Improved Dispersion of Bacterial Cellulose Fibers for the Reinforcement of Paper Made from Recycled Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bacterial Cellulose (BC)
2.3. BC Fiber Dispersion Evaluation
2.4. Adsorption of Macromolecules onto BC Fibers
2.5. The TEMPO-Mediated Oxidation of BC Fibers
2.6. Paper Handsheet Preparation and Characterization
3. Results and Discussion
3.1. BC Fiber Dispersion and Reinforcement of Paper Handsheets
3.2. Effects of Additives on BC Fiber Dispersion
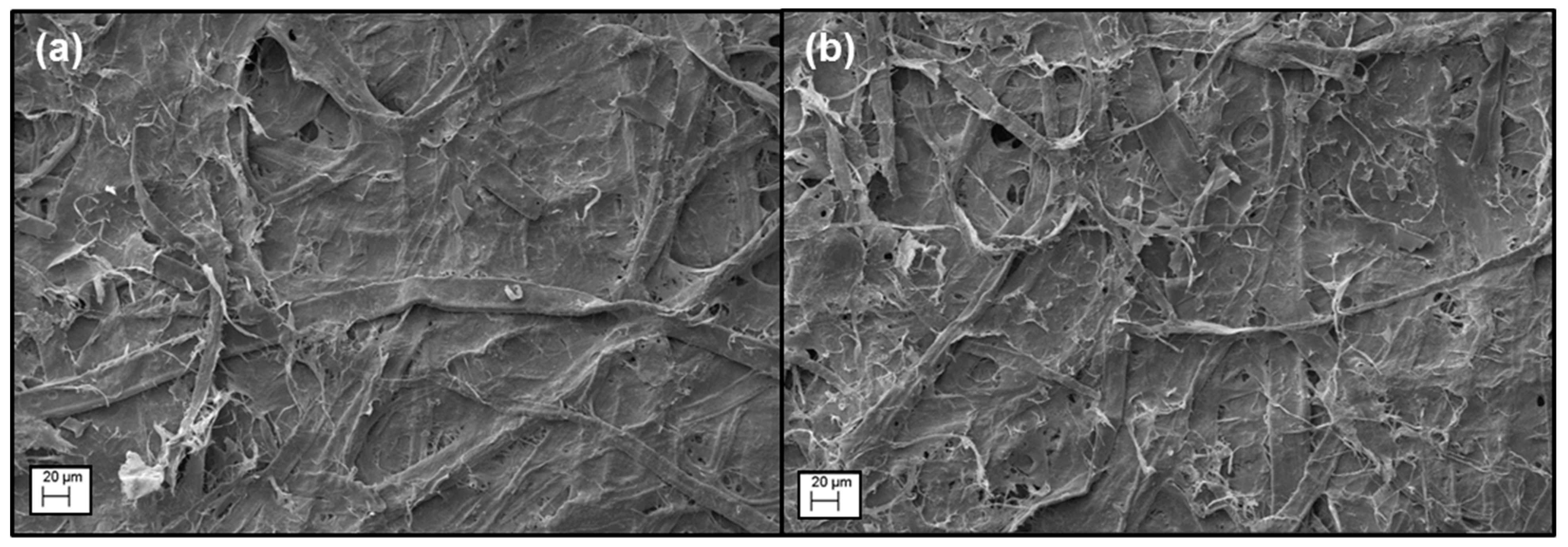
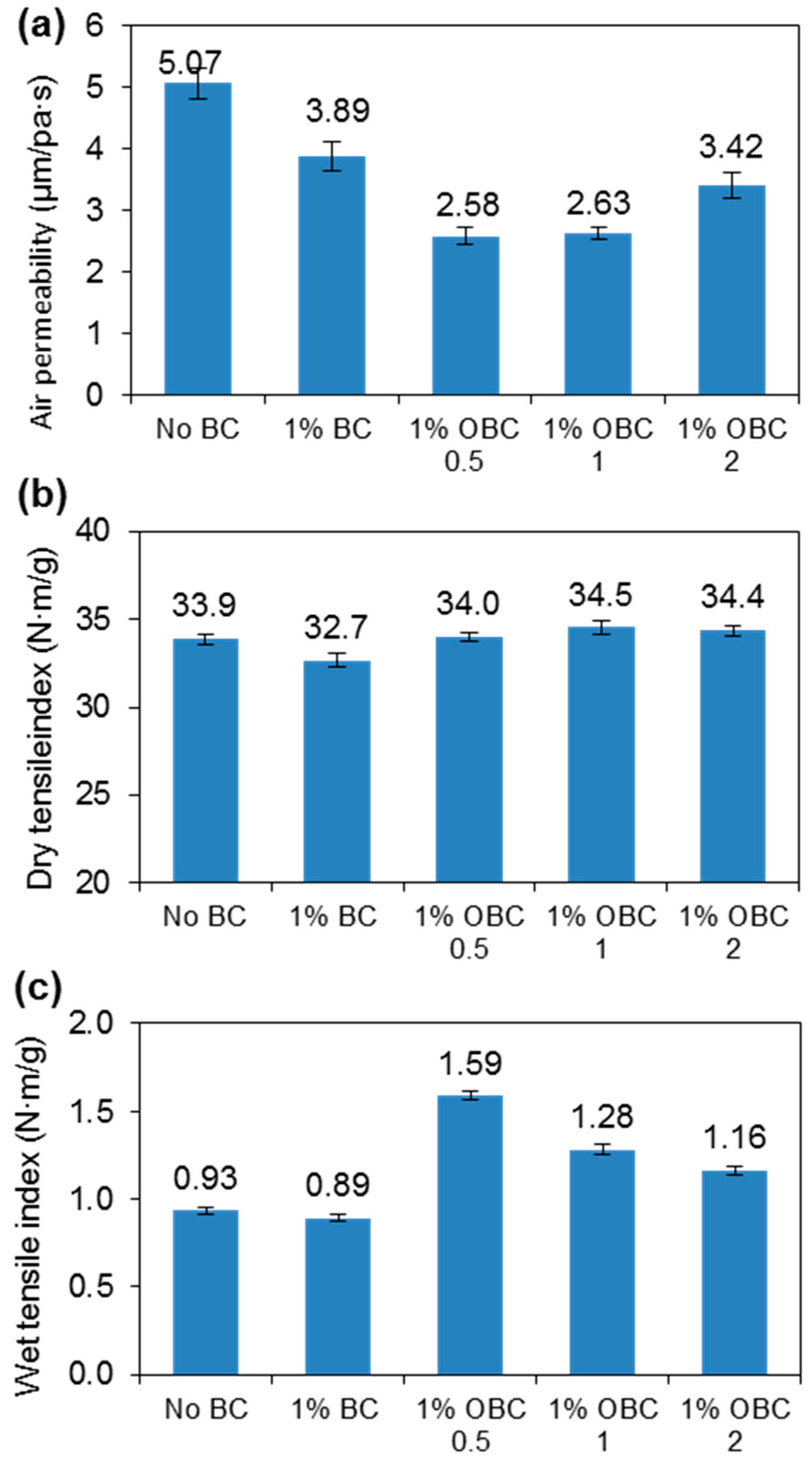
3.3. BC Fiber Dispersion by Surface Oxidation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BC | Bacterial cellulose |
| CMC | Carboxymethyl cellulose |
| OBC | Oxidized bacterial cellulose |
| PEO | Polyethylene oxide |
References
- Japan Paper Association. Available online: http://www.jpa.gr.jp/states/global-view/index.html (accessed on 16 October 2018).
