2016, 5, 948–956. [Google Scholar] [CrossRef]
- Samadi, N.; Sabzi, M.; Babaahmadi, M. Self-healing and tough hydrogels with physically cross-linked triple networks based on Agar/PVA/graphene. Int. J. Biol. Macromol. 2017, 107, 2291–2297. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ding, Y.; Wang, J. Novel Polyvinyl Alcohol (PVA)/Cellulose Nanocrystal (CNC) Supramolecular Composite Hydrogels: Preparation and Application as Soil Conditioners. Nanomaterials 2019, 9, 1397. https://doi.org/10.3390/nano9101397
Wang Z, Ding Y, Wang J. Novel Polyvinyl Alcohol (PVA)/Cellulose Nanocrystal (CNC) Supramolecular Composite Hydrogels: Preparation and Application as Soil Conditioners. Nanomaterials. 2019; 9(10):1397. https://doi.org/10.3390/nano9101397
Chicago/Turabian StyleWang, Zuo, Yaoke Ding, and Jincheng Wang. 2019. "Novel Polyvinyl Alcohol (PVA)/Cellulose Nanocrystal (CNC) Supramolecular Composite Hydrogels: Preparation and Application as Soil Conditioners" Nanomaterials 9, no. 10: 1397. https://doi.org/10.3390/nano9101397
APA StyleWang, Z., Ding, Y., & Wang, J. (2019). Novel Polyvinyl Alcohol (PVA)/Cellulose Nanocrystal (CNC) Supramolecular Composite Hydrogels: Preparation and Application as Soil Conditioners. Nanomaterials, 9(10), 1397. https://doi.org/10.3390/nano9101397





