Surfactant-Assisted Fabrication of Alumina-Doped Amorphous Silica Nanofiltration Membranes with Enhanced Water Purification Performances
Abstract
:1. Introduction
2. Experimental
2.1. Sol-Synthesis and Membrane Fabrication
2.2. Membrane Characterization
2.3. Filtration Tests
3. Results and Discussion
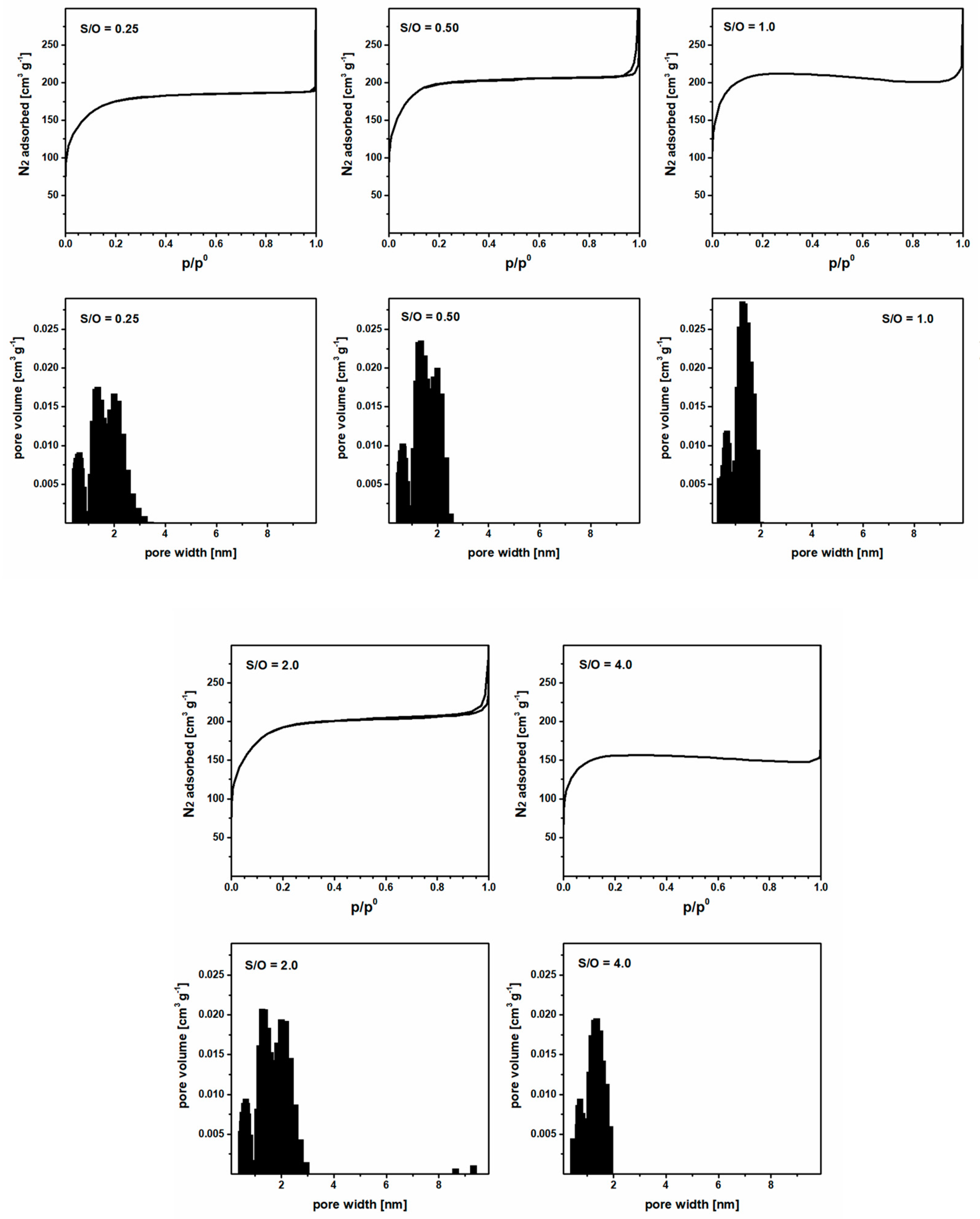
3.1. Impact of CTAB Concentration on Membrane Porosity
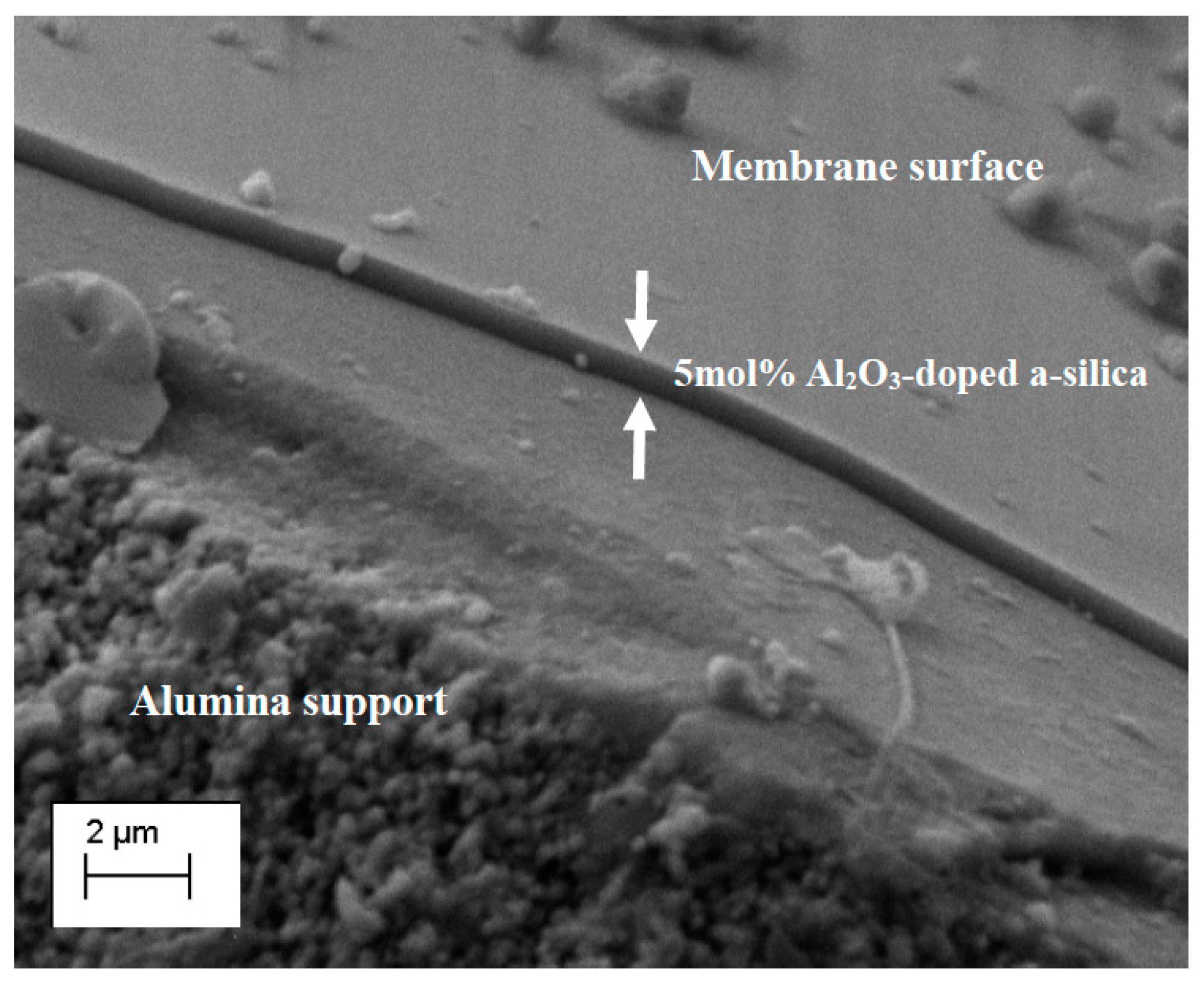
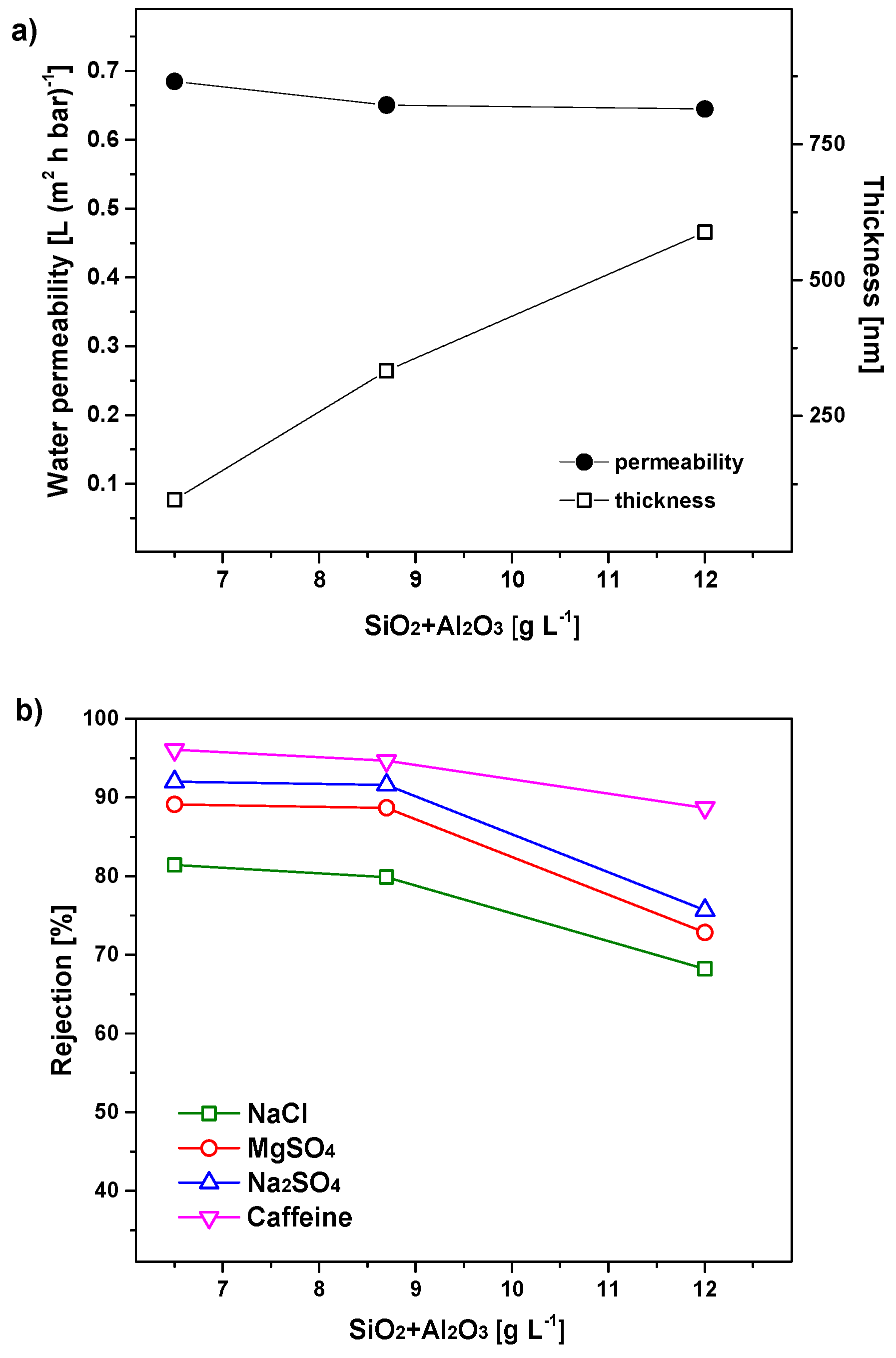
3.2. Concentration of the Coating Sol
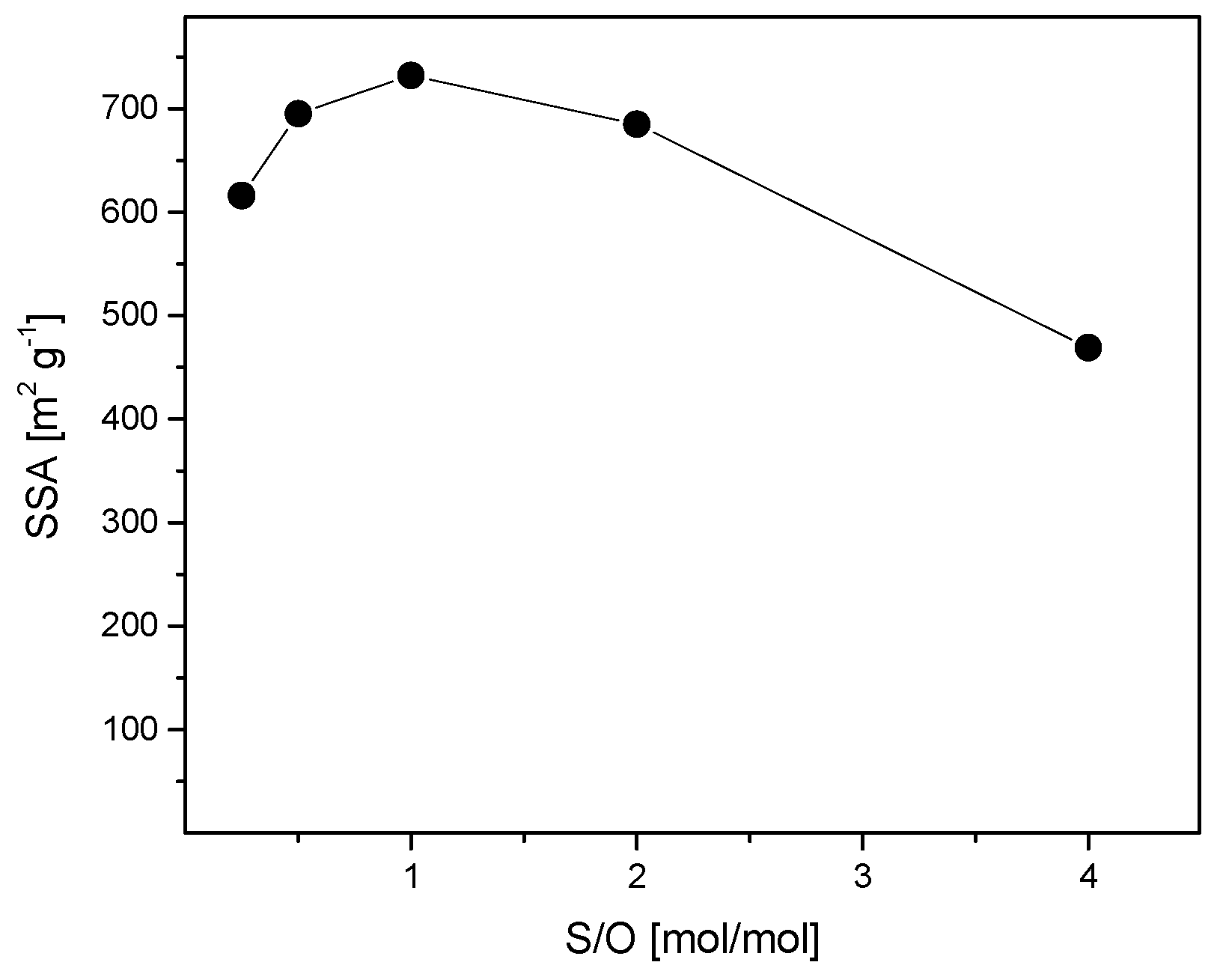
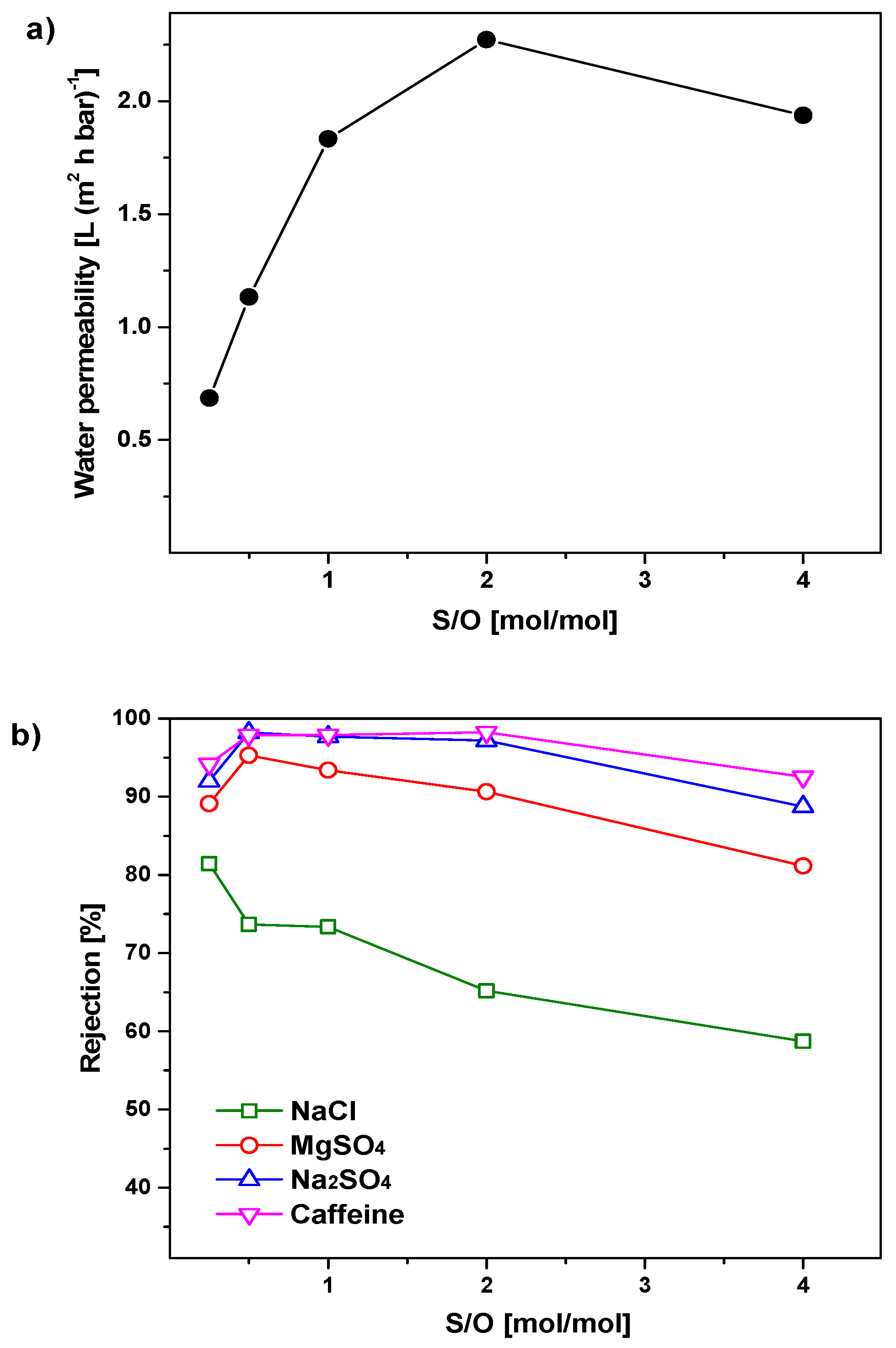
