Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications
Abstract
1. Introduction
2. Bacteria Have High Capacity for Cellulose Production
3. Different Carbon Sources Used for Bacterial Cellulose (BC) Production
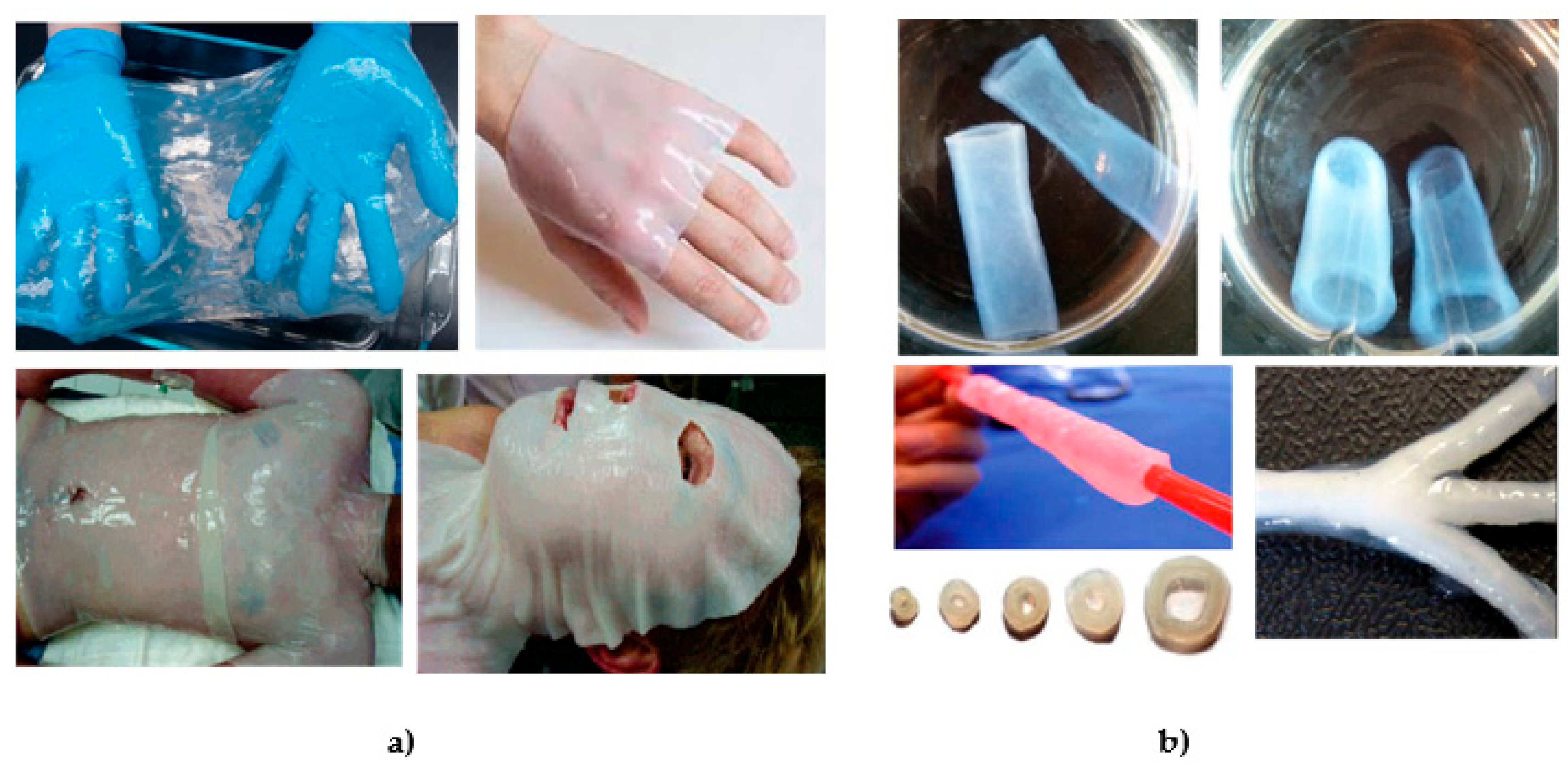
4. BC Modifications with Medical Relevance
4.1. In Situ Modifications
4.2. Ex Situ Modifications
5. BC in Regenerative Medicine
6. Perspectives and Challenges for BC
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krasteva, P.V.; Bernal-Bayard, J.; Travier, L.; Martin, F.A.; Kaminski, P.A.; Karimova, G.; Fronzes, R.; Ghigo, J.M. Insights into the structure and assembly of a bacterial cellulose secretion system. Nat. Commun. 2017, 8, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Han, Y.H.; Chen, J.L.; Zhang, D.C.; Shi, X.X.; Ye, Y.X.; Chen, D.L.; Li, M. Insights into bacterial cellulose biosynthesis from different carbon sources and the associated biochemical transformation pathways in Komagataeibacter sp. W1. Polymers 2018, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Trček, J.; Barja, F. Updates on quick identification of acetic acid bacteria with a focus on the 16S–23S rRNA gene internal transcribed spacer and the analysis of cell proteins by MALDI-TOF mass spectrometry. Int. J. Food Microbiol. 2015, 196, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Škraban, J.; Cleenwerck, I.; Vandamme, P.; Fanedl, L.; Trček, J. Genome sequences and description of novel exopolysaccharides producing species Komagataeibacter pomaceti sp. nov. and reclassification of Komagataeibacter kombuchae (Dutta and Gachhui 2007) Yamada et al. 2013 as a later heterotypic synonym of Komagataeibacter. Syst. Appl. Microbiol. 2018, 41, 581–592. [Google Scholar] [PubMed]
- Slapšak, N.; Cleenwerck, I.; de Vos, P.; Trček, J. Gluconacetobacter maltaceti sp. nov., a novel vinegar producing acetic acid bacterium. Syst. Appl. Microbiol. 2013, 36, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Zuluaga, R.; Álvarez, C.; Putaux, J.-L.; Caro, G.; Rojas, O.J.; Mondragon, I.; Gañán, P. Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydr. Polym. 2012, 89, 1033–1037. [Google Scholar] [CrossRef]
- Castro, C.; Cleenwerck, I.; Trcek, J.; Zuluaga, R.; De Vos, P.; Caro, G.; Aguirre, R.; Putaux, J.L.; Gañán, P. Gluconacetobacter medellinensis sp. nov., cellulose- and non-cellulose-producing acetic acid bacteria isolated from vinegar. Int. J. Syst. Evol. Microbiol. 2013, 63, 1119–1125. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Zhu, C.; Sun, D. Production of Bacterial Cellulose by Acetobacter Xylinum: Effects of Carbon/Nitrogen-ratio on Cell Growth and Metabolite Production. Cellulose Chem. Technol. 2016, 50, 997–1003. [Google Scholar]
- Li, J.; Chen, G.; Zhang, R.; Wu, H.; Zeng, W.; Liang, Z. Production of high crystallinity type-I cellulose from Komagataeibacter hansenii JR-02 isolated from Kombucha tea. Biotechnol. Appl. Biochem. 2019, 66, 108–118. [Google Scholar] [CrossRef]
- Morgan, J.L.W.; Strumillo, J.; Zimmer, J. Crystallographic snapshot of cellulose synthesis and membrane translocation. Nature 2012, 493, 181–186. [Google Scholar] [CrossRef]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [PubMed]
- McNamara, J.T.; Morgan, J.L.W.; Zimmer, J. A Molecular Description of Cellulose Biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; McManus, J.; Oehme, D.; Singh, A.; Yingling, Y.G.; Tien, M.; Kubicki, J.D. Simulations of Cellulose Synthesis Initiation and Termination in Bacteria. J. Phys. Chem. B 2019, 17, 3699–3705. [Google Scholar] [CrossRef] [PubMed]
- Ryngajłło, M.; Kubiak, K.; Jędrzejczak-Krzepkowska, M.; Jacek, P.; Bielecki, S. Comparative genomics of the Komagataeibacter strains—Efficient bionanocellulose producers. Microbiologyopen 2018, 8, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Jakob, F.; Pfaff, A.; Novoa-Carballal, R.; Rübsam, H.; Becker, T.; Vogel, R.F. Structural analysis of fructans produced by acetic acid bacteria reveals a relation to hydrocolloid function. Carbohydr. Polym. 2013, 92, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.U.; Jakob, F.; Behr, J.; Geissler, A.J.; Vogel, R.F. Dissection of exopolysaccharide biosynthesis in Kozakia baliensis. Microb. Cell Fact. 2016, 15, 1–13. [Google Scholar] [CrossRef][Green Version]
