Citric Acid Capped CdS Quantum Dots for Fluorescence Detection of Copper Ions (II) in Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentations
2.3. Methods
2.4. Selective Detection of Cu2+
3. Results and Discussion
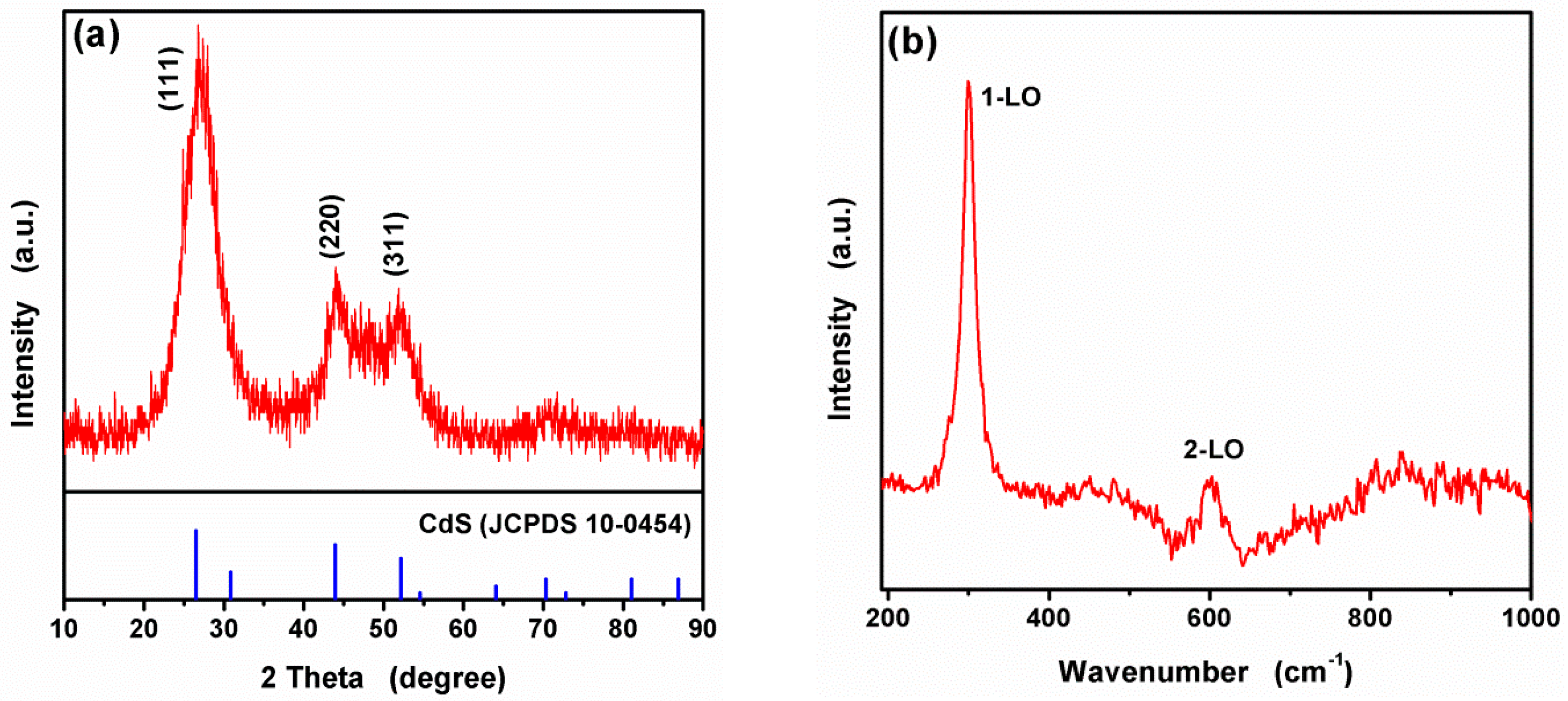
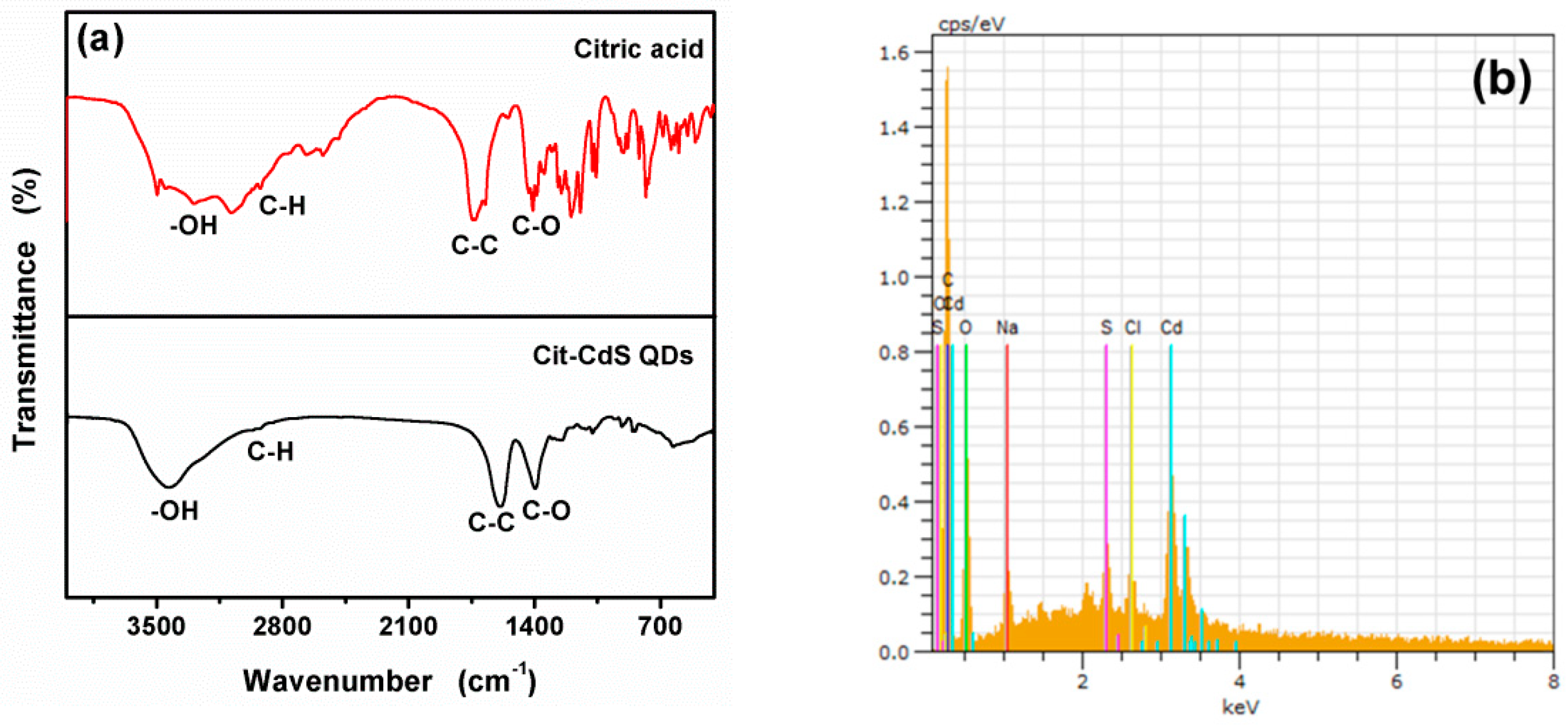
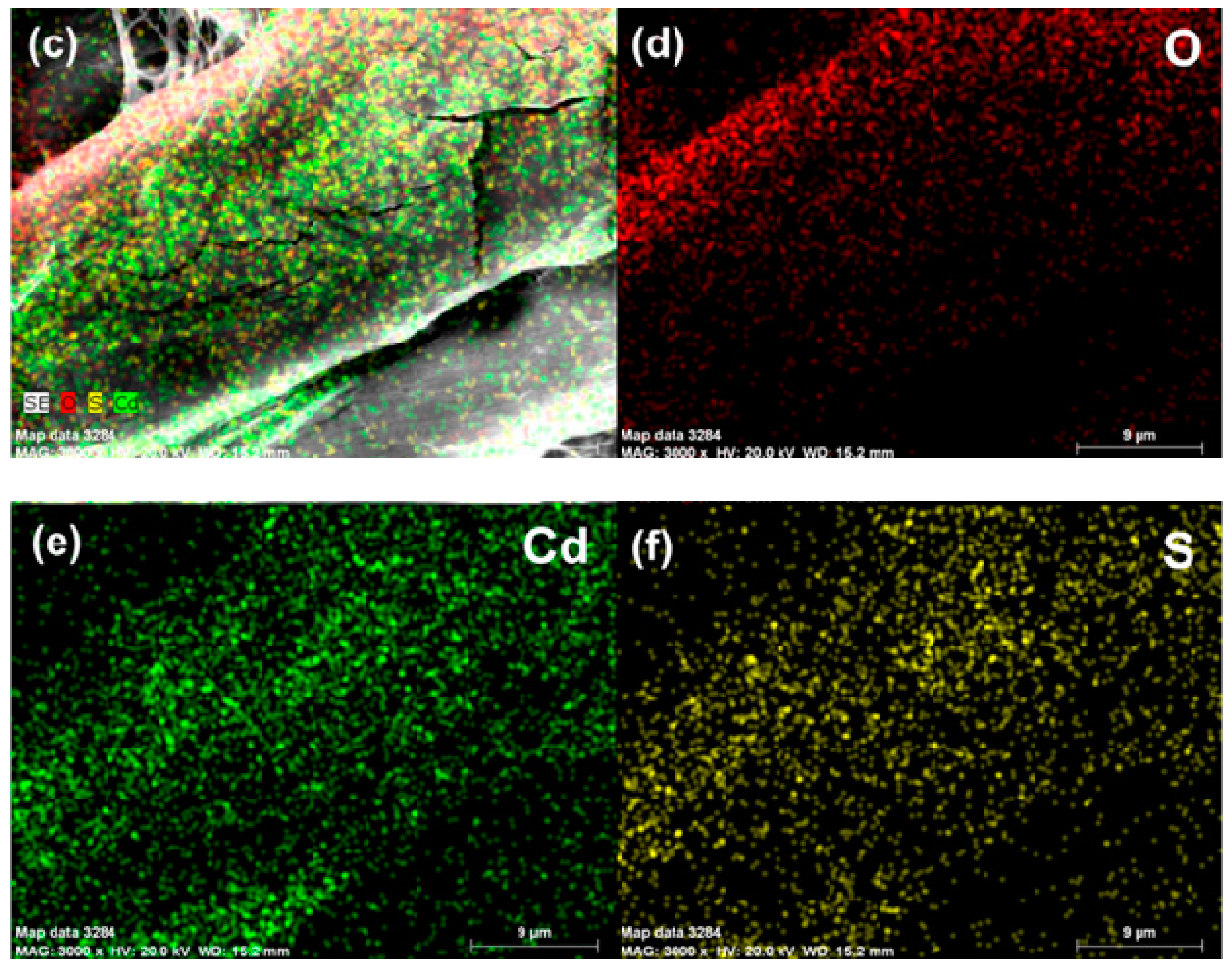
3.1. Characterization
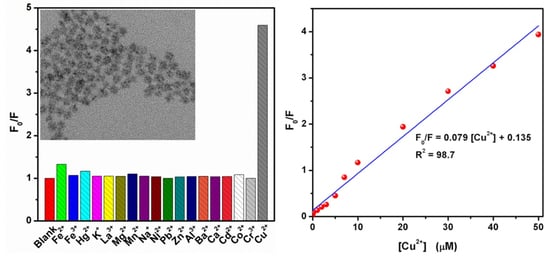
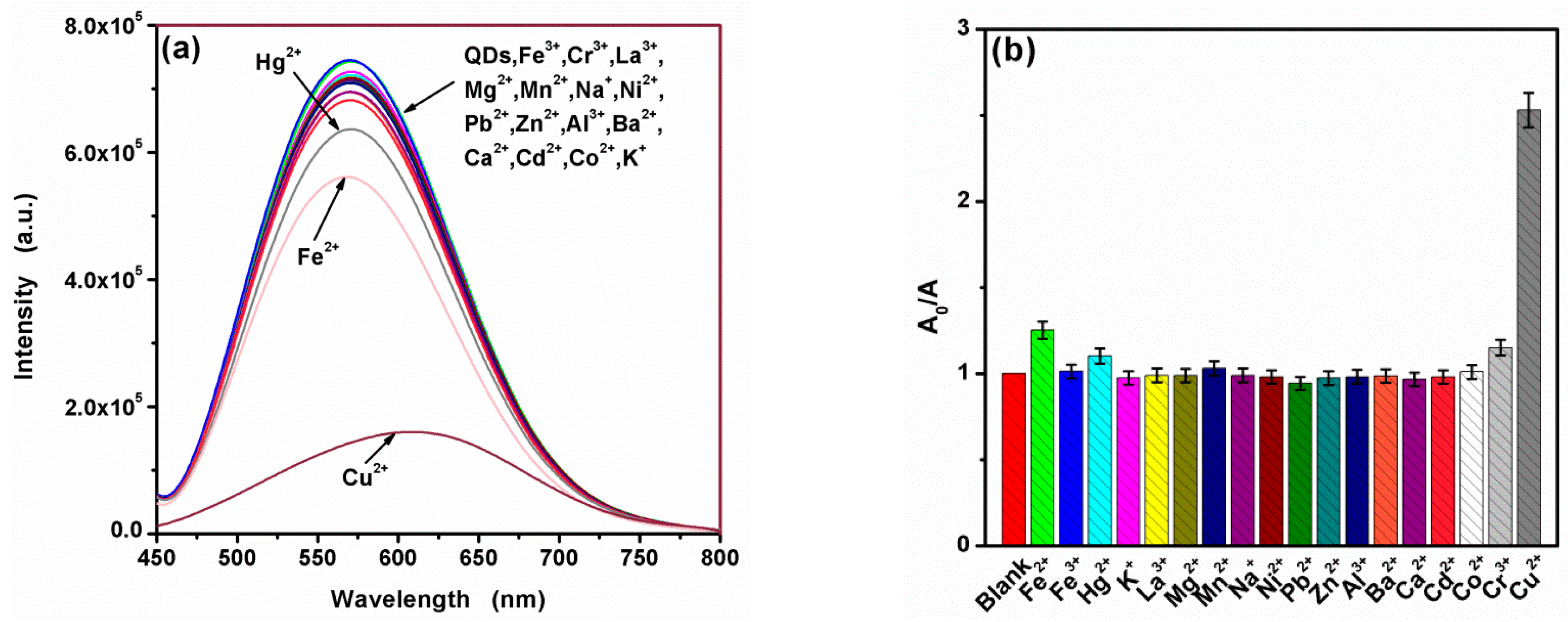
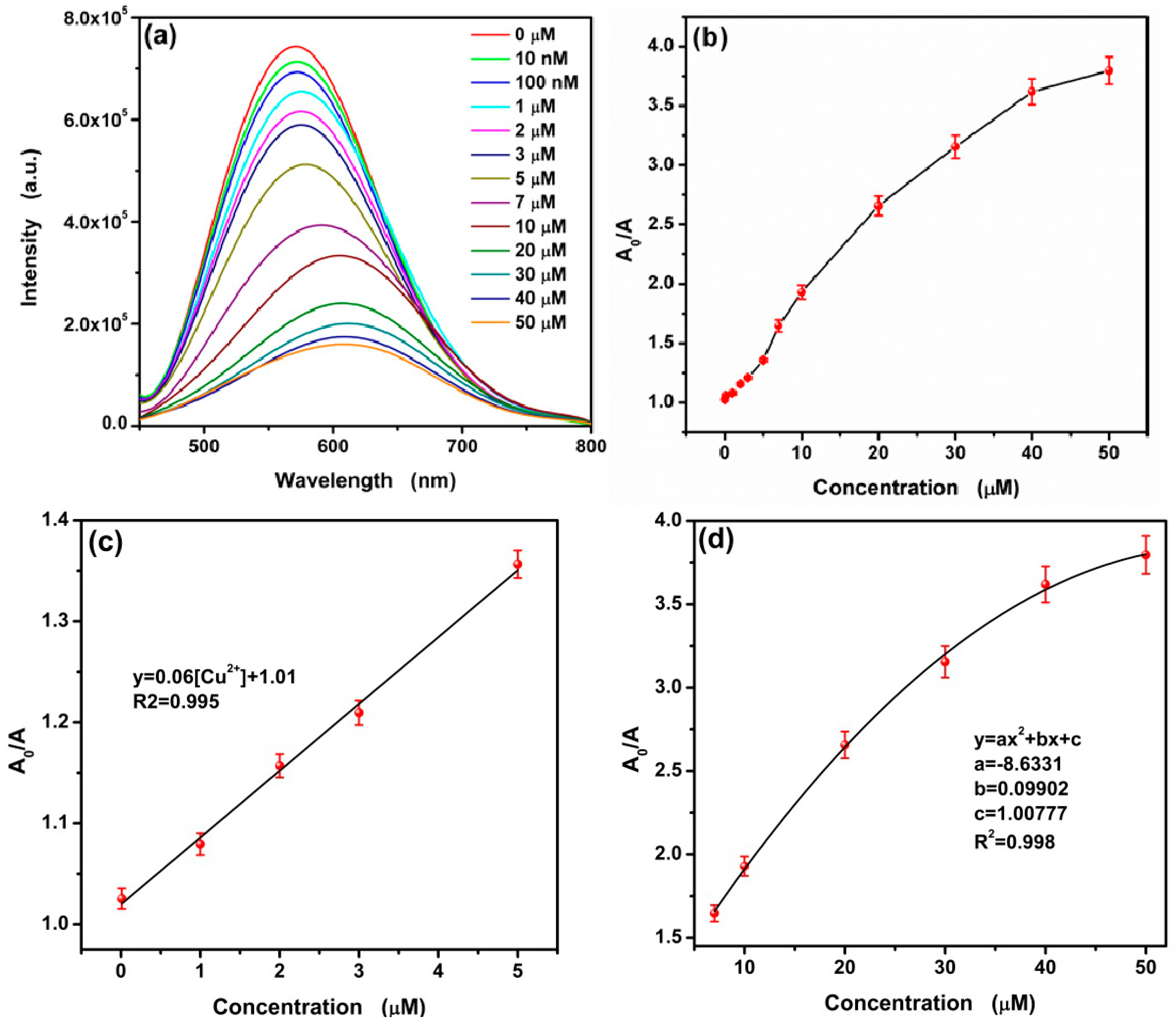
3.2. Selective Fluorescence Quenching of CA-CdS QDs by Cu2+
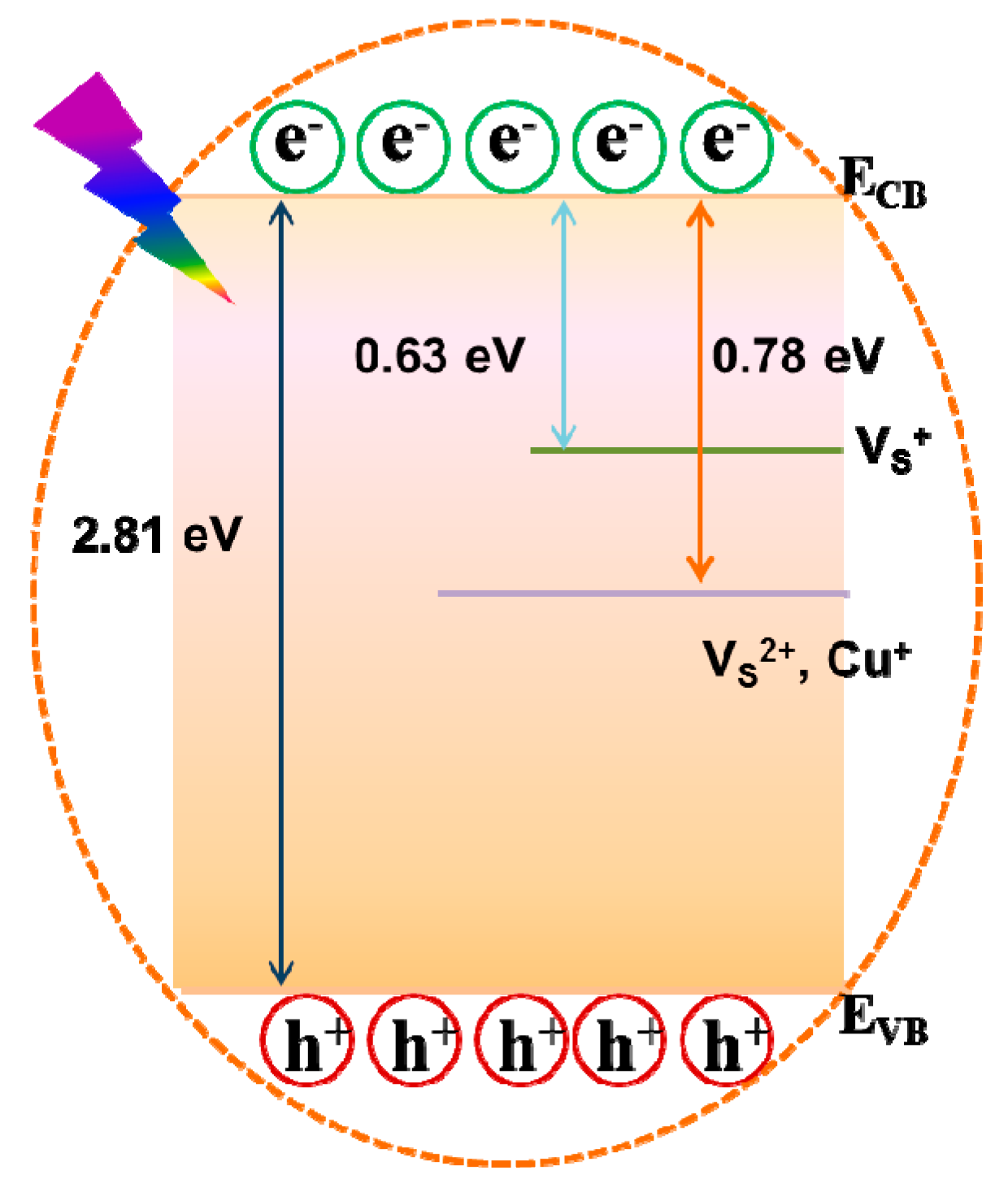
3.3. Mechanism for the Interaction Between Cu2+ and CA-CdS QDs
- In the absence of Cu2+
- In the presence of Cu2+
- Process 4: A great quantity of electrons will be excited from the VB to the CB when the sample is under UV light.