3.3. Optimization of the S/O Ratio
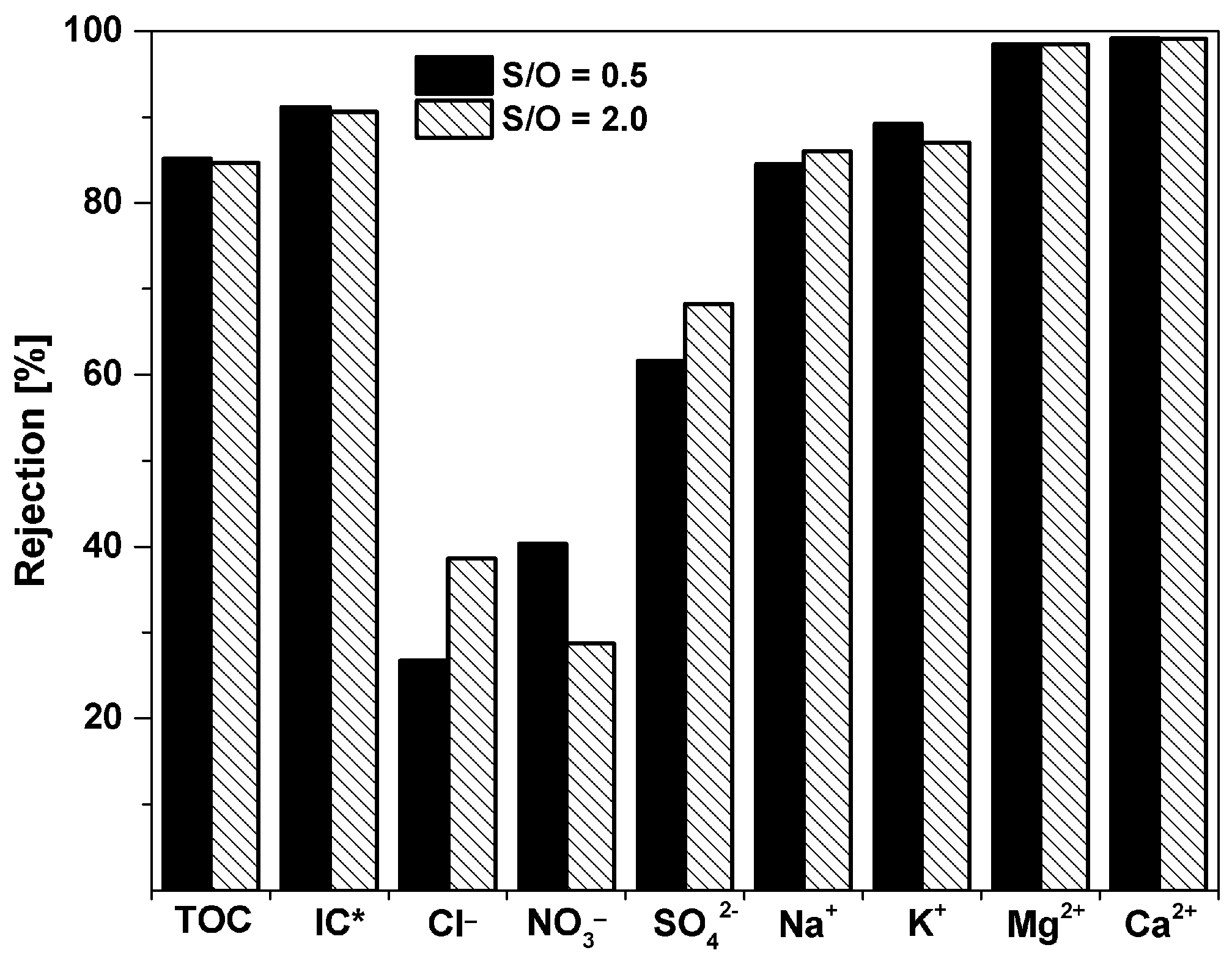
3.4. Filtration of A Wastewater Treatment Plant Effluent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shrivastava, A.; Rosenberg, S.; Peery, M. Energy efficiency breakdown of reverse osmosis and its implications on future innovation roadmap for desalination. Desalination 2015, 368, 181–192. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124, 030901. [Google Scholar] [CrossRef]
- Elma, M.; Yacou, C.; Wang, D.K.; Smart, S.; Diniz da Costa, J.C. Microporous silica based membranes for desalination. Water 2012, 4, 629–649. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marĩas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Form No. 609-00519-1206, FILMTEC NF270 Nanofiltration Elements for Commercial Systems; The Dow Chemical Company: Midlan, MI, USA; Available online: https://www.dupont.com/products/filmtecnf2704040.html (accessed on 22 September 2019).
- Form No. 609-00379-0503, FILMTEC Membranes, Nanofiltration Produces Sparkling Clean Water for Swedish Resort Community; The Dow Chemical Company: Midlan, MI, USA; Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/general/documents/609-00379.pdf (accessed on 22 September 2019).