- Araki, J. Electrostatic or steric?—Preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 2013, 9, 4125–4141. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Balea, A.; Tarres, Q.; Delgado-Aguilar, M.; Mutje, P.; Negro, C.; Blanco, A. Mechanical and chemical dispersion of nanocelluloses to improve their reinforcing effect on recycled paper. Cellulose 2018, 25, 269–280. [Google Scholar] [CrossRef]
- Kose, R.; Yamaguchi, K.; Okayama, T. Preparation of fine fiber sheets from recycled pulp fibers using aqueous counter collision. Cellulose 2016, 23, 1393–1399. [Google Scholar] [CrossRef]
- Castro, D.O.; Karim, Z.; Medina, L.; Häggström, J.O.; Carosio, F.; Svedberg, A.; Wågberg, L.; Söderberg, D.; Berglund, L.A. The use of a pilot-scale continuous paper process for fire retardant cellulose-kaolinite nanocomposites. Compos. Sci. Technol. 2018, 162, 215–224. [Google Scholar] [CrossRef]
- Balea, A.; Merayo, N.; Fuente, E.; Delgado-Aguilar, M.; Mutje, P.; Blanco, A.; Negro, C. Valorization of Corn Stalk by the Production of Cellulose Nanofibers to Improve Recycled Paper Properties. Bioresources 2016, 11, 3416–3431. [Google Scholar] [CrossRef]
- Balea, A.; Merayo, N.; Fuente, E.; Negro, C.; Delgado-Aguilar, M.; Mutje, P.; Blanco, A. Cellulose nanofibers from residues to improve linting and mechanical properties of recycled paper. Cellulose 2018, 25, 1339–1351. [Google Scholar] [CrossRef]
