Dynamic Imaging of Transferrin Receptor Molecules on Single Live Cell with Bridge Gaps-Enhanced Raman Tags
Abstract
1. Introduction
2. Materials and Methods
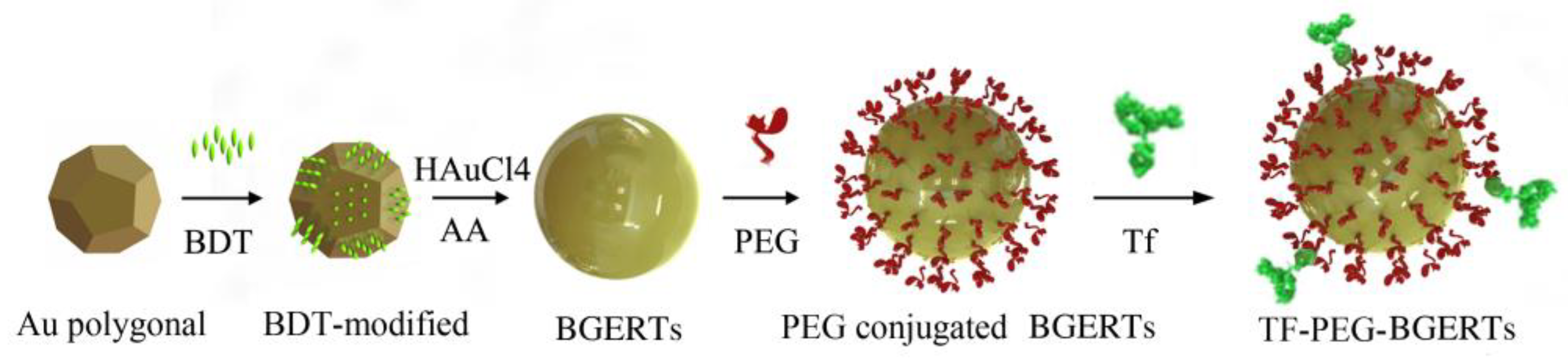
2.1. Synthesis of Functionalized Bridge Gaps-Enhanced Raman Tags (BGERTs) and Surface-Enhanced Raman Scattering (SERS) Imaging
2.2. Analysis of the Influence of the “Hotspots” Effect on the SERS Signal
2.3. Toxicity Testing
3. Results and discussion
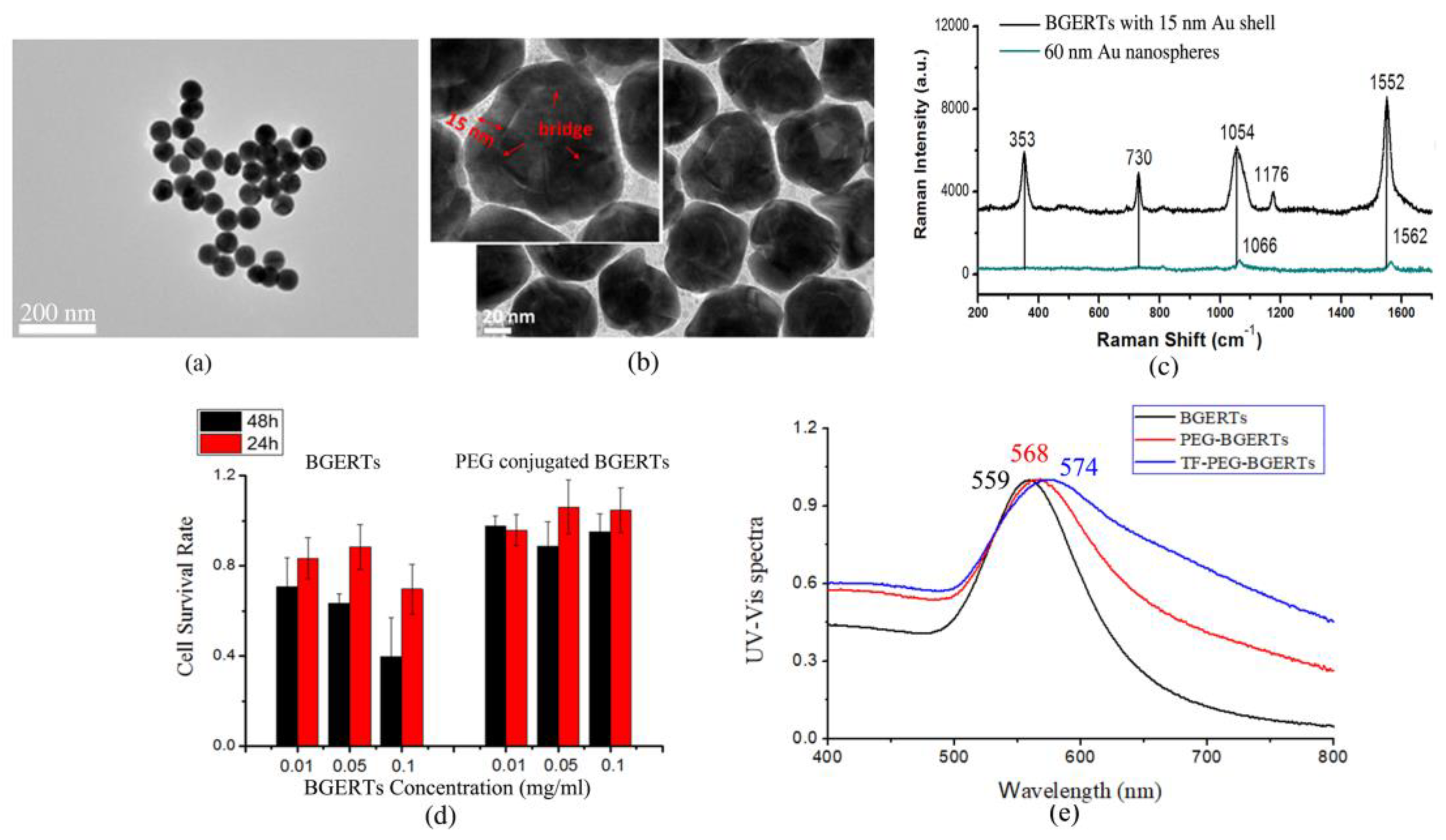
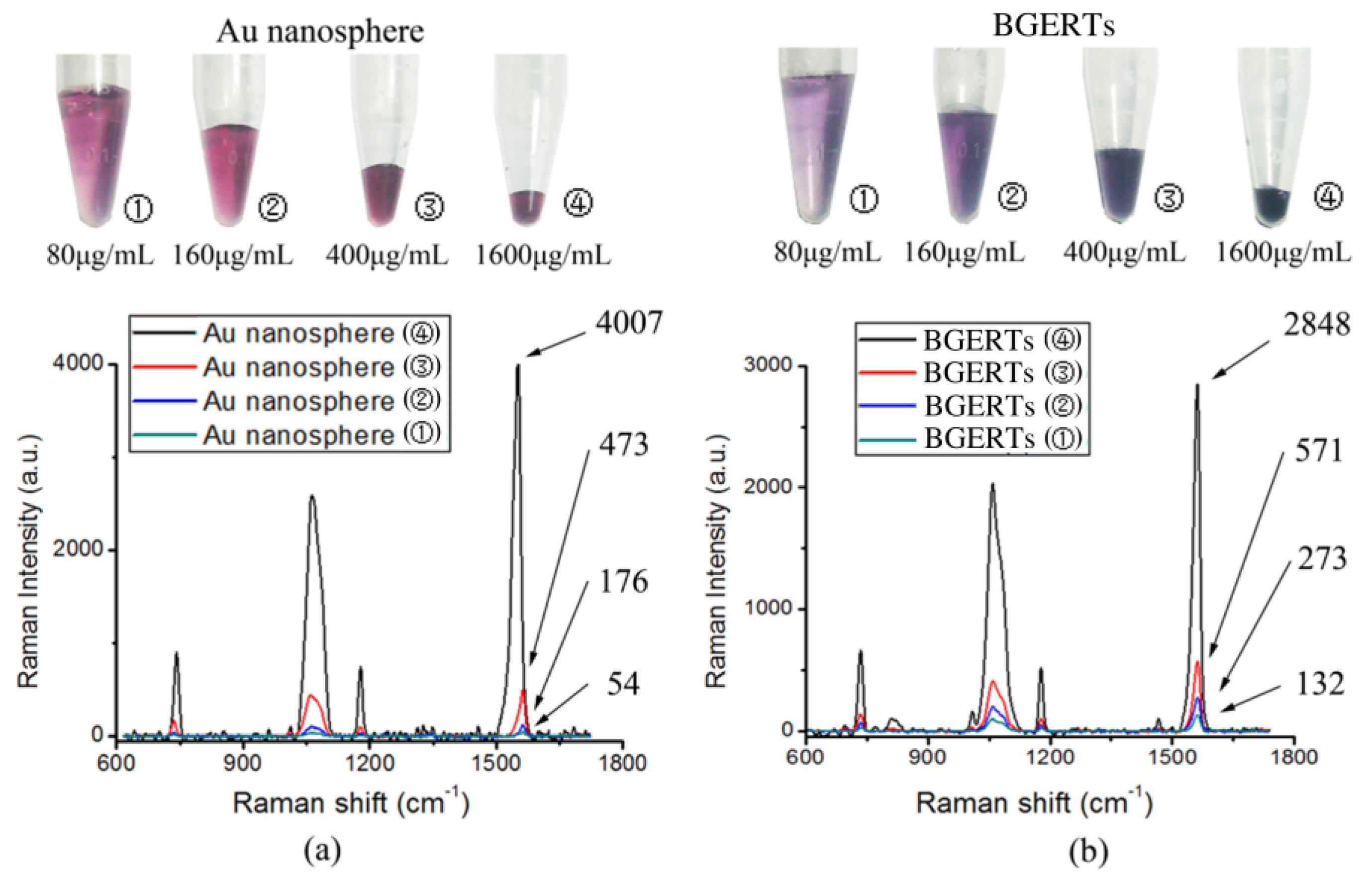
3.1. BGERTs Characterization and SERS Imaging
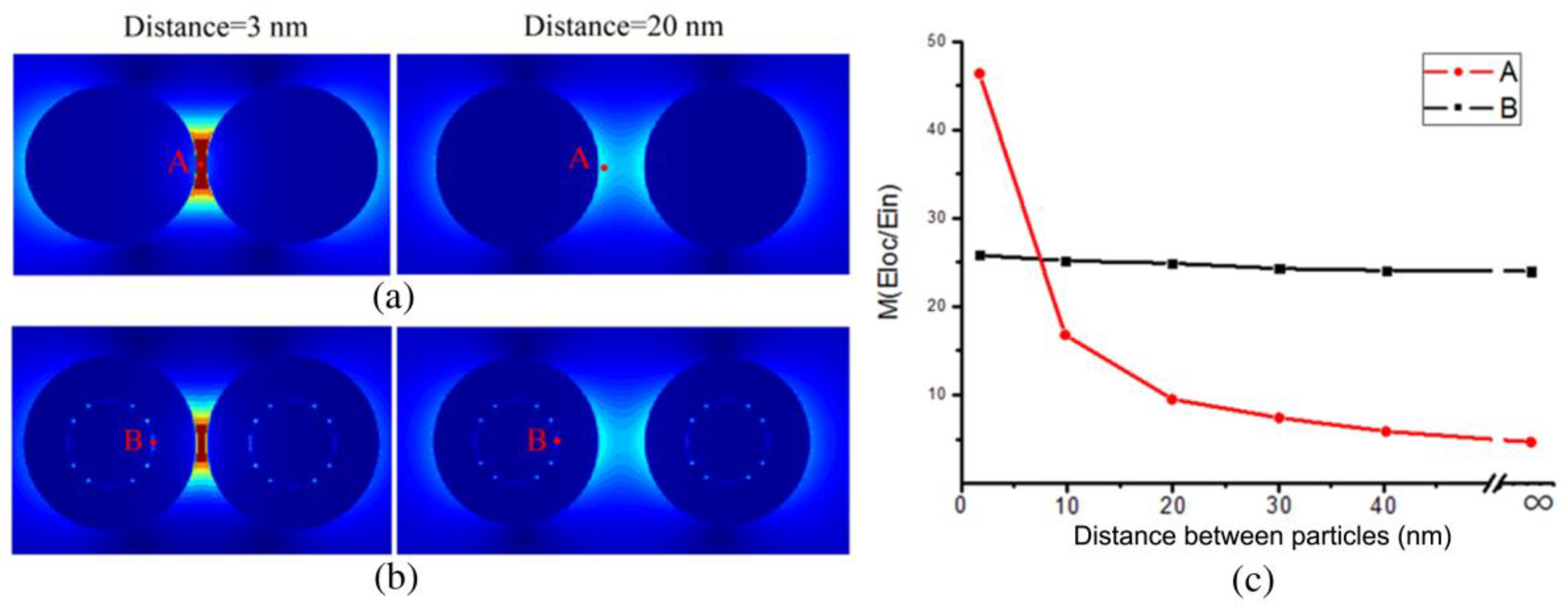
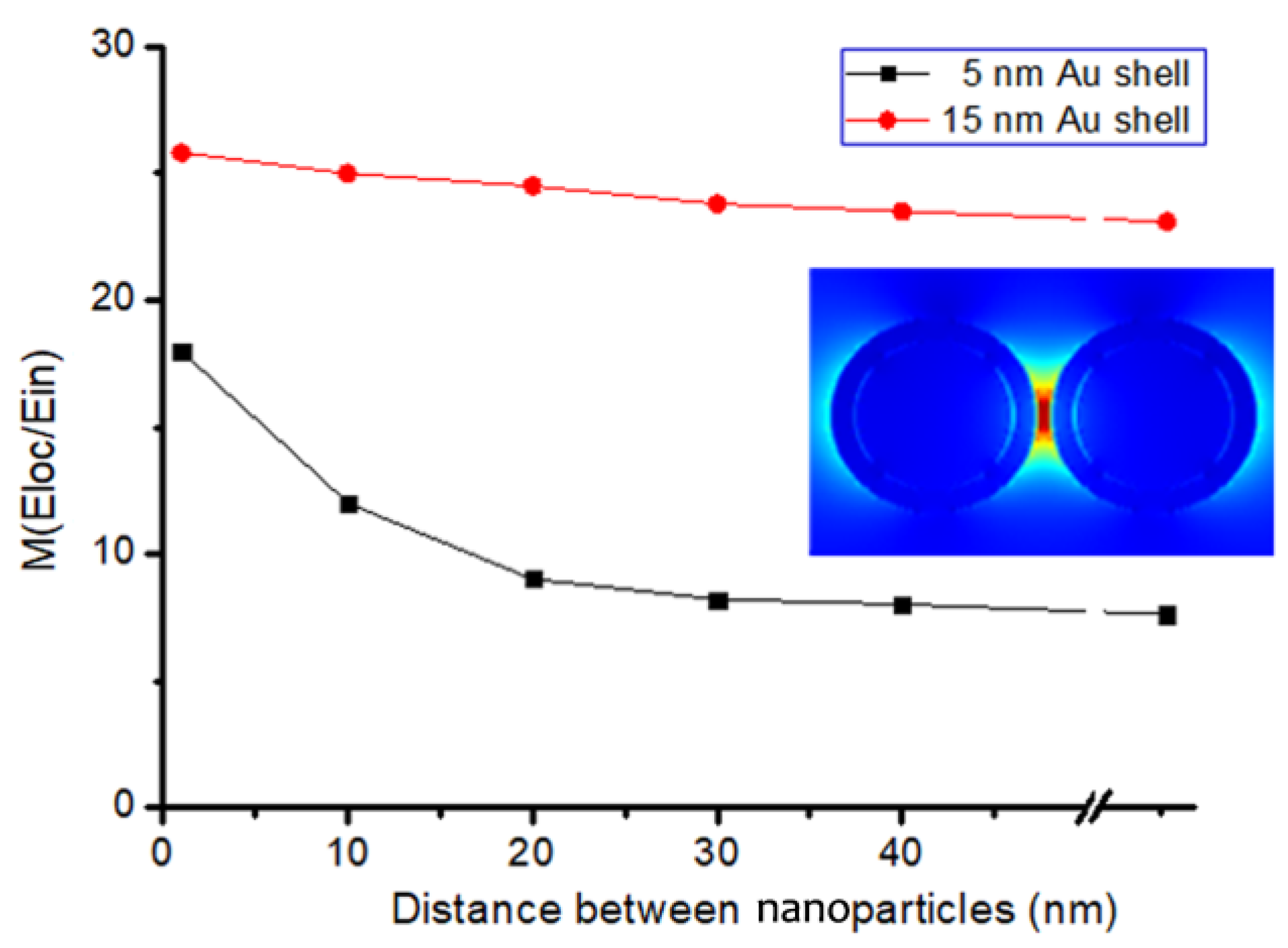
3.2. Analysis of the Influence of the “Hotspots” Effect on the Tags’ SERS Intensity
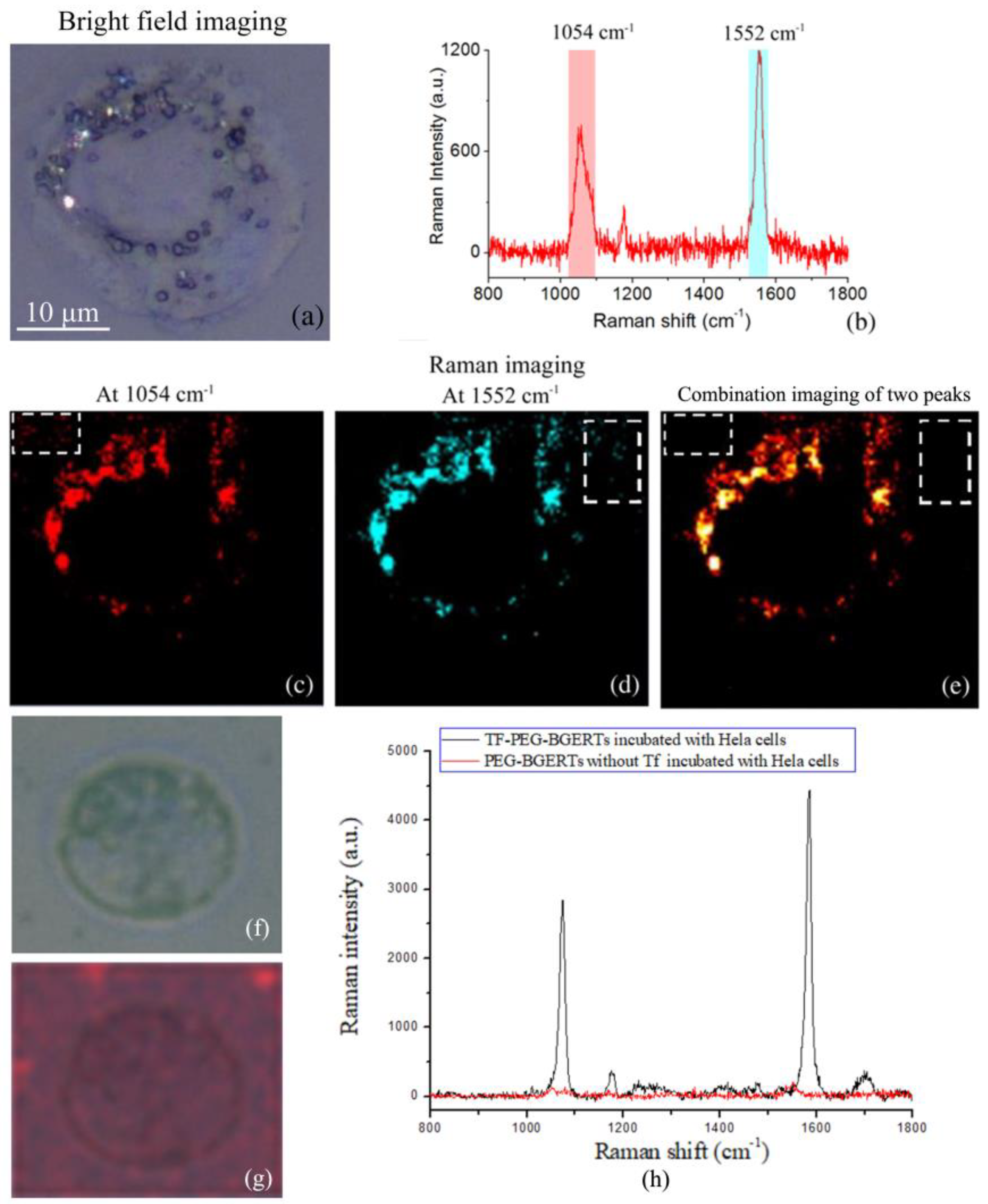
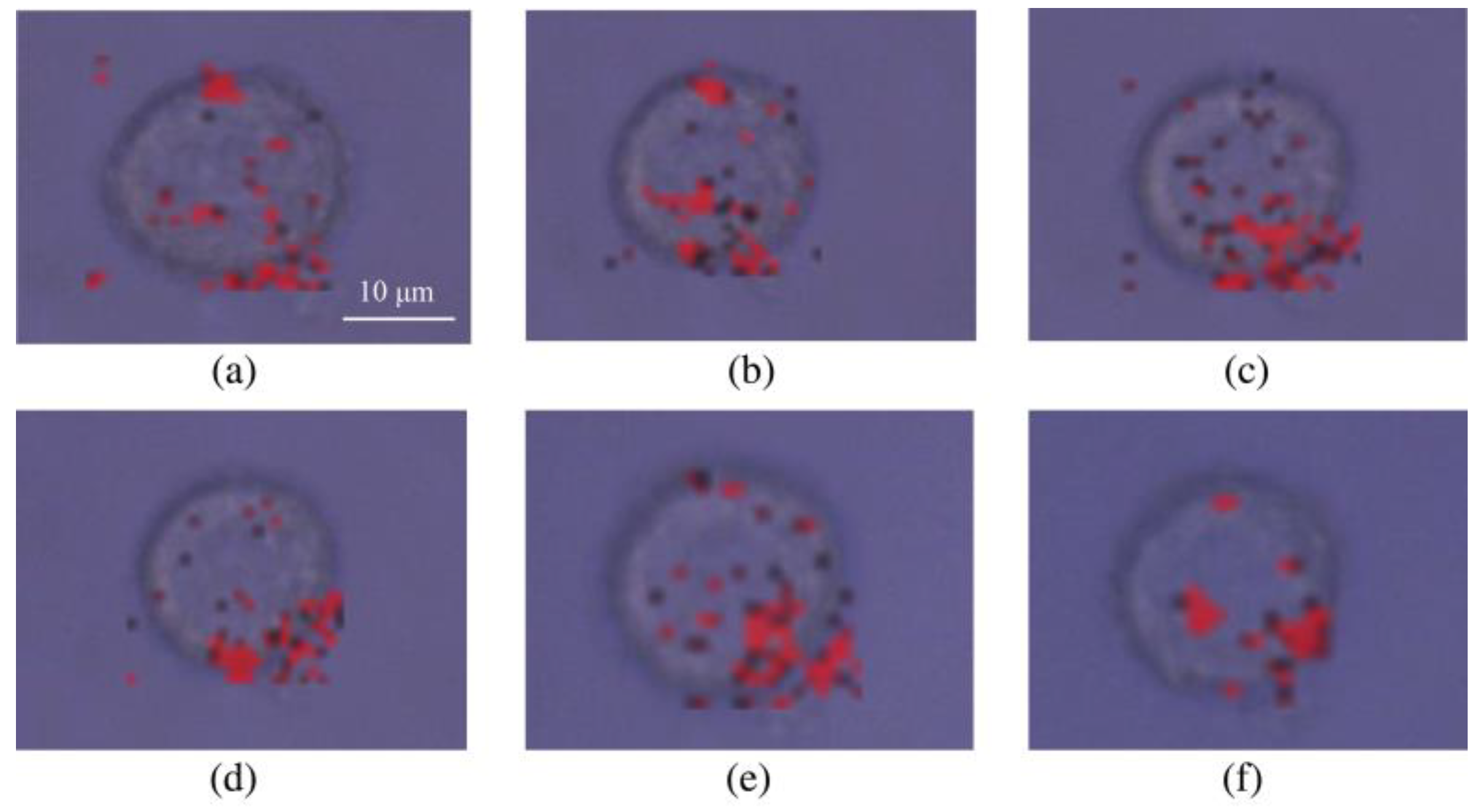
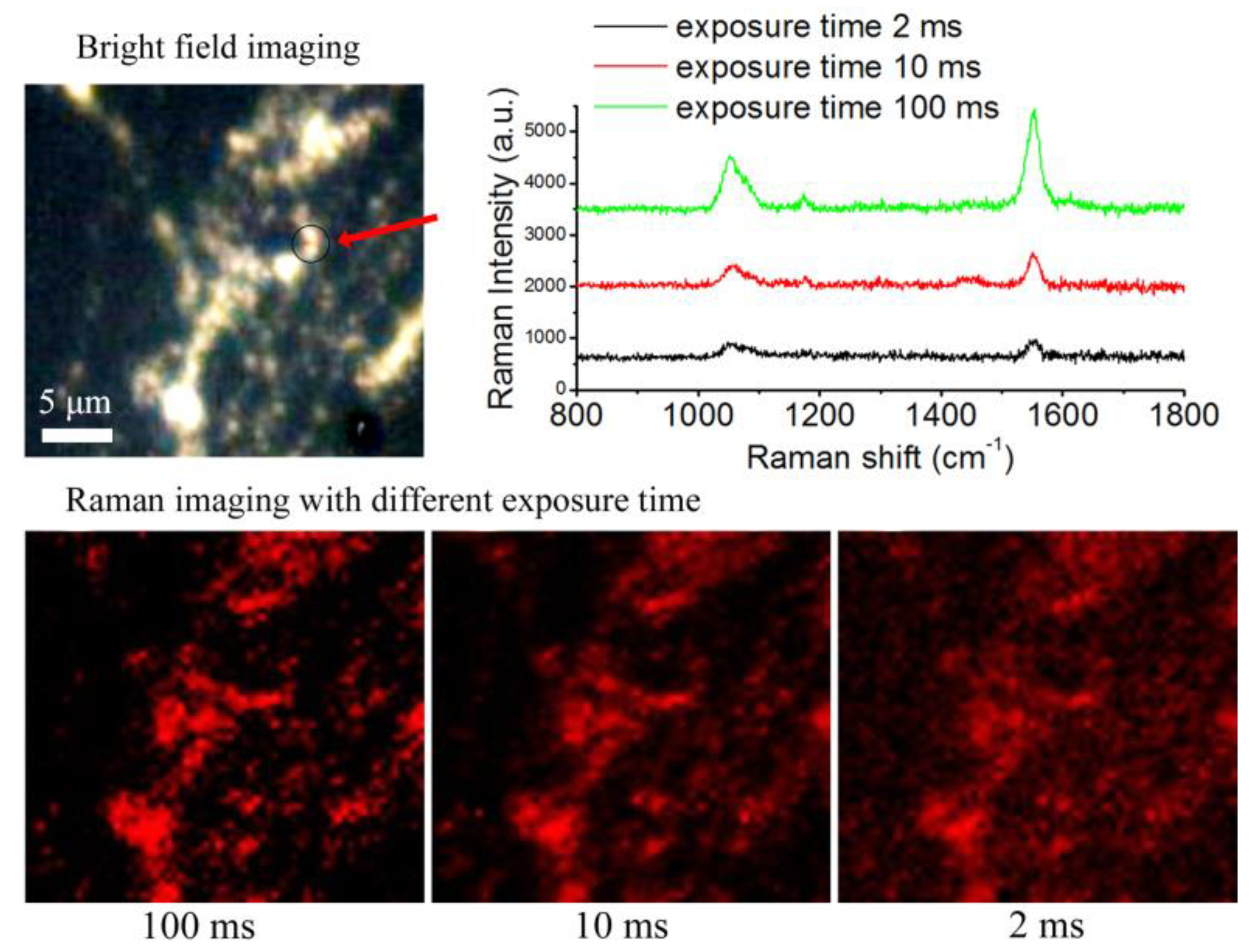
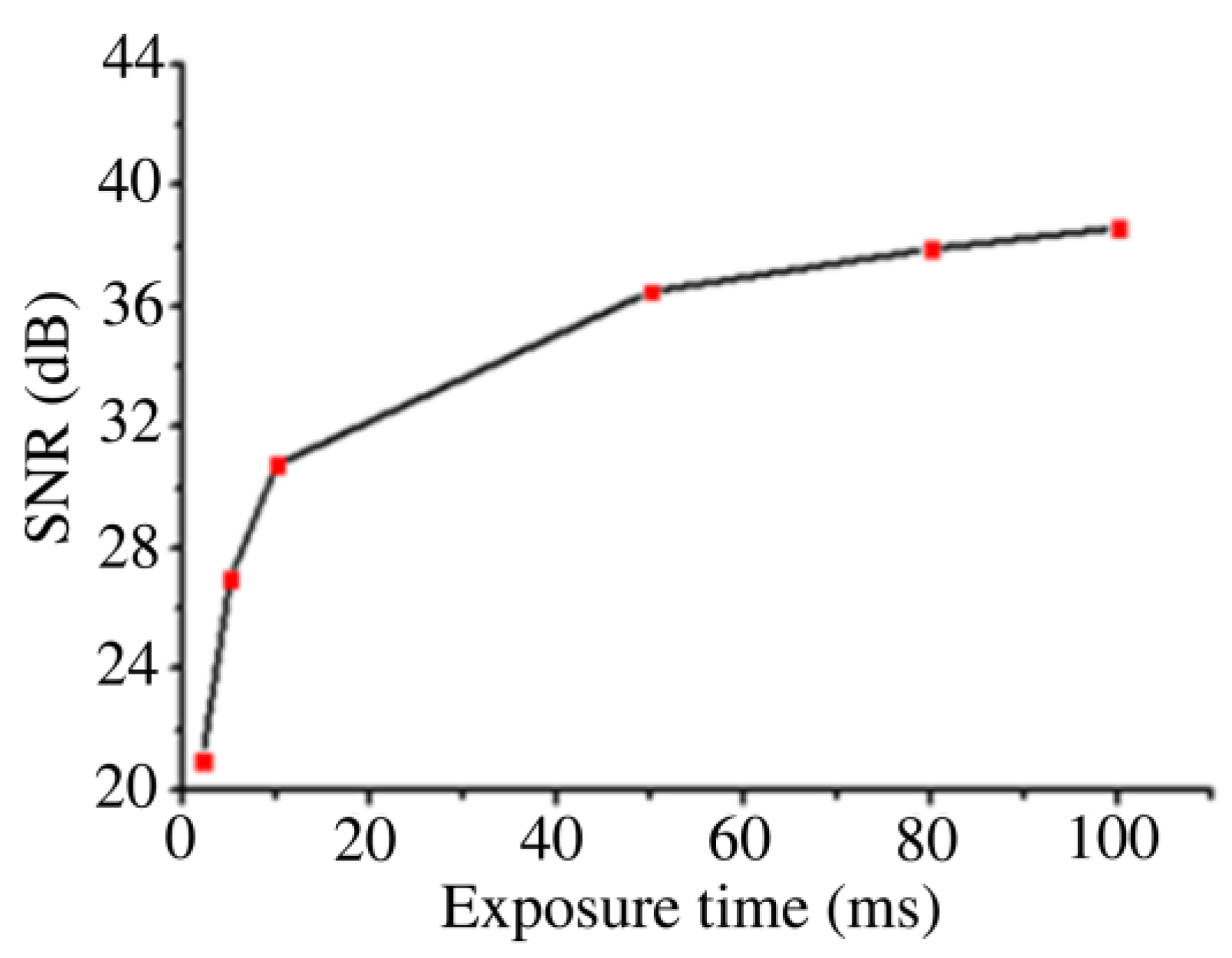
3.3. Analysis of BGERTs-Based SERS Imaging Quality
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Comsa, D.C.; Farrell, T.J.; Patterson, M.S. Quantitative fluorescence imaging of point-like sources in small animals. Phys. Med. Biol. 2008, 53, 5797. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, J.; Wachsmuth, M.; Hufnagel, L.; Knop, M. Quantitative fluorescence imaging of protein diffusion and interaction in living cells. Nat. Biotechnol. 2011, 29, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chao, J.A.; Singer, R.H. Fluorescence Fluctuation Spectroscopy Enables Quantitative Imaging of Single mRNAs in Living Cells. Biophys. J. 2012, 102, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Dan, F.; Jing, Z.; Zhu, W.S.; Manley, P.W.; Wang, Y.K.; Hood, T.; Wylie, A.; Xie, X.S. Imaging the Intracellular Distribution of Tyrosine Kinase Inhibitors in Living Cells with Quantitative Hyperspectral Stimulated Raman Scattering. Nat. Chem. 2014, 6, 614–622. [Google Scholar]
- Yan, X.; Hoek, T.A.; Vale, R.D.; Tanenbaum, M.E. Dynamics of Translation of Single mRNA Molecules In Vivo. Cell 2016, 165, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Dultz, E.; Mancini, R.; Polles, G.; Vallotton, P.; Alber, F.; Weis, K. Quantitative imaging of chromatin decompaction in living cells. Mol. Biol. Cell 2017, 29, 1763–1777. [Google Scholar] [CrossRef]
- Hai, X.; Guo, Z.; Lin, X.; Chen, X.; Wang, J. Fluorescent TPA@GQDs Probe for Sensitive Assay and Quantitative Imaging of Hydroxyl Radicals in Living Cells. ACS Appl. Mater. Interfaces 2018, 10, 5853–5861. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. Transferrin receptors and the targeted delivery of therapeutic agents against cancer. BBA Gen. Subj. 2012, 1820, 291–317. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; Desimone, J.M. The complex role of multivalency in nanoparticles targeting the transferrin receptor for cancer therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313. [Google Scholar] [CrossRef]
- Hwang, J.H.; Singhal, N.K.; Lim, D.K.; Nam, J.M. Au Nanocucumbers with Interior Nanogap for Multiple Laser Wavelength-Compatible Surface-Enhanced Raman Scattering. Bull. Korean Chem. Soc. 2015, 36, 882–886. [Google Scholar]
- Wang, Y.; Serrano, A.B.; Sentosun, K.; Bals, S.; Lizmarzán, L.M. Stabilization and Encapsulation of Gold Nanostars Mediated by Dithiols. Small 2015, 11, 4314–4320. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Khanadeev, V.A.; Tsvetkov, M.Y.; Bagratashvili, V.N.; Khlebtsov, N.G. Surface-Enhanced Raman Scattering Substrates Based on Self-Assembled PEGylated Gold and Gold–Silver Core–Shell Nanorods. J. Phys. Chem. C 2013, 117, 23162–23171. [Google Scholar] [CrossRef]
