Dual Size-Dependent Effect of Fe3O4 Magnetic Nanoparticles Upon Interaction with Lysozyme Amyloid Fibrils: Disintegration and Adsorption
Abstract
1. Introduction
2. Materials and Methods
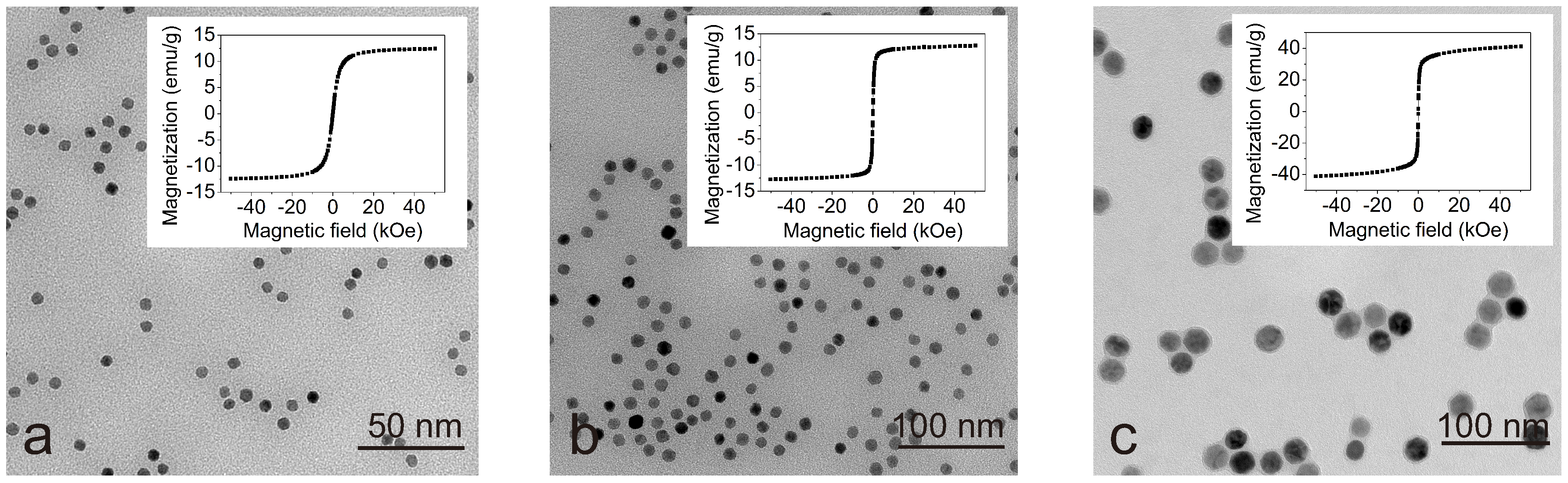
2.1. Sample Preparation
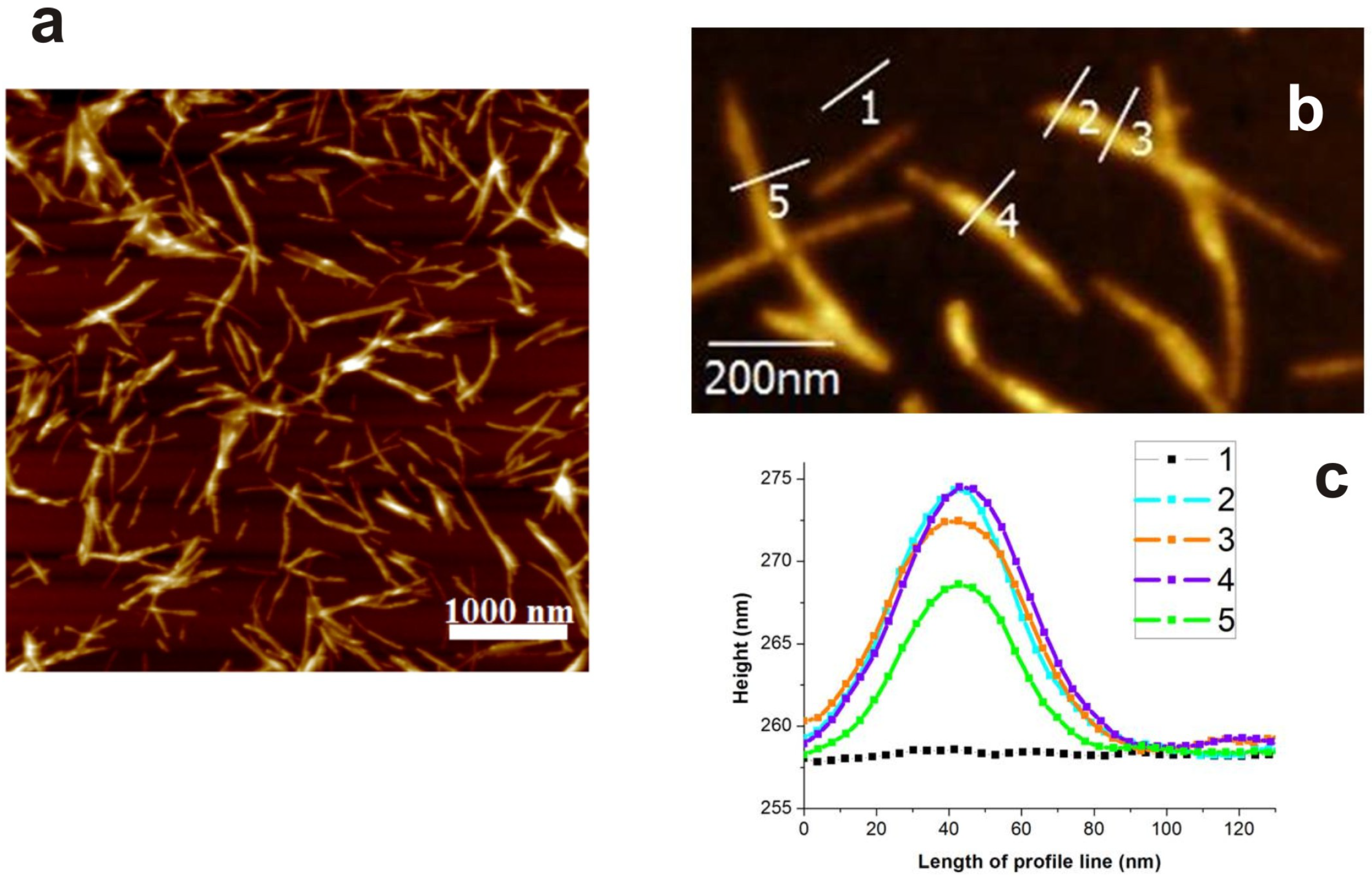
2.2. Atomic Force Microscopy
2.3. Zeta Potential
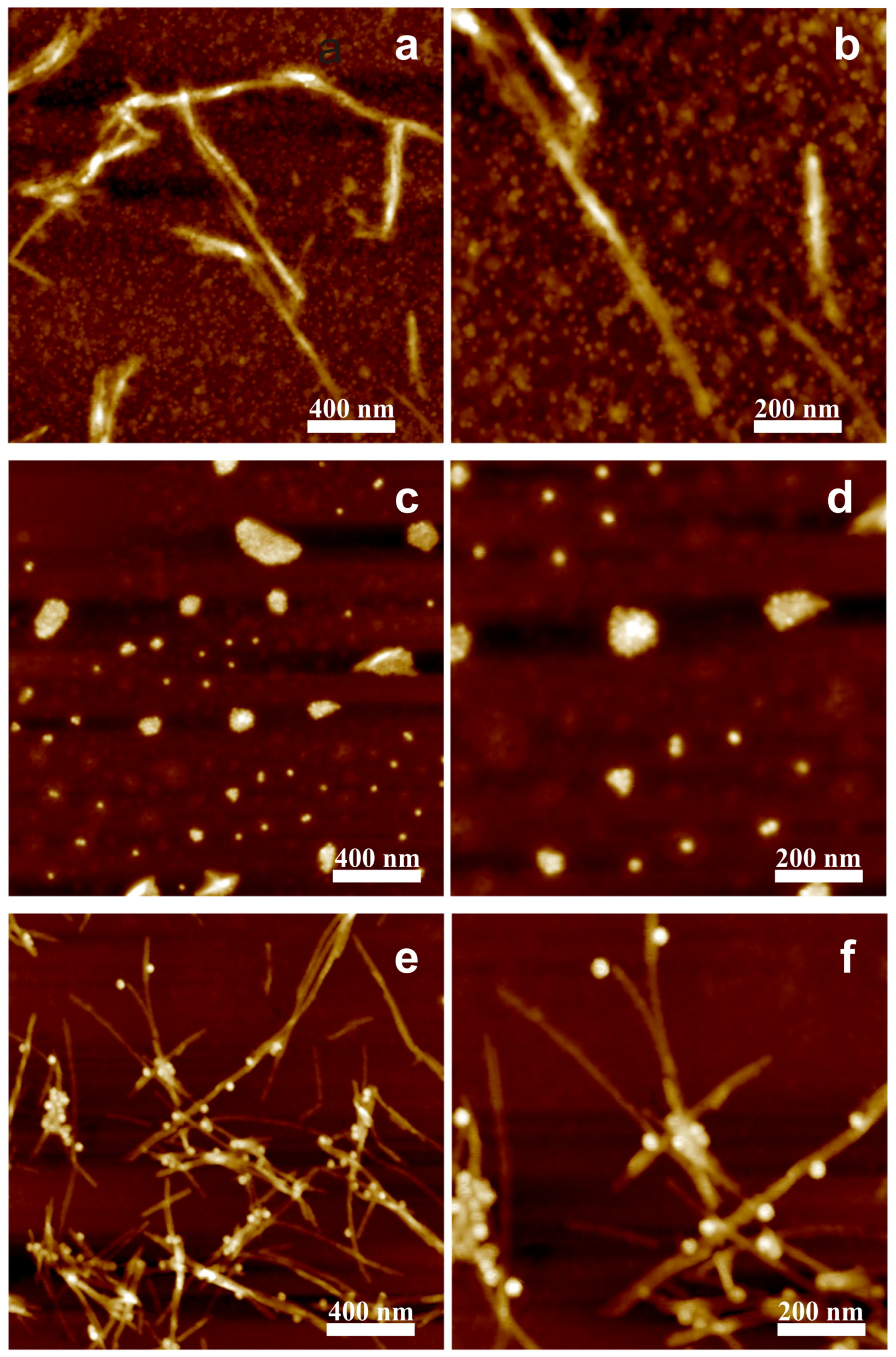
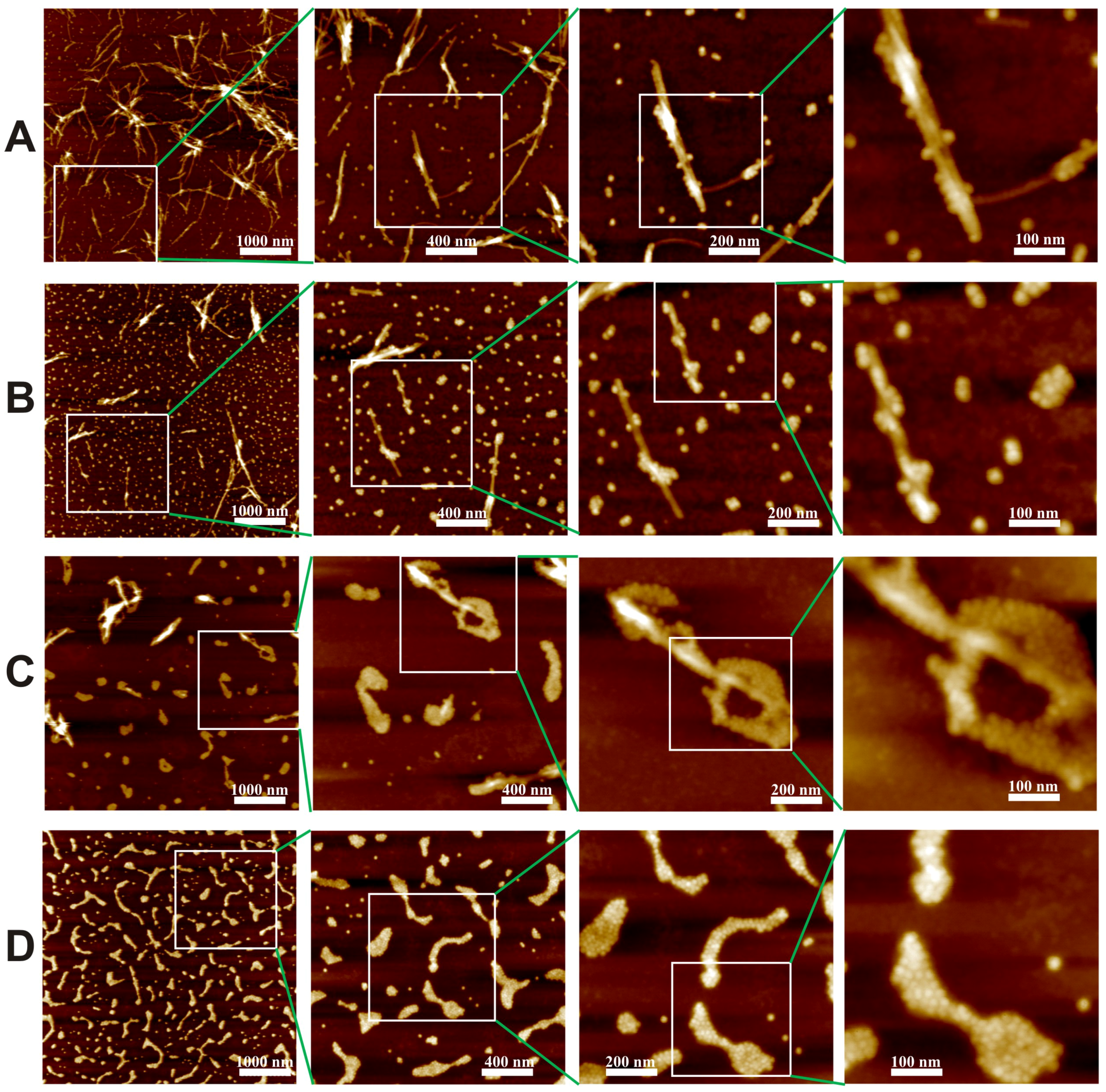
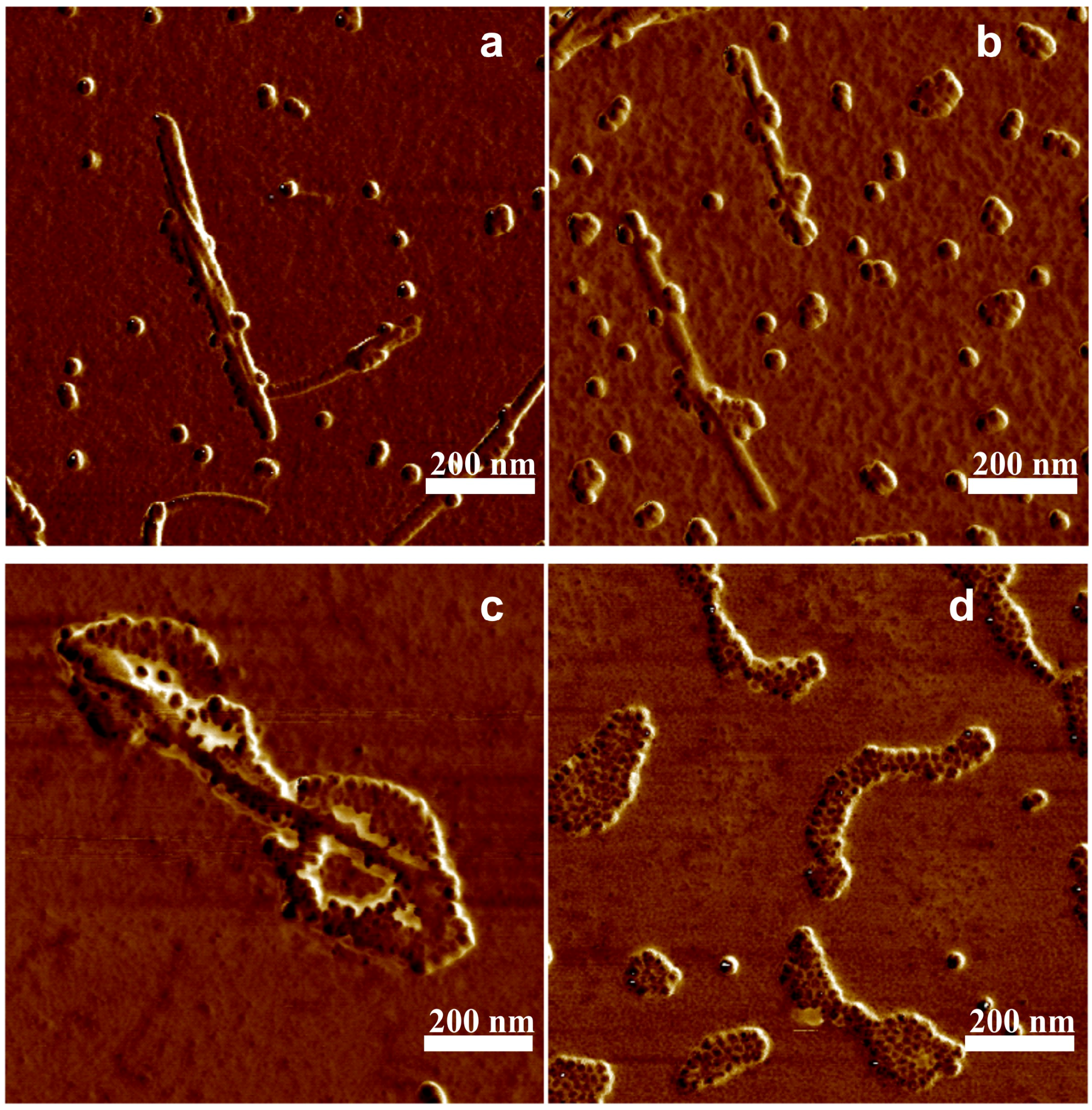
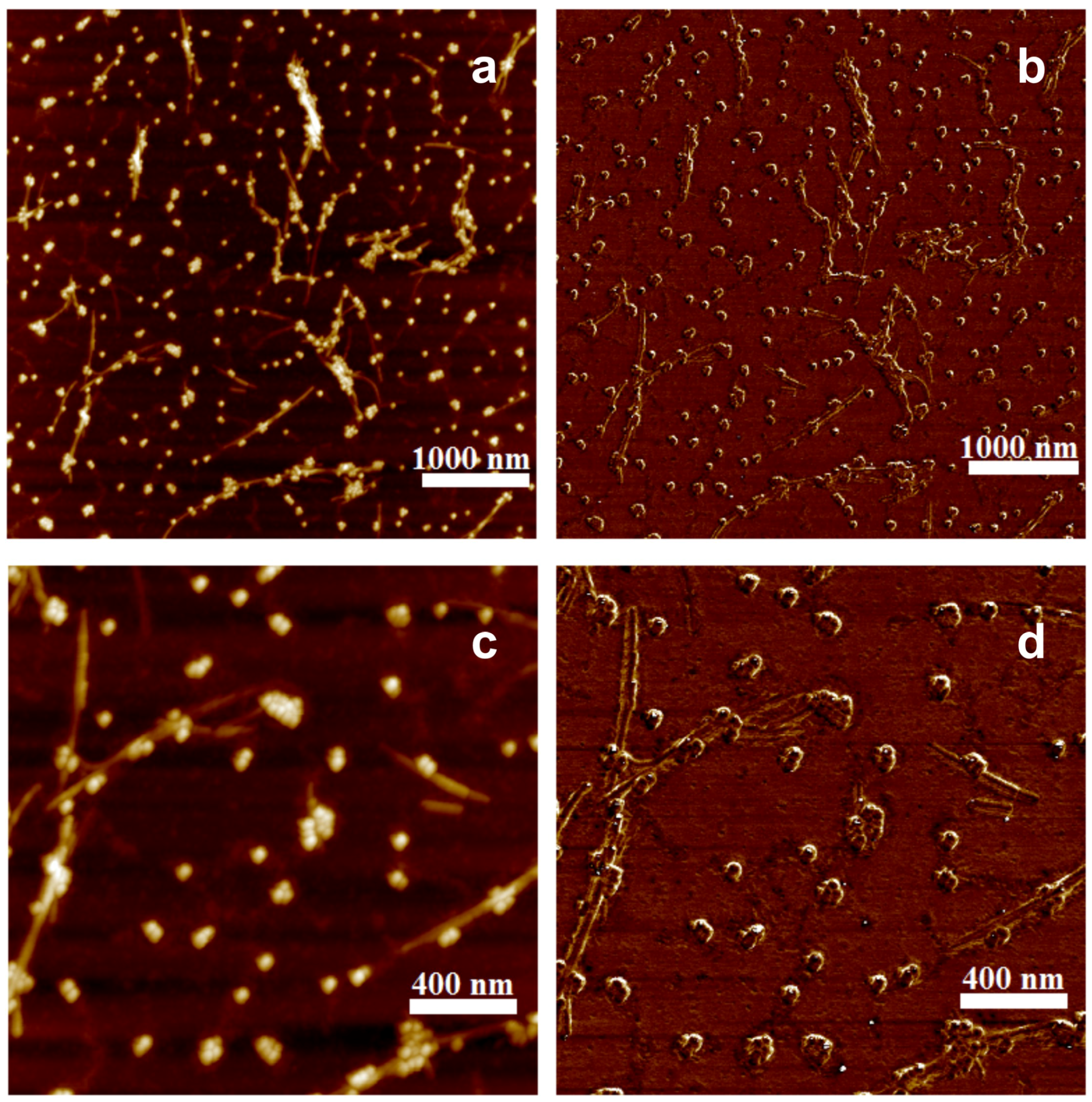
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| LAF | Lysozyme amyloid fibrils |
| HEWL | Hen egg white lysozyme |
| MNP | Magnetic nanoparticle |
| NP | nanoparticle |
| TEM | Transmission electron microscopy |
References
- Dobson, C.M. Protein aggregation and its consequences for human disease. Protein Pept. Lett. 2006, 13, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Benilova, I.; Karran, E.; De Strooper, B. The toxic AB oligomer and alzheimer’s disease: An emperor in need of clothes. Nat. Neurosci. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Comellas, G.; Lemkau, L.R.; Nieuwkoop, A.J.; Kloepper, K.D.; Ladror, D.T.; Ebisu, R.; Woods, W.S.; Lipton, A.S.; George, J.M.; Rienstra, C.M. Structured regions of α-synuclein fibrils include the early-onset Parkinson’s disease mutation sites. J. Mol. Biol. 2011, 411, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Westermark, P.; Andersson, A.; Westermark, G.T. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011, 91, 795–826. [Google Scholar] [CrossRef] [PubMed]
- Khlistunova, I.; Biernat, J.; Wang, Y.; Pickhardt, M.; von Bergen, M.; Gazova, Z.; Mandelkow, E.; Mandelkow, E.M. Inducible expression of Tau repeat domain in cell models of tauopathy: Aggregation is toxic to cells but can be reversed by inhibitor drugs. J. Biol. Chem. 2006, 281, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Estevez, A.Y.; Erlichman, J.S. Cerium oxide nanoparticles for the treatment of neurological oxidative stress diseases. In ACS Symposium Series; Andreescu, S., Hepel, M., Eds.; American Chemical Society: Washington, DC, USA, 2011; Volume 1083, pp. 255–288. [Google Scholar]
- Carroll, R.T.; Bhatia, D.; Geldenhuys, W.; Bhatia, R.; Miladore, N.; Bishayee, A.; Sutariya, V. Brain-targeted delivery of tempol- loaded nanoparticles for neurological disorders. J. Drug Target 2010, 18, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Naziroglu, M.; Muhamad, S.; Pecze, L. Nanoparticles as potential clinical therapeutic agents in alzheimer’s disease: Focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017, 10, 773–782. [Google Scholar] [CrossRef]
- Isa, L.; Jung, J.M.; Mezzenga, R. Unravelling adsorption and alignment of amyloid fibrils at interfaces by probe particle tracking. Soft Matter 2011, 7, 8127–8134. [Google Scholar] [CrossRef]
- Majorosova, J.; Petrenko, V.I.; Siposova, K.; Timko, M.; Tomasovicova, N.; Garamus, V.M.; Koralewski, M.; Avdeev, M.V.; Leszczynski, B.; Jurga, S.; et al. On the adsorption of magnetite nanoparticles on lysozyme amyloid fibrils. Colloids Surf. B Biointerfaces 2016, 146, 794–800. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chang, C.W.; Hung, H.S.; Kung, M.L.; Yeh, B.W.; Hsieh, S. Magnetite nanoparticle interactions with insulin amyloid fibrils. Nanotechnology 2016, 27, 415702. [Google Scholar] [CrossRef]
- Bharti, B.; Meissner, J.; Klapp, S.H.L.; Findenegg, G.H. Bridging interactions of proteins with silica nanoparticles: The influence of pH, ionic strength and protein concentration. Soft Matter 2014, 10, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Antosova, A.; Gazova, Z.; Fedunova, D.; Valusova, E.; Bystrenova, E.; Valle, F.; Daxnerova, Z.; Biscarini, F.; Antalik, M. Anti-amyloidogenic activity of glutathione-covered gold nanoparticles. Mater Sci. Eng. C 2012, 32, 2529–2535. [Google Scholar] [CrossRef]