- Morris, V.J.; Brownsey, G.J.; Cairns, P.; Chilvers, G.R.; Miles, M.J. Molecular origins of acetan solution properties. Int. J. Biol. Macromol. 1989, 11, 326–328. [Google Scholar] [CrossRef]
- Ishida, T.; Sugano, Y.; Nakai, T.; Shoda, M. Effects of Acetan on Production of Bacterial Cellulose by Acetobacter xylinum. Biosci. Biotechnol. Biochem. 2003, 66, 1677–1681. [Google Scholar] [CrossRef][Green Version]
- Schramm, B.M.; Hestrin, S. Factors affecting Production of Cellulose at the Air/Liquid Interface of a Culture of Acetobacter xylinum. J. Gen. Microbiol. 1954, 11, 123–129. [Google Scholar] [CrossRef]
- Naritomi, T.; Kouda, T.; Yano, H.; Yoshinaga, F. Effect of ethanol on bacterial cellulose production from fructose in continuous culture. J. Ferment. Bioeng. 1998, 85, 598–603. [Google Scholar] [CrossRef]
- Toda, K.; Asakura, T.; Fukaya, M.; Entani, E.; Kawamura, Y. Cellulose production by acetic acid-resistant Acetobacter xylinum. J. Ferment. Bioeng. 1997, 84, 228–231. [Google Scholar] [CrossRef]
- Lu, H.; Jia, Q.; Chen, L.; Zhang, L. Effect of Organic Acids on Bacterial Cellulose Produced by Acetobacter xylinum. J. Microbiol. Biotechnol. 2016, 5, 1–6. [Google Scholar]
- Li, Y.; Tian, C.; Tian, H.; Zhang, J.; He, X.; Ping, W.; Lei, H. Improvement of bacterial cellulose production by manipulating the metabolic pathways in which ethanol and sodium citrate involved. Appl. Microbiol. Biotechnol. 2012, 96, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Chi, Y.; Xu, N.; Yao, W.; Sun, Y. Effects of alcohols on bacterial cellulose production by Acetobacter xylinum 186. J. Microbiol. Biotechnol. 2011, 27, 2281–2285. [Google Scholar] [CrossRef]
- Matsuoka, M.; Tsuchida, T.; Matsushita, K.; Adachi, O.; Yoshinaga, F. A Synthetic Medium for Bacterial Cellulose Production by Acetobacter xylinum subsp. Sucrofermentans. Biosci. Biotechnol. Biochem. 2011, 60, 575–579. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresour. Technol. 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Huang, C.; Guo, H.-J.; Xiong, L.; Luo, J.; Wang, B.; Jin, X.Q.; Chen, X.F.; Chen, X.-D. Bacterial cellulose production from the litchi extract by Gluconacetobacter xylinus. Prep. Biochem. Biotechnol. 2016, 46, 39–43. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Huang, C.; Yang, X.-Y.; Xiong, L.; Guo, H.-J.; Luo, J.; Wang, B.; Zhang, H.R.; Lin, X.Q.; Chen, X.-D. Evaluating the possibility of using acetone-butanol-ethanol (ABE) fermentation wastewater for bacterial cellulose production by Gluconacetobacter xylinus. Lett. Appl. Microbiol. 2015, 60, 491–496. [Google Scholar] [CrossRef]
- Lin, D.; Lopez-Sanchez, P.; Li, R.; Li, Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 2014, 151, 113–119. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Bayrak, E.; Büyükkileci, A.O. Utilization of white grape pomace for lactic acid production. GIDA 2018, 43, 129–138. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Enciso, C.; Torres-Taborda, M.; Zuluaga, R.; Gañán, P.; Rojas, O.J.; Castro, C. Effects of alternative energy sources on bacterial cellulose characteristics produced by Komagataeibacter medellinensis. Int. J. Biol. Macromol. 2018, 117, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.M.A.S. Vitamin C enhances bacterial cellulose production in Gluconacetobacter xylinus. Carbohydr. Polym. 2014, 99, 98–100. [Google Scholar] [CrossRef]
- Rani, M.U.; Rastogi, N.K.; Appaiah, K.A.A. Statistical optimization of medium composition for bacterial cellulose production by Gluconacetobacter hansenii UAC09 using coffee cherry husk extract—An agro-industry waste. J. Microbiol. Biotechnol. 2011, 21, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural Features and Properties of Bacterial Cellulose Produced in Agitated Culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods—A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Chao, Y.P.; Sugano, Y.; Kouda, T.; Yoshinaga, F.; Shoda, M. Production of bacterial cellulose by Acetobacter xylinum with an air-lift reactor. Biotechnol. Tech. 1997, 11, 829–832. [Google Scholar] [CrossRef]
- Serafica, G.; Mormino, R.; Bungay, H. Inclusion of solid particles in bacterial cellulose. Appl. Microbiol. Biotechnol. 2002, 58, 756–760. [Google Scholar]