- Khlebtsov, B.; Panfilova, E.; Khanadeev, V.; Khlebtsov, N. Improved size-tunable synthesis and SERS properties of Au nanostars. J. Nanopart. Res. 2014, 16, 2623. [Google Scholar] [CrossRef]
- Gandra, N.; Singamaneni, S. Bilayered Raman-Intense Gold Nanostructures with Hidden Tags (BRIGHTs) for High-Resolution Bioimaging. Adv. Mater. 2013, 25, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.K.; Jeon, K.S.; Hwang, J.H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.M. Highly uniform and reproducible surface-enhanced Raman scattering from DNA-tailorable nanoparticles with 1-nm interior gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Khlebtsov, N.G. Surface Morphology of a Gold Core Controls the Formation of Hollow or Bridged Nanogaps in Plasmonic Nanomatryoshkas and Their SERS Responses. J. Phys. Chem. C 2016, 120. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Y.; Lin, L.; Gu, H.; Xiao, Z.; Ye, J. Ultra-Photostable Mesoporous Silica-Coated Gap Enhanced Raman Tags (GERTs) for High-Speed Bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 3995. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Li, Y.; Jin, X.; Huang, C.; Maier, S.A.; Ye, J. Raman photostability of off-resonant gap-enhanced Raman tags. RSC Adv. 2018, 8, 14434–14444. [Google Scholar] [CrossRef]
- Conde, J.; Bao, C.; Cui, D.; Baptista, P.V.; Tian, F. Antibody-drug gold nanoantennas with Raman spectroscopic fingerprints for in vivo tumour theranostics. J. Control. Release Off. J. Control. Release Soc. 2014, 183, 87–93. [Google Scholar] [CrossRef]
- Lee, S.; Chon, H.; Lee, J.; Ko, J.; Chung, B.H.; Lim, D.W.; Choo, J. Rapid and sensitive phenotypic marker detection on breast cancer cells using surface-enhanced Raman scattering (SERS) imaging. Biosens. Bioelectron. 2014, 51, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.W.; So, P.T.; Dasari, R.R.; Lim, D.K. High resolution live cell Raman imaging using subcellular organelle-targeting SERS-sensitive gold nanoparticles with highly narrow intra-nanogap. Nano Lett. 2015, 15, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zheng, Z.; Xu, Y.; Lin, J.; Chen, G.; Weng, C.; Lin, D.; Qiu, S.; Cheng, M.; Huang, Z. A noninvasive cancer detection strategy based on gold nanoparticle surface-enhanced raman spectroscopy of urinary modified nucleosides isolated by affinity chromatography. Biosens. Bioelectron. 