- Process 5: The photogenerated electrons in the shallow or deep trap recombinated with the hole in the VB of CA-Cd QDs sent out yellow emission (~570 nm).
- Process 6: The same process as the process 4.
- Process 7: The ultra-small Cu2S are formed on the surface of CA-CdS QDs result in the presence of Cu+ sites. An electron was trapped by Cu+ sites.
- Process 8: Recombination of shallow or deep trapped electrons in the CB with hole in the VB of CA-CdS QDs and some electron were trapped by Cu2+ sites with a new nonradiative channel.
- Process 9: Recombination of shallow or deep trapped electrons in the CB with hole in the VB of CA-CdS QDs and sent out an orange emission (~610 nm).
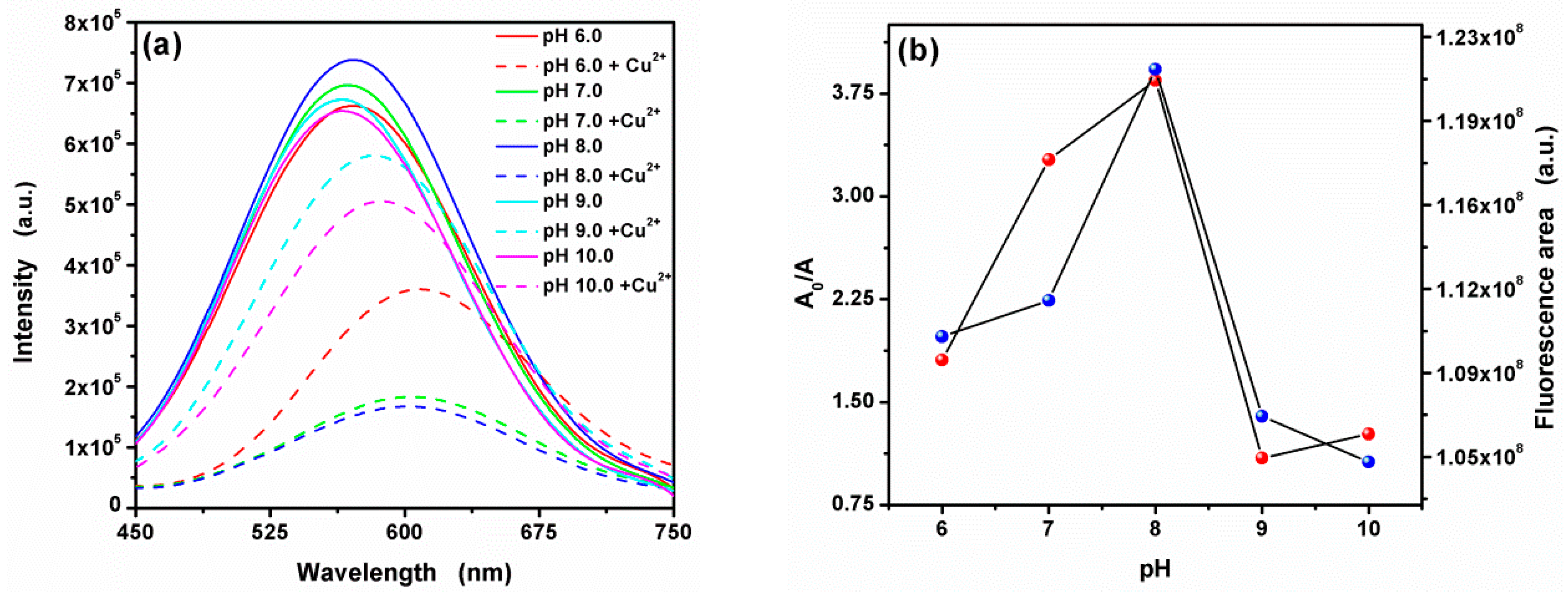
3.4. Effect of pH on the Fluorescence Quenching by Cu2+
3.5. Sensitive Fluorescence Quenching of CA-CdS QDs by Cu2+