- Merayo, N.; Balea, A.; de la Fuente, E.; Blanco, A.; Negro, C. Synergies between cellulose nanofibers and retention additives to improve recycled paper properties and the drainage process. Cellulose 2017, 24, 2987–3000. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and application of microbial cellulose. Polym. Degrad. Stabil. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Yoshinaga, F.; Tonouchi, N.; Watanabe, K. Research progress in production of bacterial cellulose by aeration and agitation culture and its application as a new industrial material. Biosci. Biotechnol. Biochem. 1997, 61, 219–224. [Google Scholar] [CrossRef]
- Xiang, Z.; Jin, X.; Liu, Q.; Chen, Y.; Li, J.; Lu, F. The reinforcement mechanism of bacterial cellulose on paper made from woody and non-woody fiber sources. Cellulose 2017, 24, 5147–5156. [Google Scholar] [CrossRef]
- Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Effects of physical and chemical structures of bacterial cellulose on its enhancement to paper physical properties. Cellulose 2017, 24, 3513–3523. [Google Scholar] [CrossRef]
- Xiang, Z.; Chen, Y.; Liu, Q.; Lu, F. A highly recyclable dip-catalyst produced from palladium nanoparticle-embedded bacterial cellulose and plant fibers. Green Chem. 2018, 20, 1085–1094. [Google Scholar] [CrossRef]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Amornkitbamrung, L.; Marnul, M.-C.; Palani, T.; Hribernik, S.; Kovalcik, A.; Kargl, R.; Stana-Kleinschek, K.; Mohan, T. Strengthening of paper by treatment with a suspension of alkaline nanoparticles stabilized by trimethylsilyl cellulose. Nano-Struct. Nano-Objects 2018, 16, 363–370. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Halloysite Nanotubes for Cleaning, Consolidation and Protection. Chem. Rec. 2018, 18, 940–949. [Google Scholar] [CrossRef]