2017, 91, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Hu, Z.; Yu, G.; Yang, D.; Zhao, J. Label and label-free based surface-enhanced Raman scattering for pathogen bacteria detection: A review. Biosens. Bioelectron. 2017, 94, 131. [Google Scholar] [CrossRef] [PubMed]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Duyne, R.P.V. Probing the Structure of Single-Molecule Surface-Enhanced Raman Scattering Hot Spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef] [PubMed]
- Mcmahon, J.M.; Henry, A.I.; Wustholz, K.L.; Natan, M.J.; Freeman, R.G.; Van Duyne, R.P.; Schatz, G.C. Gold nanoparticle dimer plasmonics: Finite element method calculations of the electromagnetic enhancement to surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2009, 394, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Mukherjee, S.; Mirin, N.A.; Levit, S.D.; Nordlander, P.; Halas, N.J. Nanosphere-in-a-Nanoshell: A Simple Nanomatryushka. J. Phys. Chem. C 2010, 114, 7378–7383. [Google Scholar] [CrossRef]
- Ciceron, A.O.; Cordula, U.; Knight, M.W.; Skyrme, U.A.; Oara, N.; Bishnoi, S.W.; Shaunak, M.; Goodman, A.M.; Heather, C.; Tamika, M. Au Nanomatryoshkas as Efficient Near-Infrared Photothermal Transducers for Cancer Treatment: Benchmarking against Nanoshells. ACS Nano 2014, 8, 6372–6381. [Google Scholar]
- Lin, L.; Gu, H.; Ye, J. Plasmonic multi-shell nanomatryoshka particles as highly tunable SERS tags with built-in reporters. Chem. Commun. 2015, 51, 17740–17743. [Google Scholar] [CrossRef]
- Oh, J.W.; Lim, D.K.; Kim, G.H.; Suh, Y.D.; Nam, J.M. Thiolated DNA-Based Chemistry and Control in the Structure and Optical Properties of Plasmonic Nanoparticles with Ultrasmall Interior Nanogap. J. Am. Chem. Soc. 2014, 136, 14052–14059. [Google Scholar] [CrossRef]
- Bao, Z.; Zhang, Y.; Tan, Z.; Yin, X.; Di, W.; Ye, J. Gap-enhanced Raman tags for high-contrast sentinel lymph node imaging. Biomaterials 2018, 163, 105–115. [Google Scholar] [CrossRef]
- Wu, Z.S.; Wang, Y.P. Electromagnetic scattering for multilayered sphere: Recursive algorithms. Radio Sci. 1991, 26, 1393–1401. [Google Scholar] [CrossRef]