- Lu, H.; Jiang, X. Structure and Properties of Bacterial Cellulose Produced Using a Trickling Bed Reactor. Appl. Biochem. Biotechnol. 2014, 172, 3844–3861. [Google Scholar] [CrossRef] [PubMed]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetically responsive and flexible bacterial cellulose membranes. Carbohydr. Polym. 2018, 192, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Keshk, S.M.A.S.; Nada, A.M.A. Heterogeneous Derivatization of Bacterial and Plant Cellulose. Biosci. Biotechnol. Res. Asia 2016, 1, 39–42. [Google Scholar]
- Rebelo, A.R.; Archer, A.J.; Chen, X.; Liu, C.; Yang, G.; Liu, Y. Dehydration of bacterial cellulose and the water content effects on its viscoelastic and electrochemical properties. Sci. Technol. Adv. Mater. 2018, 19, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Hesse, S.; Kabrelian, V.; Klemm, D. Controlling the water content of never dried and reswollen bacterial cellulose by the addition of water-soluble polymers to the culture medium. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 463–470. [Google Scholar] [CrossRef]
- Bottan, S.; Robotti, F.; Jayathissa, P.; Hegglin, A.; Bahamonde, N.; Heredia-Guerrero, J.A.; Bayer, I.S.; Scarpellini, A.; Merker, H.; Lindebnblatt, N.; et al. Surface-structured bacterial cellulose with guided assembly-based biolithography (GAB). ACS Nano 2015, 9, 206–219. [Google Scholar] [CrossRef]
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L. Regenerated chitin fibers reinforced with bacterial cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313. [Google Scholar] [CrossRef]
- Qiu, Y.; Qiu, L.; Cui, J.; Wei, Q. Bacterial cellulose and bacterial cellulose-vaccarin membranes for wound healing. Mater. Sci. Eng. C 2016, 59, 303–309. [Google Scholar] [CrossRef]
- Wu, C.-N.; Fuh, S.-C.; Lin, S.-P.; Lin, Y.-Y.; Chen, H.-Y.; Liu, J.-M.; Cheng, K.-C. TEMPO-Oxidized Bacterial Cellulose Pellicle with Silver Nanoparticles for Wound Dressing. Biomacromolecules 2018, 19, 544–554. [Google Scholar] [CrossRef]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial cellulose-zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Ullah, H.; Ul-Islam, M.; Khan, R.; Khan, S.; Ahmad, F.; Khan, T.; Wahid, F. Bacterial cellulose–TiO2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv. 2017, 7, 47662–47668. [Google Scholar] [CrossRef]
- Fürsatz, M.; Skog, M.; Sivlér, P.; Palm, E.; Aronsson, C.; Skallberg, A.; Greczynski, G.; Khalaf, H.; Bengtsson, T.; Aili, D. Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide ε-poly-L-Lysine. Biomed. Mater. 2018, 13, 025014. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xie, J.; Deng, Y.; Bian, Y.; Hong, F. Hydrothermal synthesis of bacterial cellulose/AgNPs composite: A ‘green’ route for antibacterial application. Carbohydr. Polym. 2012, 87, 2482–2487. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Yang, Y.-N.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef]
- de Lima Fontes, M.; Meneguin, A.B.; Tercjak, A.; Gutierrez, J.; Cury, B.S.F.; dos Santos, A.M.; Ribeiro, S.J.L.; Barud, H.S. Effect of in situ modification of bacterial cellulose with carboxymethylcellulose on its nano/microstructure and methotrexate release properties. Carbohydr. Polym. 2018, 179, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Hobzova, R.; Hrib, J.; Sirc, J.; Karpushkin, E.; Michalek, J.; Janouskova, O.; Gatenholm, P. Embedding of Bacterial Cellulose Nanofibers within PHEMA Hydrogel Matrices: Tunable Stiffness Composites with Potential for Biomedical Applications. J. Nanomater. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Lv, X.; Yang, J.; Feng, C.; Li, Z.; Chen, S.; Xie, M.; Xu, Y. Bacterial Cellulose-Based Biomimetic Nanofibrous Scaffold with Muscle Cells for Hollow Organ Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 19–29. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Zhang, Y.; Wan, Y. Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. C 2010, 30, 214–218. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, W.; Zheng, M.; Huo, M.; Zhong, C. Bacterial cellulose/hyaluronic acid composite hydrogels with improved viscoelastic properties and good thermodynamic stability. Plast. Rubber Compos. 2018, 47, 165–175. [Google Scholar] [CrossRef]
- Gonçalves, S.; Rodrigues, I.P.; Padrão, J.; Silva, J.P.; Sencadas, V.; Lanceros-Mendez, S.; Rodrigues, L.R. Acetylated bacterial cellulose coated with urinary bladder matrix as a substrate for retinal pigment epithelium. Colloids Surf. B 2016, 139, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arias, S.L.; Shetty, A.R.; Senpan, A.; Echeverry-Rendón, M.; Reece, L.M.; Allain, J.P. Fabrication of a Functionalized Magnetic Bacterial Nanocellulose with Iron Oxide Nanoparticles. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; Zhang, H. A natural in situ fabrication method of functional bacterial cellulose using a microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef]