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination-Development to date and future potential. J. Memb. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Farahbakhsh, K.; Svrcek, C.; Guest, R.K.; Smith, D.W. A review of the impact of chemical pretreatment on low-pressure water treatment membranes. J. Environ. Eng. Sci. 2004, 4, 237–253. [Google Scholar] [CrossRef]
- Tsuru, T. Inorganic porous membranes for liquid phase separation. Sep. Purif. Methods. 2001, 30, 191–220. [Google Scholar] [CrossRef]
- Lin, Y.S. Microporous and dense inorganic membranes: Current status and prospective. Sep. Purif. Technol. 2001, 25, 39–55. [Google Scholar] [CrossRef]
- Farsi, A.; Malvache, C.; De Bartolis, O.; Magnacca, G.; Kristensen, P.K.; Christensen, M.L.; Boffa, V. Design and fabrication of silica-based nanofiltration membranes for water desalination and detoxification. Microporous Mesoporous Mater. 2017, 237, 117–126. [Google Scholar] [CrossRef]
- Wijaya, S.; Duke, M.C.; Diniz da Costa, J.C. Carbonised template silica membranes for desalination. Desalination 2009, 236, 291–298. [Google Scholar] [CrossRef]
- Boffa, V.; Parmeggiani, L.; Nielsen, A.H.; Magnacca, G. Hydrophilicity and surface heterogeneity of TiO2-doped silica materials for membrane applications. Microporous Mesoporous Mater. 2016, 221, 81–90. [Google Scholar] [CrossRef]
- Fotou, G.P.; Lin, Y.S.; Pratsinis, S.E. Hydrothermal stability of pure and modified microporous silica membranes. J. Mater. Sci. 1995, 30, 2803–2808. [Google Scholar] [CrossRef]
- Boffa, V.; Blank, D.H.A.; ten Elshof, J.E. Hydrothermal stability of microporous silica and niobia-silica membranes. J. Memb. Sci. 2008, 319, 256–263. [Google Scholar] [CrossRef]
- Waldman, R.Z.; Choudhury, D.; Mandia, D.J.; Elam, J.W.; Nealey, P.F.; Martinson, A.B.F.; Darling, S.B. Sequential Infiltration Synthesis of Al2O3 in Polyethersulfone Membranes. JOM 2019, 71, 212–223. [Google Scholar] [CrossRef]
- Lin, C.X.C.; Ding, L.P.; Smart, S.; Diniz da Costa, J.C. Cobalt oxide silica membranes for desalination. J. Colloid Interface Sci. 2012, 368, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Boffa, V.; Magnacca, G.; Jørgensen, L.B.; Wehner, A.; Dörnhöfer, A.; Yue, Y. Toward the effective design of steam-stable silica-based membranes. Microporous Mesoporous Mater. 2013, 179, 242–249. [Google Scholar] [CrossRef]
- Gu, Y.; Hacarlioglu, P.; Oyama, S.T. Hydrothermally stable silica–alumina composite membranes for hydrogen separation. J. Membr. Sci. 2008, 310, 28–37. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; McPherson, B.; Lee, R. Enhanced water permeation of reverse osmosis through MFI-type zeolite membranes with high aluminum contents. Ind. Eng. Chem. Res. 2007, 46, 1584–1589. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2017, 434, 60–80. [Google Scholar] [CrossRef]
- Wen, J.; Wilkes, G.L. Organic/inorganic hybrid network materials by the sol-gel approach. Chem. Mater. 1996, 8, 1667–1681. [Google Scholar] [CrossRef]
- De Lange, R.S.A.; Kumar, K.N.P.; Hekkink, J.H.A.; van de Velde, G.M.H.; Keizer, K.; Burggraaf, A.J.; Dokter, W.H.; van Garderen, H.F.; Beelen, T.P.M. Microporous SiO2 and SiO2/MOx (M=Ti, Zr, Al) for ceramic membrane applications: A microstructural study of the sol-stage and the consolidated state. J. Sol.-Gel Sci. Technol. 1994, 2, 489–495. [Google Scholar] [CrossRef]
- Boffa, V.; Castricum, H.L.; Garcia, R.; Schmuhl, R.; Petukhov, A.V.; Blank, D.H.A.; Ten Elshof, J.E. Structure and growth of polymeric niobia-silica mixed-oxide sols for microporous molecular sieving membranes: A saxs study. Chem. Mater. 2009, 21, 1822–1828. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Effects of surfactants on the rate of chemical reactions. J. Chem. 2014, 2014, 564–567. [Google Scholar] [CrossRef]