- Winter, H.T.; Cerclier, C.; Delorme, N.; Bizot, H.; Quemener, B.; Cathala, B. Improved Colloidal Stability of Bacterial Cellulose Nanocrystal Suspensions for the Elaboration of Spin-Coated Cellulose-Based Model Surfaces. Biomacromolecules 2010, 11, 3144–3151. [Google Scholar] [CrossRef]
- Mishima, T.; Hisamatsu, M.; York, W.S.; Teranishi, K.; Yamada, T. Adhesion of β-D-glucans to cellulose. Carbohydr. Res. 1998, 308, 389–395. [Google Scholar] [CrossRef]
- Pirich, C.L.; de Freitas, R.A.; Woehl, M.A.; Picheth, G.F.; Petri, D.F.S.; Sierakowski, M.R. Bacterial cellulose nanocrystals: Impact of the sulfate content on the interaction with xyloglucan. Cellulose 2015, 22, 1773–1787. [Google Scholar] [CrossRef]
- Azzam, F.; Heux, L.; Putaux, J.L.; Jean, B. Preparation By Grafting Onto, Characterization, and Properties of Thermally Responsive Polymer-Decorated Cellulose Nanocrystals. Biomacromolecules 2010, 11, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Kloser, E.; Gray, D.G. Surface Grafting of Cellulose Nanocrystals with Poly(ethylene oxide) in Aqueous Media. Langmuir 2010, 26, 13450–13456. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.W.Y.; An, X.; Zhu, X.; Cheng, X.; Zheng, L.; Nasrallah, J.E. Improving the colloidal stability of Cellulose nano-crystals by surface chemical grafting with polyacrylic acid. J. Bioresour. Bioprod. 2016, 1, 114–119. [Google Scholar]