- Orelma, H.; Morales, L.O.; Johansson, L.S.; Hoeger, I.C.; Filpponen, I.; Castro, C.; Laine, J. Affibody conjugation onto bacterial cellulose tubes and bioseparation of human serum albumin. RSC Adv. 2014, 4, 51440–51450. [Google Scholar] [CrossRef]
- Romanov, D.P.; Khripunov, A.K.; Baklagina, Y.G.; Severin, A.V.; Lukasheva, N.V.; Tolmachev, D.A.; Klechkovskaya, V.V. Nanotextures of composites based on the interaction between hydroxyapatite and cellulose Gluconacetobacter xylinus. Glas. Phys. Chem. 2014, 40, 367–374. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Bañó, M.C. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009, 5, 1605–1615. [Google Scholar] [CrossRef]
- Wan, Y.; Gao, C.; Han, M.; Liang, H.; Ren, K.; Wang, Y.; Luo, H. Preparation and characterization of bacterial cellulose/heparin hybrid nanofiber for potential vascular tissue engineering scaffolds. Polym. Adv. Technol. 2011, 22, 2643–2648. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.; Huang, Y. Immobilisation of heparin on bacterial cellulose-chitosan nano-fibres surfaces via the cross-linking technique. IET Nanobiotechnol. 2012, 6, 52. [Google Scholar] [CrossRef]
- Brackmann, C.; Zaborowska, M.; Sundberg, J.; Gatenholm, P.; Enejder, A. In situ imaging of collagen synthesis by osteoprogenitor cells in microporous bacterial cellulose scaffolds. Tissue Eng. Part C Methods 2012, 18, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomaterials 2010, 31, 8889–8901. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.R.; Pértile, R.A.N.; Rambo, C.R.; Porto, L.M. Enriched glucose and dextrin mannitol-based media modulates fibroblast behavior on bacterial cellulose membranes. Mater. Sci. Eng. C 2013, 33, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
- Butchosa, N.; Brown, C.; Larsson, P.T.; Berglund, L.A.; Bulone, V.; Zhou, Q. Nanocomposites of bacterial cellulose nanofibers and chitin nanocrystals: Fabrication, characterization and bactericidal activity. Green Chem. 2013, 15, 3404. [Google Scholar] [CrossRef]
- Rühs, P.A.; Storz, F.; Gómez, Y.A.L.; Haug, M.; Fischer, P. 3D bacterial cellulose biofilms formed by foam templating. NPJ Biofilms Microbiomes 2018, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Barud, H.G.; Barud, H.d.S.; Cavicchioli, M.; do Amaral, T.S.; Junior, O.B.d.O.; Santos, D.M.; Ribeiro, S.J.L. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaffold for tissue regeneration. Carbohydr. Polym. 2015, 128, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kim, J. Preparation and Characterization of Novel Bacterial Cellulose/Gelatin Scaffold for Tissue Regeneration Using Bacterial Cellulose Hydrogel. J. Nanotechnol. Eng. Med. 2010, 1, 021002. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Zhang, A.; Huang, Y.; Tavakoli, J.; Tang, Y. Novel Bacterial Cellulose/Gelatin Hydrogels as 3D Scaffolds for Tumor Cell Culture. Polymers 2018, 10, 581. [Google Scholar] [CrossRef]
- Andrade, F.K.; Silva, J.P.; Carvalho, M.; Castanheira, E.M.S.; Soares, R.; Gama, M. Studies on the hemocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2011, 98A, 554–566. [Google Scholar] [CrossRef]
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of Nanocellulose with a Xyloglucan–RGD Conjugate Enhances Adhesion and Proliferation of Endothelial Cells: Implications for Tissue Engineering. Biomacromolecules 2007, 8, 3697–3704. [Google Scholar] [CrossRef]
- Véliz, D.S.; Alam, C.; Toivola, D.M.; Toivakka, M.; Alam, P. On the non-linear attachment characteristics of blood to bacterial cellulose/kaolin biomaterials. Colloids Surf. B 2014, 116, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.E.; Wan, W.K. The polyvinyl alcohol–bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2006, 79B, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Huang, M. Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Katepetch, C.; Rujiravanit, R.; Tamura, H. Formation of nanocrystalline ZnO particles into bacterial cellulose pellicle by ultrasonic-assisted in situ synthesis. Cellulose 2013, 20, 1275–1292. [Google Scholar] [CrossRef]