3.6. Application in Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Strausak, D.; Mercer, J.F.B.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Res. Bull. 2011, 55, 175–185. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, T.; Deng, M.; Tang, X.; Han, S.; Liu, A.; Bai, Y.; Qu, D.; Huang, X.; Qiu, F. Selective and sensitive detection of copper (II) based on fluorescent zinc-dopedAgInS2 quantum dots. J. Lumin. 2018, 201, 182–188. [Google Scholar] [CrossRef]
- Lin, Z.; Gao, S.; Lin, J.; Lin, W.; Qiu, S.; Guo, L.; Qiu, B.; Chen, G. Visual detection of copper (II) based on the aggregation of gold nano-particles via click chemistry. Anal. Methods 2012, 5, 612–615. [Google Scholar] [CrossRef]
- Zhao, H.; Xue, G.; Nan, T.; Tan, G.; Li, Z.; Li, Q.; Zhang, Q.; Wang, B. Detection of copper ions using microcantilever immunosensors and enzyme-linked immunosorbent assay. Anal. Chim. Acta 2010, 676, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Li, Y.; Li, W.; Lin, C.; Han, W.; Yang, M. Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resour. Conserv. Recycl. 2010, 54, 985–990. [Google Scholar] [CrossRef]
- Chan, Y.; Chen, J.; Liu, Q.; Wark, S.E.; Son, D.H.; Batteas, J.D. Ultrasensitive copper(II) detection using plasmon-enhanced and photo-brightened luminescence of CdSe quantum dots. Anal. Chem. 2010, 82, 3671–3678. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Sohrabi, A.; Abbasi, S. Determination of copper by adsorptive stripping voltammetry in the presence of calcein blue. Electroanalysis 2007, 19, 1609–1615. [Google Scholar] [CrossRef]
- Chandra, R.; Ghorai, A.; Patra, G.K. A simple benzildihydrazone derived colorimetric and fluorescent ‘on–off-on’ sensor for sequential detection of copper(II) and cyanide ions in aqueous solution. Sens. Actuators B 2018, 255, 701–711. [Google Scholar] [CrossRef]