- Zaman, M.; Xiao, H.N.; Chibante, F.; Ni, Y.H. Synthesis and characterization of cationically modified nanocrystalline cellulose. Carbohydr. Polym. 2012, 89, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hasani, M.; Cranston, E.D.; Westman, G.; Gray, D.G. Cationic surface functionalization of cellulose nanocrystals. Soft Matter 2008, 4, 2238–2244. [Google Scholar] [CrossRef]
- Sun, C.H.; Gunasekaran, S. Effects of protein concentration and oil-phase volume fraction on the stability and rheology of menhaden oil-in-water emulsions stabilized by whey protein isolate with xanthan gum. Food Hydrocoll. 2009, 23, 165–174. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Tovar, R.G.; Fischer, W.J.; Eckhart, R.; Bauer, W. White Water Recirculation Method as a Means to Evaluate the Influence of Fines on the Properties of Handsheets. Bioresources 2015, 10, 7242–7251. [Google Scholar]
- Pereira, P.H.F.; Waldron, K.W.; Wilson, D.R.; Cunha, A.P.; de Brito, E.S.; Rodrigues, T.H.S.; Rosa, M.F.; Azeredo, H.M.C. Wheat straw hemicelluloses added with cellulose nanocrystals and citric acid. Effect on film physical properties. Carbohydr. Polym. 2017, 164, 317–324. [Google Scholar] [CrossRef]
- Xiang, Z.Y.; Runge, T. Emulsifying properties of succinylated arabinoxylan-protein gum produced from corn ethanol residuals. Food Hydrocoll. 2016, 52, 423–430. [Google Scholar] [CrossRef]
- Xiang, Z.Y.; Anthony, R.; Lan, W.; Runge, T. Glutaraldehyde crosslinking of arabinoxylan produced from corn ethanol residuals. Cellulose 2016, 23, 307–321. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
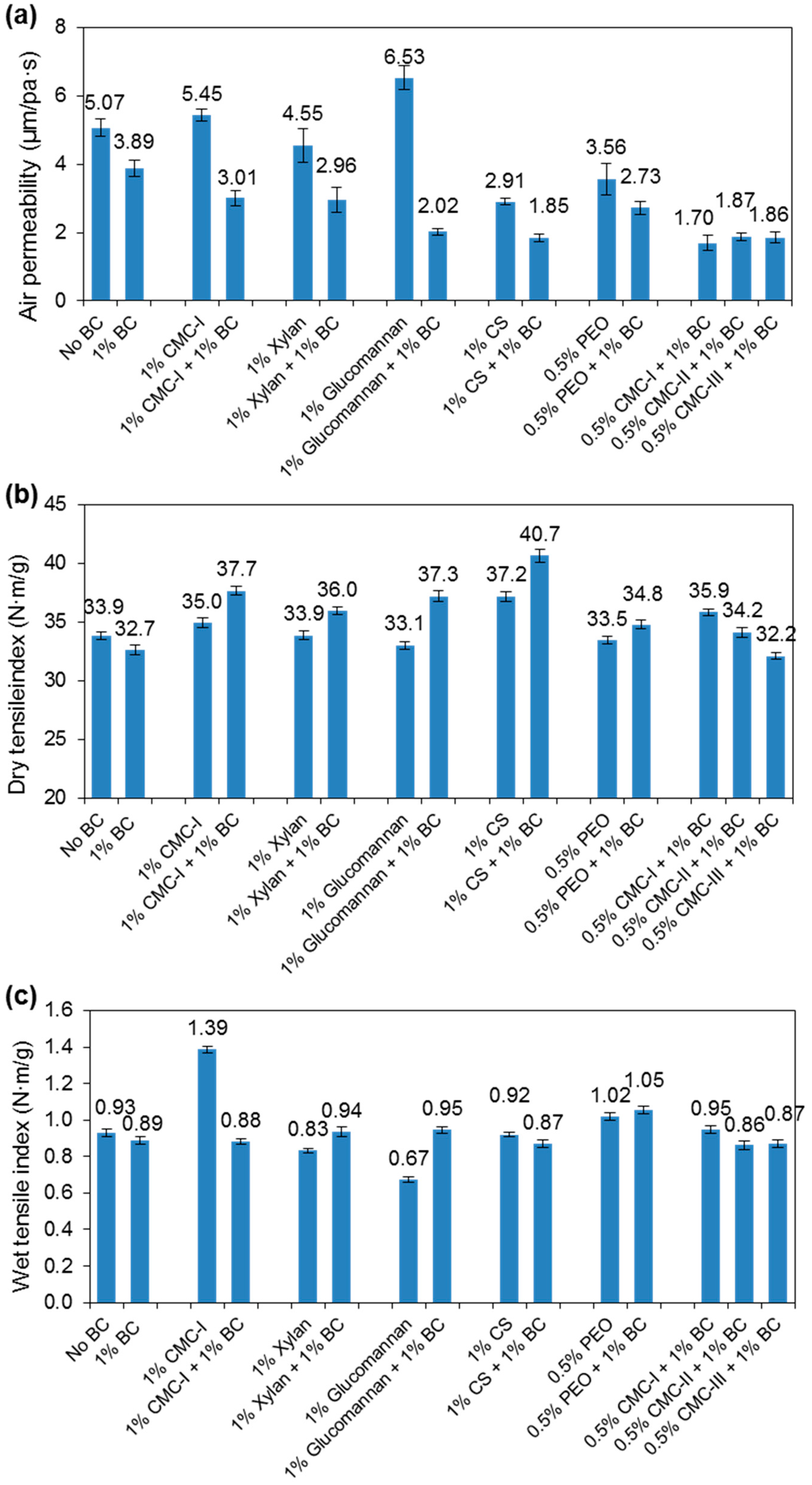
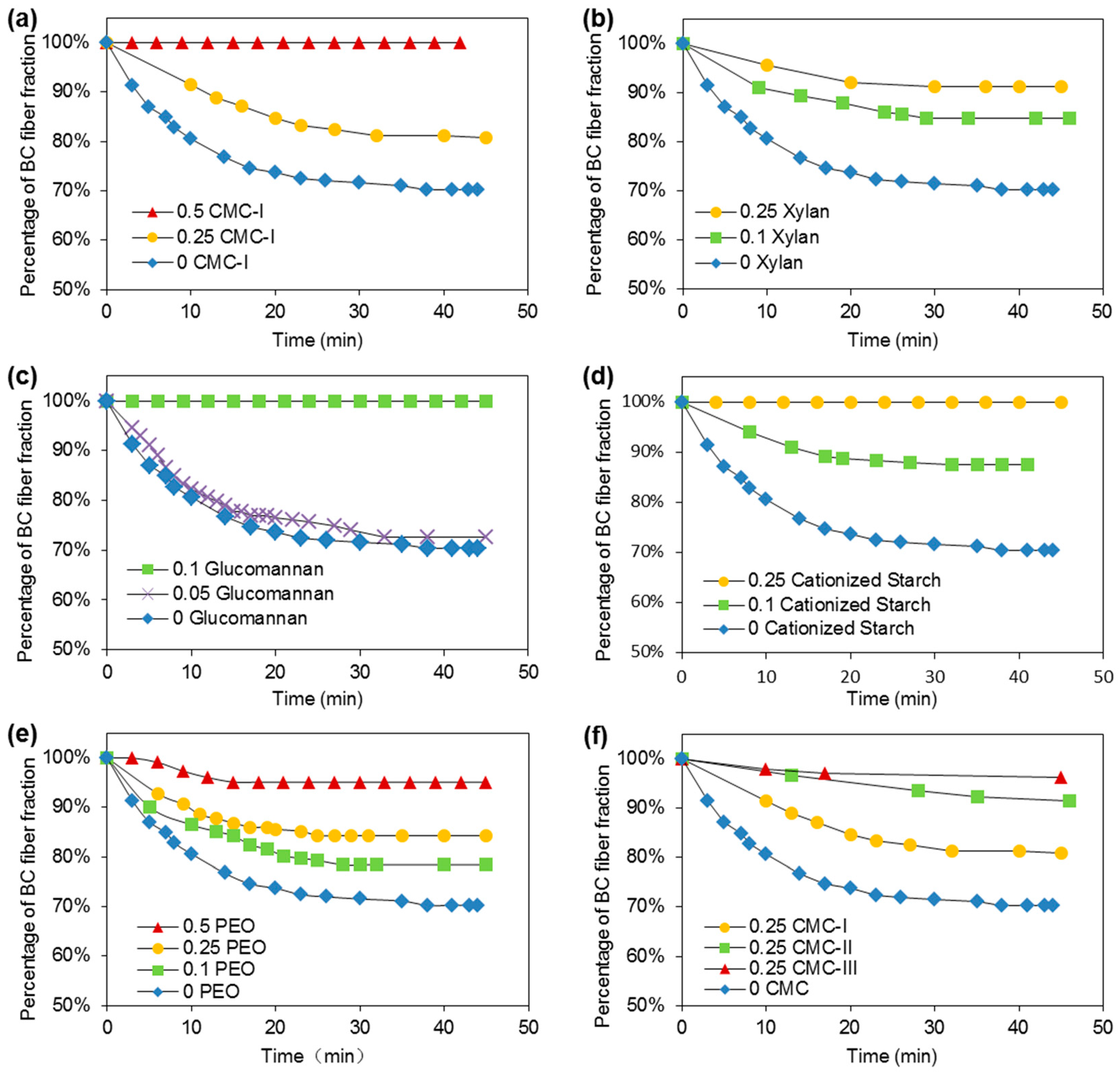
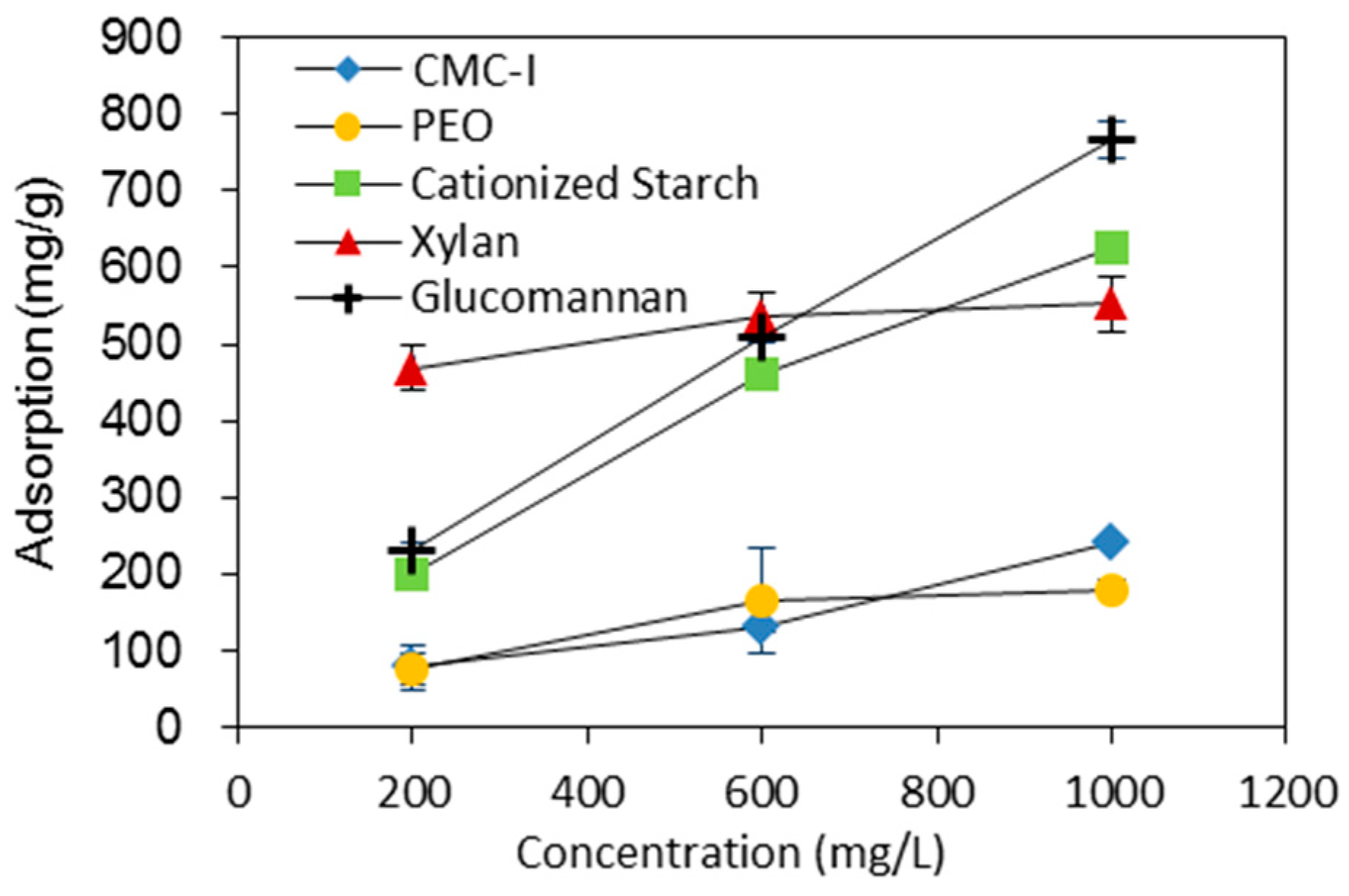