- Yang, G.; Xie, J.; Hong, F.; Cao, Z.; Yang, X. Antimicrobial activity of silver nanoparticle impregnated bacterial cellulose membrane: Effect of fermentation carbon sources of bacterial cellulose. Carbohydr. Polym. 2012, 87, 839–845. [Google Scholar] [CrossRef]
- Shi, Q.; Li, Y.; Sun, J.; Zhang, H.; Chen, L.; Chen, B.; Wang, Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials 2012, 33, 6644–6649. [Google Scholar] [CrossRef]
- Rouabhia, M.; Asselin, J.; Tazi, N.; Messaddeq, Y.; Levinson, D.; Zhang, Z. Production of Biocompatible and Antimicrobial Bacterial Cellulose Polymers Functionalized by RGDC Grafting Groups and Gentamicin. ACS Appl. Mater. Interfaces 2014, 6, 1439–1446. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.Z.; Luo, H.L.; Gao, C.; Huang, Y. Immobilization of gelatin on bacterial cellulose nanofibers surface via crosslinking technique. Mater. Sci. Eng. C 2012, 32, 536–541. [Google Scholar] [CrossRef]
- Oshima, T.; Taguchi, S.; Ohe, K.; Baba, Y. Phosphorylated bacterial cellulose for adsorption of proteins. Carbohydr. Polym. 2011, 83, 953–958. [Google Scholar] [CrossRef]
- Gorgieva, S.; Hribernik, S. Microstructured and Degradable Bacterial Cellulose–Gelatin Composite Membranes: Mineralization Aspects and Biomedical Relevance. Nanomaterials 2019, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Girandon, L.; Kokol, V. Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C 2017, 73, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Vivod, V.; Maver, U.; Gradišnik, L.; Dolenšek, J.; Kokol, V. Internalization of (bis)phosphonate-modified cellulose nanocrystals by human osteoblast cells. Cellulose 2017, 24, 10. [Google Scholar] [CrossRef]
- Napavichayanun, S.; Yamdech, R.; Aramwit, P. The safety and efficacy of bacterial nanocellulose wound dressing incorporating sericin and polyhexamethylene biguanide: In vitro, in vivo and clinical studies. Arch. Dermatol. Res. 2016, 308, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-R.; Kanninen, L.; Kuisma, T.; Niklander, J.; Noon, L.A.; Burks, D.; Yliperttula, M. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 2014, 23, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Ndong Ntoutoume, G.M.A.; Grassot, V.; Brégier, F.; Chabanais, J.; Petit, J.-M.; Granet, R.; Sol, V. PEI-cellulose nanocrystal hybrids as efficient siRNA delivery agents—Synthesis, physicochemical characterization and in vitro evaluation. Carbohydr. Polym. 2017, 164, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Haldane, D.; Pircher, N.; Liebner, F.; French, A.; Condon, B.D. Protease Biosensors Based on Peptide-Nanocellulose Conjugates: From Molecular Design to Dressing Interface. Int. J. Med. Nano Res. 2016, 3, 1. [Google Scholar]
- Barbosa, A.; Robles, E.; Ribeiro, J.; Lund, R.; Carreño, N.; Labidi, J. Cellulose Nanocrystal Membranes as Excipients for Drug Delivery Systems. Materials 2016, 9, 1002. [Google Scholar] [CrossRef]
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M.N. Bacterial Cellulose: A Sustainable Source to Develop Value-Added Products—A Review. BioResources 2016, 11, 2. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2006, 76A, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Czaja, W.; Krystynowicz, A.; Kawecki, M.; Wysota, K.; Sakiel, S.; Wróblewski, P.; Glik, J.; Nowak, M.; Bielecki, S. Biomedical Applications of Microbial Cellulose in Burn Wound Recovery. In Cellulose: Molecular and Structural Biology; Springer: Dordrecht, The Netherlands, 2007; pp. 307–321. [Google Scholar]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C. Science and Principles of Biodegradable and Bioresorbable Medical Polymers: Materials and Properties; Woodhead Publishing: Sawston, Cambridge, UK, 2016; pp. 295–316. [Google Scholar]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lina, F.; Chandra, P.; Adrianna, M.; Wankei, W. Bacterial cellulose production using a novel microbe. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Bodin, A.; Bäckdahl, H.; Fink, H.; Gustafsson, L.; Risberg, B.; Gatenholm, P. Influence of cultivation conditions on mechanical and morphological properties of bacterial cellulose tubes. Biotechnol. Bioeng. 2007, 97, 425–434. [Google Scholar] [CrossRef]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Nanoderm. Available online: http://nanoderm.ca (accessed on 6 September 2019).