- Mege, S.; Vereist, M.; Lecante, P.; Perez, E.; Ansart, F.; Savariault, J.M. Surfactant effects in vanadium alkoxide derived gels. J. Non. Cryst. Solids 1998, 238, 37–44. [Google Scholar] [CrossRef]
- Liu, S.; Boffa, V.; Yang, D.; Fan, Z.; Meng, F.; Yue, Y. Clarifying the gel-to-glass transformation in Al2O3-SiO2 systems. J. Non. Cryst. Solids 2018, 492, 77–83. [Google Scholar] [CrossRef]
- Olivier, J.P. Modeling physical adsorption on porous and nonporous solids using density functional theory. J. Porous Mater. 1995, 2, 9–17. [Google Scholar] [CrossRef]
- Farsi, A.; Boffa, V.; Qureshi, H.F.; Nijmeijer, A.; Winnubst, L.; Christensen, M.L. Modeling water flux and salt rejection of mesoporous γ-alumina and microporous organosilica membranes. J. Memb. Sci. 2014, 470, 307–315. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.S.; Rouquerol, J. Siemieniewska, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Strawbridge, I.; James, P.F. The factors affecting the thickness of sol-gel derived silica coatings prepared by dipping. J. Non. Cryst. Solids 1986, 86, 381–393. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol–Gel Science: The Physics and Chemistry of Sol–Gel Processing, 2nd ed.; Academic Press: San Diego, CA, USA, 1990; pp. 788–797. [Google Scholar]
- Zhu, B.; Myat, D.T.; Shin, J.; Na, Y.; Moon, I.; Connor, G.; Maeda, S.; Morris, G.; Gray, S.; Duke, M. Application of robust MFI-type zeolite membrane for desalination of saline wastewater. J. Memb. Sci. 2015, 475, 167–174. [Google Scholar] [CrossRef]


| S/O # | Reaction Time (h) | TEOS:Ethanol (mol:mol) | Al2O3:SiO2 (mol%) | Al2O3 + SiO2 Concentration § (g·L−1) |
|---|---|---|---|---|
| Membrane samples with different sol dilutions | ||||
| 0.25 | 15 | 1:4 | 5% | 11.8 |
| 0.25 | 15 | 1:4 | 5% | 8.7 |
| 0.25 | 10 | 1:4 | 5% | 6.5 |
| Membrane samples with different CTAB concentrations | ||||
| 0.25 | 10 | 1:4 | 5% | 6.5 |
| 0.5 | 10 | 1:4 | 5% | 6.5 |
| 1 | 10 | 1:8 * | 5% | 6.5 |
| 2 | 20 | 1:12 * | 5% | 6.5 |
| 4 | 40 | 1:32 * | 5% | 6.5 |
| Wastewater Treatment Plan Effluent (ppm) | Permeate S/O = 0.5 (ppm) | Permeate S/O = 2 (ppm) | |
|---|---|---|---|
| Cl− | 92.1 | 67.5 | 56.5 |
| NO3− | 20.2 | 12.0 | 14.4 |
| SO42− | 40.9 | 15.7 | 13.0 |
| Na+ | 103 | 16.0 | 14.4 |
| K+ | 27.2 | 2.95 | 3.53 |
| Mg2+ | 7.1 | 0.11 | 0.11 |
| Ca2+ | 92 | 0.77 | 0.82 |
| TOC (total organic carbon) | 8.51 | 1.26 | 1.31 |
| TC (total carbon) | 69.0 | 6.59 | 6.97 |
| IMI | 3 × 10−5 | - | - |
| CBZ | 8.4 × 10−4 | 2.3 × 10−5 | 3.4 × 10−5 |
| BZT | 1.7 × 10−4 | - | - |
| MBZT | 1.7 × 10−3 | 1.5 × 10−5 | 2.0 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Janowska, K.; Boffa, V.; Fabbri, D.; Magnacca, G.; Calza, P.; Yue, Y. Surfactant-Assisted Fabrication of Alumina-Doped Amorphous Silica Nanofiltration Membranes with Enhanced Water Purification Performances. Nanomaterials 2019, 9, 1368. https://doi.org/10.3390/nano9101368
Ma X, Janowska K, Boffa V, Fabbri D, Magnacca G, Calza P, Yue Y. Surfactant-Assisted Fabrication of Alumina-Doped Amorphous Silica Nanofiltration Membranes with Enhanced Water Purification Performances. Nanomaterials. 2019; 9(10):1368. https://doi.org/10.3390/nano9101368
Chicago/Turabian StyleMa, Xianzheng, Katarzyna Janowska, Vittorio Boffa, Debora Fabbri, Giuliana Magnacca, Paola Calza, and Yuanzheng Yue. 2019. "Surfactant-Assisted Fabrication of Alumina-Doped Amorphous Silica Nanofiltration Membranes with Enhanced Water Purification Performances" Nanomaterials 9, no. 10: 1368. https://doi.org/10.3390/nano9101368
APA StyleMa, X., Janowska, K., Boffa, V., Fabbri, D., Magnacca, G., Calza, P., & Yue, Y. (2019). Surfactant-Assisted Fabrication of Alumina-Doped Amorphous Silica Nanofiltration Membranes with Enhanced Water Purification Performances. Nanomaterials, 9(10), 1368. https://doi.org/10.3390/nano9101368