- Gupta, V.K.; Yola, M.L.; Atar, N.; Ustundag, Z.; Solak, A.O. A novel sensitive Cu(II) and Cd(II) nanosensor platform: Graphene oxide terminated p-aminophenyl modified glassy carbon surface. Electrochim. Acta 2013, 112, 541–548. [Google Scholar] [CrossRef]
- Lutsenko, S. Human copper homeostasis: A network of interconnected pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, P.; Li, J.; Tang, S. Fluorescent ion-imprinted sensor for selective and sensitive detection of copper (II) ions. Sens. Actuators B 2018, 255, 2095–2104. [Google Scholar] [CrossRef]
- Shen, Q.; Li, W.; Tang, S.; Hu, Y.; Nie, Z.; Huang, Y.; Yao, S. A simple “clickable” biosensor for colorimetric detection of copper (II) ions based on unmodified gold nanoparticles. Biosens. Bioelectron. 2013, 41, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, Z.; Qu, W.; Shao, H.; Jiang, X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens. Bioelectron. 2011, 26, 4064–4069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Xing, X.; Wang, Y. Grey level replaces fluorescent intensity: Fluorescent paper sensor based on ZnO nanoparticles for quantitative detection of Cu2+ without photoluminescence spectrometer. Sens. Actuators B 2018, 255, 2356–2366. [Google Scholar] [CrossRef]
- Liu, C.; Bai, R.; Ly, Q.S. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2018, 42, 1511–1522. [Google Scholar] [CrossRef]
- Standards for Drinking Water Quality (No. GB5749-2006); Standards Press of China: Beijing, China, 2007. Available online: http://www.nhc.gov.cn/zwgkzt/wsbysj/200804/30005.shtml (accessed on 27 December 2018).
- Standards for Surface Water Environment Quality (No. GB3838-2002); Standards Press of China: Beijing, China, 2002. Available online: http://kjs.mee.gov.cn/hjbhbz/bzwb/shjbh/shjzlbz/200206/t20020601_66497.shtml (accessed on 27 December 2018).