- Blackburn, R. Biodegradable and Sustainable Fibres; Woodhead Publishing: Cambridge, UK, 2005. [Google Scholar]
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Almasi, H.; Jafarzadeh, P.; Mehryar, L. Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr. Polym. 2018, 186, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Park, J.K. Bacterial cellulose-titanium dioxide nanocomposites: Nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 2015, 22, 565–579. [Google Scholar] [CrossRef]
- Bayazidi, P.; Almasi, H.; Asl, A.K. Immobilization of lysozyme on bacterial cellulose nanofibers: Characteristics, antimicrobial activity and morphological properties. Int. J. Biol. Macromol. 2018, 107, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Gidley, M.J.; Dykes, G.A. Potential of a nisin-containing bacterial cellulose film to inhibit Listeria monocytogenes on processed meats. Food Microbiol. 2008, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Jebali, A.; Hekmatimoghaddam, S.; Behzadi, A.; Rezapor, I.; Mohammadi, B.H.; Jasemizad, T.; Sayadi, M. Antimicrobial activity of nanocellulose conjugated with allicin and lysozyme. Cellulose 2013, 20, 2897–2907. [Google Scholar] [CrossRef]
- JPiasecka-Zelga, P.; Szulc, J.; Wietecha, J.; Ciechańska, D. An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018, 116, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.M.C.O.; Mesquita, L.A.F.; Souza, D.; Irioda, A.C.; Francisco, J.C.; Souza, C.F.; Carvalho, K.A.T. Regeneration of Skin Tissue Promoted by Mesenchymal Stem Cells Seeded in Nanostructured Membrane. Transplant. Proc. 2014, 46, 1882–1886. [Google Scholar] [CrossRef]
- Loh, E.Y.X.; Mohamad, N.; Fauzi, M.B.; Ng, M.H.; Ng, S.F.; Amin, M.C.I.M. Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing. Sci. Rep. 2018, 8, 2875. [Google Scholar] [CrossRef]
- Ravi, S.; Chaikof, E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2010, 5, 107–120. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, Y.S. The role of bacterial cellulose in artificial blood vessels. Mol. Cell. Toxicol. 2017, 13, 257–261. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; He, Y.; Duan, T. In-situ biopreparation of biocompatible bacterial cellulose/graphene oxide composites pellets. Appl. Surf. Sci. 2015, 338, 22–26. [Google Scholar] [CrossRef]
- Andrade, F.K.; Costa, R.; Domingues, L.; Soares, R.; Gama, M. Improving bacterial cellulose for blood vessel replacement: Functionalization with a chimeric protein containing a cellulose-binding module and an adhesion peptide. Acta Biomater. 2010, 6, 4034–4041. [Google Scholar] [CrossRef]
- Leitão, A.F.; Gupta, S.; Silva, J.P.; Reviakine, I.; Gama, M. Hemocompatibility study of a bacterial cellulose/polyvinyl alcohol nanocomposite. Colloids Surf. B 2012, 111, 493–502. [Google Scholar]
- Novaes, A.B.J.; Novaes, A.B. Bone formation over a TiAl6V4 (IMZ) implant placed into an extraction socket in association with membrane therapy (Gengiflex). Clin. Oral Implants Res. 1993, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Saska, S.; Teixeira, L.N.; Tambasco de Oliveira, P.; Minarelli Gaspar, A.M.; Lima Ribeiro, S.J.; Messaddeq, Y.; Marchetto, R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Shin, Y.M.; Kim, S.E.; Jeong, S.I.; Jeong, J.-O.; Park, J.-S.; Lim, Y.-M. Characterization of hydroxyapatite-coated bacterial cellulose scaffold for bone tissue engineering. Biotechnol. Bioprocess. Eng. 2015, 20, 948–955. [Google Scholar] [CrossRef]