| Additives | CMC-I | Xylan | Glucomannan | Cationized Starch | PEO |
|---|---|---|---|---|---|
| Viscosity (mPa·s) | 30.9 ± 1.9 | 28.9 ± 0.6 | 29.4 ± 1.3 | 29.9 ± 0.8 | 33.1 ± 2.3 |
| Weight Ratio | Zeta potential (mV) of Macromolecule Additives | |||||
|---|---|---|---|---|---|---|
| None | CMC-I | Xylan | Glucomannan | Cationized Starch | PEO | |
| 0.1 | −21.2 ± 0.6 | −34.2 ± 1.8 | −22.9 ± 0.9 | −23.5 ± 1.3 | −20.0 ± 0.8 | −26.6 ± 2.0 |
| 0.25 | - | −56.8 ± 2.1 | −24.9 ± 1.1 | - | −13.2 ± 0.4 | −13.2 ± 1.4 |
| 0.5 | - | −60.6 ± 1.7 | −24.3 ± 1.0 | - | 29.9 ± 2.2 | −10.3 ± 0.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, Z.; Zhang, J.; Liu, Q.; Chen, Y.; Li, J.; Lu, F. Improved Dispersion of Bacterial Cellulose Fibers for the Reinforcement of Paper Made from Recycled Fibers. Nanomaterials 2019, 9, 58. https://doi.org/10.3390/nano9010058
Xiang Z, Zhang J, Liu Q, Chen Y, Li J, Lu F. Improved Dispersion of Bacterial Cellulose Fibers for the Reinforcement of Paper Made from Recycled Fibers. Nanomaterials. 2019; 9(1):58. https://doi.org/10.3390/nano9010058
Chicago/Turabian StyleXiang, Zhouyang, Jie Zhang, Qingguo Liu, Yong Chen, Jun Li, and Fachuang Lu. 2019. "Improved Dispersion of Bacterial Cellulose Fibers for the Reinforcement of Paper Made from Recycled Fibers" Nanomaterials 9, no. 1: 58. https://doi.org/10.3390/nano9010058
APA StyleXiang, Z., Zhang, J., Liu, Q., Chen, Y., Li, J., & Lu, F. (2019). Improved Dispersion of Bacterial Cellulose Fibers for the Reinforcement of Paper Made from Recycled Fibers. Nanomaterials, 9(1), 58. https://doi.org/10.3390/nano9010058