- Tang, S.; Chang, Y.; Chia, G.H.; Lee, H.K. Selective extraction and release using (EDTA-Ni)-layered double hydroxide coupled with catalytic oxidation of 3, 3′-, 5, 5′- tetramethylbenzidine for sensitive detection of copper ion. Anal. Chim. Acta 2015, 885, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.W.; Cate, D.M.; Miller-Lionberg, D.D.; Reilly, T.; Volckens, J.; Henry, C.S. Solid-phase extraction coupled to a paper-based technique for trace copper detection in drinking water. Environ. Sci. Technol. 2018, 52, 3567–3573. [Google Scholar] [CrossRef]
- dos Santos, W.N.L.; da Silva, E.G.P.; Fernandes, M.S.; Araujo, G.R.O.; Costa, A.C.S.; Vale, M.G.R.; Ferreira, S.L.C. Determination of copper in powdered chocolate samples by slurry-sampling flame atomic-absorption spectrometry. Anal. Bioanal. Chem. 2015, 382, 1099–1102. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Lemos, V.A.; Moreira, B.C.; Costa, A.C.S.; Santelli, R.E. An on-line continuous flow system for copper enrichment and determination by flame atomic absorption spectroscopy. Anal. Chim. Acta 2000, 403, 259–264. [Google Scholar] [CrossRef]
- Zhu, Y.; Inagaki, K.; Chiba, K. Determination of Fe, Cu, Ni, and Zn in seawater by ID-ICP-MS after preconcentration using a syringe-driven chelating column. J. Anal. At. Spectrom. 2009, 24, 1179–1183. [Google Scholar] [CrossRef]
- Becker, J.S.; Zoriy, M.; Becker, J.S.; Pickhardt, C.; Damoc, E.; Juhacz, G.; Palkovits, M.; Przybylski, M. Determination of phosphorus-, copper-, and zinc-containing human brain proteins by LA-ICPMS and MALDI-FTICR-MS. Anal. Chem. 2005, 77, 5751–5860. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, M.D.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Direct determination of toxic trace metals in honey and sugars using inductively coupled plasma atomic emission spectrometry. Talanta 2005, 65, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Faraji, M.; Yamini, Y.; Shariati, S. Application of cotton as a solid phase extraction sorbent for on-line preconcentration of copper in water samples prior to inductively coupled plasma optical emission spectrometry determination. J. Hazard. Mater. 2009, 166, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Jakmunee, J.; Junsomboon, J. Determination of cadmium, lead, copper and zinc in the acetic acid extract of glazed ceramic surfaces by anodic stripping voltammetric method. Talanta 2008, 77, 172–175. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Amini, M.K.; Dehghan, M.; Kordi, E.; Sheikh-Mohseni, M.A. Preparation of Cu (II) imprinted polymer electrode and its application for potentiometric and voltammetric determination of Cu (II). J. Iran. Chem. Soc. 2014, 11, 257–262. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Li, Q.; Wang, Z.; Xing, X.; Wang, Y. Portably colorimetric paper sensor based on ZnS quantum dots forsemi-quantitative detection of Co2+ through the measurement of grey level. Sens. Actuators B 2018, 260, 1068–1075. [Google Scholar] [CrossRef]
- Boonmee, C.; Noipa, T.; Tuntulani, T.; Ngeontae, W. Cysteamine capped CdS quantum dots as a fluorescence sensor for the determination of copper ion exploiting fluorescence enhancement and long-wave spectral shifts. Spectrochim. Acta A 2016, 169, 161–168. [Google Scholar] [CrossRef]
- Shamirian, A.; Ghai, A.; Snee, P.T. QD-based FRET probes at a glance. Sensors 2015, 15, 13028–13051. [Google Scholar] [CrossRef]
- Koneswaran, M.; Narayanaswamy, R. Mercaptoacetic acid capped CdS quantum dots as fluorescence single shot probe for mercury (II). Sens. Actuators B 2009, 139, 91–96. [Google Scholar] [CrossRef]
- Gui, W.; Chen, X.; Ma, Q. A novel detection method of human serum albumin based on CuInZnS quantum dots-Co2+ sensing system. Anal. Bioanal. Chem. 2017, 409, 3871–3876. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Wen, S.; Liu, S.; Heng, J.; Qin, D.; Hou, L.; Wu, H.; Xu, W.; Huang, W. CdTe nanocrystal hetero-junction solar cells with high open circuit voltage based on Sb-doped TiO2 electron acceptor materials. Nanomaterials 2017, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Eck, M.; Van Pham, C.; Zufle, S.; Sessler, M.; Scheunemann, D.; Erdem, E.; Weber, S.; Borchert, H.; Ruhstaller, B. Improved efficiency of bulk heterojunction hybrid solar cells by utilizing CdSe quantum dot–graphene nanocomposites. Phys. Chem. Chem. Phys. 2014, 16, 12251–12260. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yan, C.; Li, J.; Ding, X.; Rao, L.; Li, Z. Improvement in luminous efficacy and thermal performance using quantum dots spherical shell for white light emitting diodes. Nanomaterials 2018, 8, 618. [Google Scholar] [CrossRef]
- Pham, C.V.; Repp, S.; Thomann, R.; Krueger, M.; Weber, S.; Erdem, E. Charge transfer and surface defect healing within ZnO nanoparticle decorated graphene hybridmaterials. Nanoscale 2016, 8, 9682–9687. [Google Scholar] [CrossRef] [PubMed]