- Kang, Y.J. Method of Tissue Repair Using a Composite Material. Patent Number WO2016169416A, December 2011. [Google Scholar]
- Zaborowska, M.; Bodin, A.; Bäckdahl, H.; Popp, J.; Goldstein, A.; Gatenholm, P. Microporous bacterial cellulose as a potential scaffold for bone regeneration. Acta Biomater. 2010, 6, 2540–2547. [Google Scholar] [CrossRef]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and differentiation of SH-SY5Y neuroblastoma cells on bacterial nanocellulose scaffolds. Artif. Cell. Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Amin, M.C.I.M.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 8. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, A.J.; Freire, C.S.; Neto, C.P. Do bacterial cellulose membranes have potential in drug-delivery systems? Expert Opin. Drug Deliv. 2014, 11, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.C.I.M.; Ahmad, N.; Pandey, M.; Xin, C.J. Stimuli-responsive bacterial cellulose-g-poly(acrylic acid-co-acrylamide) hydrogels for oral controlled release drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.d.F.S.; Nigoghossian, K.; Primo, F.L.; Saska, S.; Capote, T.S.d.O.; Caminaga, R.M.S.; Tedesco, A.C. Bacterial Cellulose Membranes as a Potential Drug Delivery System for Photodynamic Therapy of Skin Cancer. J. Braz. Chem. Soc. 2016, 27, 1949–1959. [Google Scholar] [CrossRef]
- Hoffman, W.L.; Jump, A.A.; Ruggles, A.O.; Kelly, P.J. Antibodies bound to nitrocellulose in acidic buffers retain biological activity. Electrophoresis 1993, 14, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H. Nanocomposite biomaterial mimicking aortic heart valve leaflet mechanical behaviour. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Tronser, T.; Laromaine, A.; Roig, A.; Levkin, P.A. Bacterial Cellulose Promotes Long-Term Stemness of mESC. ACS Appl. Mater. Interfaces 2018, 10, 16260–16269. [Google Scholar] [CrossRef] [PubMed]


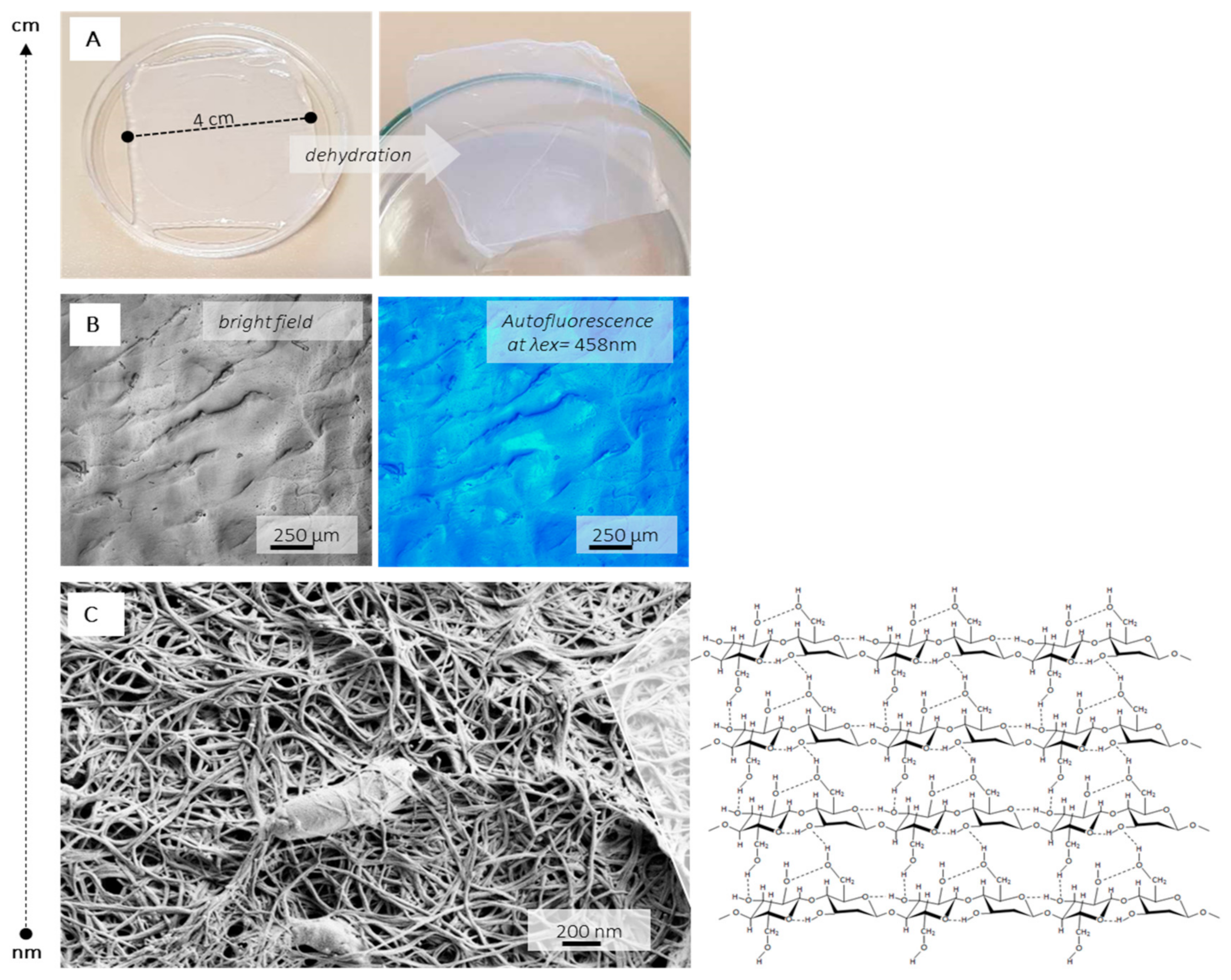

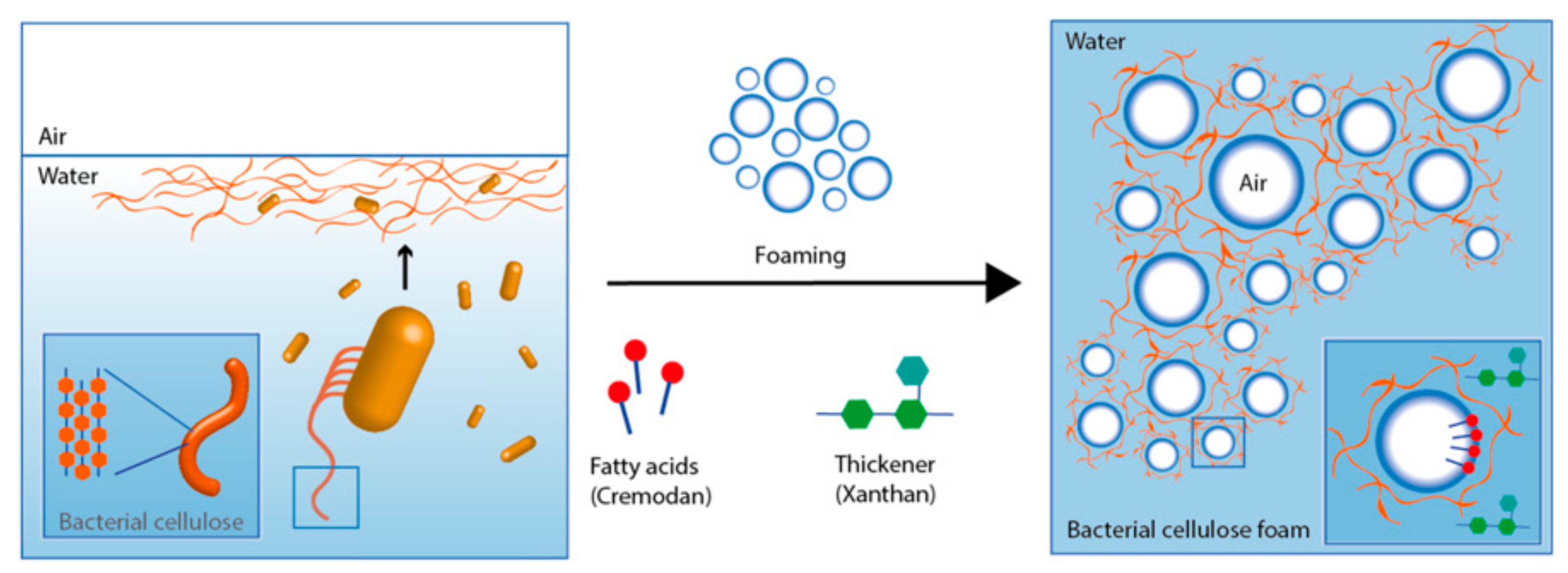

| Method for BC Production | Basic Characteristics of The Process and The Cellulose |
|---|---|
| Static production [36] | Most commonly used method at the lab scale. Duration of the process is up to two weeks. Cellulose is in the form of hydrogel sheet. |
| Production in shaking culture [37,38] | Increased delivery of oxygen to bacteria. Might result in reduced genetic stability of bacteria and lower BC production. Production of cellulose of different particle sizes and various shapes (mainly of spherical structure). Suitable for economic scale production. |
| Production in airlift bioreactor [38,39] | Efficient oxygen supply with low power supply. Cellulose produced in pellet. |
| Production in rotating disc bioreactors [40] | Production of homogenous cellulose. Cellulose yield is compared to the static process. |
| Production in trickling bed reactor [41] | Provides high oxygen concentration and low shear force. Produce BC in form of irregular sheets. |
| Modification | Application | Resulting Properties |
|---|---|---|
| BC nanocrystals/Regenerated Chitin fibers (BCNC/RC) [48] | Suture biomaterials | Biocompatible surgical sutures increasing strength of BCNC/RC filaments; Enzymatic degradation possible; Degradation rate can be tuned by varying concentration of BCNCs in the yarn; Chitin can promote cell proliferation (in vivo). |
| BC with modified topography [47] | Wound dressing | Improved cell alignment; Promotion of fibroblast infiltration and new collagen deposition in the wound bed. |