- Bellova, A.; Bystrenova, E.; Koneracka, M.; Kopcansky, P.; Valle, F.; Tomasovicova, N.; Timko, M.; Bagelova, J.; Biscarini, F.; Gazova, Z. Effect of Fe3O4 magnetic nanoparticles on lysozyme amyloid aggregation. Nanotechnology 2010, 21, 065103. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, A.; Dong, K.; Li, W.; Ren, J.; Qu, X. Chemically exfoliated WS2 nanosheets efficiently inhibit amyloid b-peptide aggregation and can be used for photothermal treatment of alzheimer’s disease. Nano Res. 2015, 8, 3216–3227. [Google Scholar] [CrossRef]
- Ban, D.K.; Paul, S. Nano zinc oxide inhibits fibrillar growth and suppresses cellular toxicity of lysozyme amyloid. ACS Appl. Mater. Interfaces 2016, 8, 31587–31601. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ge, C.; Liu, J.; Chong, Y.; Gu, Z.; Jimenez-Cruz, C.A.; Chai, Z.; Zhou, R. Destruction of amyloid fibrils by graphene through penetration and extraction of peptides. Nanoscale 2015, 7, 18725–18737. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Pate, K.M.; Soto-Ortega, D.D.; Lohse, S.; Munnik, N.; Lim, M.; Jackson, L.S.; Lyles, V.D.; Jones, L.; Glassgow, N.; et al. Influence of gold nanoparticle surface chemistry and diameter upon alzheimer’s disease amyloid-b protein aggregation. J. Biol. Eng. 2017, 11, 1–11. [Google Scholar] [CrossRef]
- Skaat, H.; Chen, R.; Grinberg, I.; Margel, S. Engineered polymer nanoparticles containing hydrophobic dipeptide for inhibition of amyloid-b fibrillation. Biomacromolecules 2012, 13, 2662–2670. [Google Scholar] [CrossRef]