- Joo, S.W.; Han, S.W.; Kim, K. Adsorption of 1,4-Benzenedithiol on Gold and Silver Surfaces: Surface-Enhanced Raman Scattering Study. J. Colloid Interface Sci. 2001, 240, 391–399. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, J.; Tang, P.; Lu, X.; Tian, J.; Zhong, L. Dynamic Imaging of Transferrin Receptor Molecules on Single Live Cell with Bridge Gaps-Enhanced Raman Tags. Nanomaterials 2019, 9, 1373. https://doi.org/10.3390/nano9101373
Zhang Q, Li J, Tang P, Lu X, Tian J, Zhong L. Dynamic Imaging of Transferrin Receptor Molecules on Single Live Cell with Bridge Gaps-Enhanced Raman Tags. Nanomaterials. 2019; 9(10):1373. https://doi.org/10.3390/nano9101373
Chicago/Turabian StyleZhang, Qinnan, Jiaosheng Li, Ping Tang, Xiaoxu Lu, Jindong Tian, and Liyun Zhong. 2019. "Dynamic Imaging of Transferrin Receptor Molecules on Single Live Cell with Bridge Gaps-Enhanced Raman Tags" Nanomaterials 9, no. 10: 1373. https://doi.org/10.3390/nano9101373
APA StyleZhang, Q., Li, J., Tang, P., Lu, X., Tian, J., & Zhong, L. (2019). Dynamic Imaging of Transferrin Receptor Molecules on Single Live Cell with Bridge Gaps-Enhanced Raman Tags. Nanomaterials, 9(10), 1373. https://doi.org/10.3390/nano9101373