| Vaccarin impregnated on BC [49] | Neovascularization; Stratified squamous epithelium; Dense new- born subcutaneous tissue formation of collagen fibers and hyperplastic fibrous connective tissue. | |
| 2,2,6,6-Tetramethylpiperidinyloxy (TEMPO)-Oxidized BC with Ag nanoparticles [50] | Antimicrobial activity; Ag+ release with a rate of 12.2%/day at 37 °C in 3 days; Biocompatible. | |
| BC/ZnO nanocomposite [51] | Antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Citrobacter freundii; Significant healing of 66% after 15 days related to day 0. | |
| BC/TiO2 nanocomposite [52] | Antimicrobial activity against Escherichia coli and Staphylococcus aureus. | |
| BC/ε -poly-L-Lysine (ε-PLL) nanocomposite [53] | Antimicrobial activity (broad-spectrum) without affecting the beneficial structural and mechanical properties; Modification with non-toxic biopolymer ε-PLL inhibited growth of S. epidermidis on the membranes but did not affect the cytocompatibility to cultured human fibroblast. | |
| BC/Ag nanoparticle composite [54,55] | Environmentally benign and facile approach; Sustained release of Ag; Prolonged antibacterial performance against Staphylococcus aureus. | |
| Silymarin (SMN)-zein nanoparticle/BC nanocomposite [56] | Change of wettability and swelling; Antioxidant and antibacterial activity; Air-dried SMN-zein/BC nanocomposite slow down the lipid oxidation. | |
| BC/Octenidin/Poloxamer hybrid system [57] | Drug deliveryWound treatment | Long term controlled release of octenidine; Improved mechanical and antimicrobial properties; Ready-to-use system with Poloxamer-loaded BC for advanced treatment of infected wounds; Non toxicity in test with shell-less hen’s egg model. |
| BC/CMC/Methotrexate [58] | Impact of DS-CMC on methotrexate loading; Topical treatment of psoriasis; Decrease of the elastic modulus as the degree of substitution (DS) of CMC increased; | |
| BC/PHEMA Hydrogel matrice [59] | Biomedical application | New modification: in situ ultraviolet (UV) radical polymerization; Tensile strength increased; Nontoxic; Rat mesenchymal stem cells (rMSCs) proliferation; Tissue replacement and wound healing. |
| BC with tuned porosity [60] | Tissue engineering | Higher pore size than native BC to allow muscle cell ingrowth; Small decrease in mechanical strength. |
| BC/PVA composite [61] BC/Hyaluronic acid (HA) [62] | Artificial cornea | Higher visible light transmittance than plain BC. |
| BC/urinary bladder matrix [63] | Retinal pigment epithelium | Higher adhesion and proliferation of retinal pigment epithelium cells than uncoated BC; Closer recapitulation of the in vivo cell phenotype than uncoated BC. |
| BC/iron oxide nanoparticles [64] | Blood vessels | Introduction of magnetic domains; Young's modulus correspond to values for blood vessels. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. https://doi.org/10.3390/nano9101352
Gorgieva S, Trček J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials. 2019; 9(10):1352. https://doi.org/10.3390/nano9101352
Chicago/Turabian StyleGorgieva, Selestina, and Janja Trček. 2019. "Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications" Nanomaterials 9, no. 10: 1352. https://doi.org/10.3390/nano9101352
APA StyleGorgieva, S., & Trček, J. (2019). Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials, 9(10), 1352. https://doi.org/10.3390/nano9101352