- Liao, Y.H.; Chang, Y.J.; Yoshiike, Y.; Chang, Y.C.; Chen, Y.R. Negatively charged gold nanoparticles inhibit alzheimer’s amyloid-b fibrillation, induce fibril dissociation, and mitigate neurotoxicity. Small 2012, 8, 3621–3639. [Google Scholar] [CrossRef]
- Siposova, K.; Kubovcikova, M.; Bednarikova, Z.; Koneracka, M.; Zavisova, V.; Antosova, A.; Kopcansky, P.; Daxnerova, Z.; Gazova, Z. Depolymerization of insulin amyloid fibrils by albumin-modified magnetic fluid. Nanotechnology 2012, 23, 055101. [Google Scholar] [CrossRef]
- Palmal, S.; Maity, A.R.; Singh, B.K.; Basu, S.; Jana, N.R. Inhibition of amyloid fibril growth and dissolution of amyloid fibrils by curcumin-gold nanoparticles. Chem. Eur. J. 2014, 20, 6184–6191. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.; Kalipillai, P.; Santhosh, P.B.; Mani, E. Role of surface charge of inhibitors on amyloid beta fibrillation. J. Phys. Chem. C 2017, 121, 6339–6348. [Google Scholar] [CrossRef]
- Alavez, S.; Vantipalli, M.C.; Zucker, D.J.S.; Klang, I.M.; Lithgow, G.J. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 2011, 472, 226–229. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.H.; Lee, H.; Nam, J.M. How do the size, charge and shape of nanoparticles affect amyloid B aggregation on brain lipid bilayer. Sci. Rep. 2016, 6, 19548. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.J.; Sperling, R.A.; Li, J.K.; Yang, T.Y.; Li, P.Y.; Zanella, M.; Chang, W.H.; Parak, W.J. Design of amphiphilic polymer for nanoparticle coating and functionalization. Small 2008, 4, 334–341. [Google Scholar] [CrossRef]
- Yin, X.; Shi, M.; Wu, J.; Pan, Y.T.; Gray, D.L.; Bertke, J.A.; Yang, H. Quantitative analysis of different formation modes of platinum nanocrystals controlled by ligand chemistry. Nanoletters 2017, 17, 6146–6150. [Google Scholar] [CrossRef] [PubMed]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, S. Size-dependent properties of magnetic iron oxide nanocrystals. Nanoscale 2011, 3, 225. [Google Scholar] [CrossRef] [PubMed]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94. [Google Scholar] [CrossRef]
- Tomašovičová, N.; Hu, P.S.; Zeng, C.L.; Huráková, M.; Csach, K.; Majorošová, J.; Kubovčíková, M.; Kopčanský, P. Dynamic morphogenesis of dendritic structures formation in hen egg white lysozyme fibrils doped with magnetic nanoparticles. Colloids Surf. B Biointerfaces 2018, 161, 457–463. [Google Scholar] [CrossRef]
- Bolisetty, S.; Vallooran, J.J.; Adamcik, J.; Mezzenga, R. Magnetic-Responsive Hybrids of Fe3O4 Nanoparticles with beta-Lactoglobulin Amyloid Fibrils and Nanoclusters. ACS Nano 2013, 7, 6146–6155. [Google Scholar] [CrossRef]
- Bolisetty, S.; Harnau, L.; Jung, J.M.; Mezzenga, R. Gelation, Phase Behavior, and Dynamics of beta-Lactoglobulin Amyloid Fibrils at Varying Concentrations and Ionic Strengths. Biomacromolecules 2012, 13, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Saxena, S.K.; Mishra, S.; Yogi, P.; Sagdeo, P.R.; Kumar, R. Ecofriendly gold nanoparticles-Lysozyme interaction: Thermodynamical perspectives. J. Photochem. Photobiol. B Biol. 2017, 174, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Drescher, S.; Mey, I.; Wahab, M.; Graf, G.; Garamus, V.M.; Hause, G.; Mogel, H.J.; Janshoff, A.; Dobner, B.; et al. Helical nanofibers of self-assembled bipolar phospholipids as template for gold nanoparticles. J. Phys. Chem. B 2008, 112, 4506–4511. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, H.; Catts, V.S.; Katayama, Y.; Shoji, H.; Takagi, T.; Huang, F.L.; Nakao, A.; Mori, Y.; Huang, K.P.; Ishii, S.; et al. Decreased brain pH as a shared Endophenotype of psychiatric disorders. Neuropsychopharmacology 2018, 43, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.; Rao, R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc. Natl. Acad. Sci. USA 2018, 115, 6640–6649. [Google Scholar] [CrossRef] [PubMed]
| Sample | 5 nm MNPs | 10 nm MNPs | 20 nm MNPs | LAF |
| Zeta potential | −58.9 mV | −55.4 mV | −31.5 mV | 44.8 mV |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomašovičová, N.; Hu, P.-S.; Zeng, C.-L.; Majorošová, J.; Zakutanská, K.; Kopčanský, P. Dual Size-Dependent Effect of Fe3O4 Magnetic Nanoparticles Upon Interaction with Lysozyme Amyloid Fibrils: Disintegration and Adsorption. Nanomaterials 2019, 9, 37. https://doi.org/10.3390/nano9010037
Tomašovičová N, Hu P-S, Zeng C-L, Majorošová J, Zakutanská K, Kopčanský P. Dual Size-Dependent Effect of Fe3O4 Magnetic Nanoparticles Upon Interaction with Lysozyme Amyloid Fibrils: Disintegration and Adsorption. Nanomaterials. 2019; 9(1):37. https://doi.org/10.3390/nano9010037
Chicago/Turabian StyleTomašovičová, Natália, Po-Sheng Hu, Cyun-Lun Zeng, Jozefína Majorošová, Katarína Zakutanská, and Peter Kopčanský. 2019. "Dual Size-Dependent Effect of Fe3O4 Magnetic Nanoparticles Upon Interaction with Lysozyme Amyloid Fibrils: Disintegration and Adsorption" Nanomaterials 9, no. 1: 37. https://doi.org/10.3390/nano9010037
APA StyleTomašovičová, N., Hu, P.-S., Zeng, C.-L., Majorošová, J., Zakutanská, K., & Kopčanský, P. (2019). Dual Size-Dependent Effect of Fe3O4 Magnetic Nanoparticles Upon Interaction with Lysozyme Amyloid Fibrils: Disintegration and Adsorption. Nanomaterials, 9(1), 37. https://doi.org/10.3390/nano9010037