- Repp, S.; Weber, S.; Erdem, E. Defect dvolution of nonstoichiometric ZnO quantum dots. J. Phys. Chem. C 2016, 120, 25124–25130. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Z.; Huang, G.; Liu, X. ZnSe quantum dots based fluorescence sensors for Cu2+ ions. Sens. Actuators A 2014, 205, 72–78. [Google Scholar] [CrossRef]
- Geng, S.; Lin, S.; Li, N.; Luo, H. Polyethylene glycol capped ZnO quantum dots as a fluorescent probe for determining copper(II) ion. Sens. Actuators B 2017, 253, 137–143. [Google Scholar] [CrossRef]
- Elmizadeh, H.; Soleimani, M.; Faridbod, F.; Bardajee, G.R. Ligand-capped CdTe quantum dots as a fluorescent nanosensor for detection of copper ions in environmental water sample. J. Fluoresc. 2017, 27, 2323–2333. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, W.E. Functionalized ME-capped CdSe quantum dots based luminescence probe for detection of Ba2+ ions. Sens. Actuators B 2012, 164, 76–81. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Y.; Yang, H.; Li, Z. Ultrasensitive cysteine sensing using citrate-capped CdS quantum dots. Talanta 2011, 83, 943–947. [Google Scholar] [CrossRef]
- Khataee, A.; Hasanzadeh, A.; Iranifam, M.; Joo, S.W. A novel flow-injection chemiluminescence method for determination of baclofen using l-cysteine capped CdS quantum dots. Sens. Actuators B 2015, 215, 271–282. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, Y.; Sun, S. Facile synthesis l-cysteine capped CdS:Eu quantum dots and their Hg2+ sensitive properties. Appl. Surf. Sci. 2013, 276, 333–339. [Google Scholar] [CrossRef]
- Tayebi, M.; Yaraki, M.T.; Ahmadieh, M.; Tahriri, M.; Vashaee, D.; Tayebi, L. Determination of total aflatoxin using cysteamine-capped CdS quantum dots as a fluorescence probe. Colloid Polym. Sci. 2016, 294, 1453–1462. [Google Scholar] [CrossRef]
- Noipa, T.; Ngamdee, K.; Tuntulani, T.; Ngeontae, W. Cysteamine CdS quantum dots decorated with Fe3+ as a fluorescence sensor for the detection of PPi. Spectrochim. Acta A 2014, 118, 17–23. [Google Scholar] [CrossRef]
- Maulu, A.; Navarro-Arenas, J.; Rodriguez-Canto, P.J.; Sanchez-Royo, J.F.; Abargues, R.; Suarez, I.; Martinez-Pastor, J.P. Charge transport in trap-sensitized infrared PbS quantum-dot-based photoconductors: Pros and cons. Nanomaterials 2018, 8, 677. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, X.; Yang, Y.; Zhao, R.; Zou, T.; Wang, Z.; Wang, Y. One-step hydrothermal synthesis of thioglycolic acid capped CdS quantum dots as fluorescence determination of cobalt ion. Sci. Rep. 2018, 8, 8953. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wang, J.; Zhao, K.; Xue, H.; Ta, C. A simple and rapid label-free fluorimetric “turn off-on” sensor for cadmium detection using glutathione-capped CdS quantum dots. Chem. Res. Chin. 2016, 32, 570–575. [Google Scholar] [CrossRef]
- Gattás-Asfura, K.A.; Leblanc, R.M. Peptide-coated CdS quantum dots for the optical detection of copper(II) and silver(I). Chem. Commun. 2003, 21, 2684–2685. [Google Scholar] [CrossRef]
- Thambidurai, M.; Muthukumarasamy, N.; Agilan, S.; Arul, N.S.; Murugan, N.; Balasundaraprabhu, R. Structural and optical characterization of Ni-doped CdS quantum dots. J. Mater. Sci. 2011, 46, 3200–3206. [Google Scholar] [CrossRef]
- Ahamad, T.; Khan, M.A.M.; Kumar, S.; Ahamed, M.; Shahabuddin, M.; Alhazaa, A.N. CdS quantum dots: Growth, microstructural, optical and electrical characteristics. J. Mater. Sci. 2016, 122, 179–186. [Google Scholar] [CrossRef]
- Thongtema, T.; Phuruangratb, A.; Thongtemb, S. Characterization of nano- and micro-crystalline CdS synthesized using cyclic microwave radiation. J. Phys. Chem. Solids 2008, 69, 1346–1349. [Google Scholar] [CrossRef]
- Mehtala, J.G.; Zemlyanov, D.Y.; Max, J.P.; Kadasala, N.; Zhao, S.; Wei, A. Citrate-stabilized gold nanorods. Langmuir 2014, 30, 13727–13730. [Google Scholar] [CrossRef] [PubMed]
- Cheraghipour, E.; Javadpour, S.; Mehdizadeh, A.R. Citrate capped superparamagnetic iron oxide nanoparticles used for hyperthermia therapy. J. Biomed. Sci. Eng. 2012, 5, 715–719. [Google Scholar] [CrossRef]
- Thottoli, A.K.; Unni, A.K.A. Effect of trisodium citrate concentration on the particle growth of ZnS nanoparticles. J. Nanostruct. Chem. 2013, 3, 56–67. [Google Scholar] [CrossRef]
- Rajeshwer, K.; de Tacconi, N.R.; Chenthamarakshan, C.R. Semiconductor-based composite materials: Preparation, properties, and performance. Chem. Mater. 2001, 13, 2765–2782. [Google Scholar] [CrossRef]
- Kumar, D.S.; Kumar, B.J.; Mahesh, H.M. Optical properties of colloidal aqueous synthesized 3 mercaptopropionic acid stabilized CdS quantum dots. AIP Conf. Proc. 2016, 1728, 020162. [Google Scholar] [CrossRef]
- Michalow, K.A.; Logvinovich, D.; Weidenkaff, A.; Amberg, M.; Fortunato, G.; Heel, A.; Graule, T.; Rekas, M. Synthesis, characterization and electronic structure of nitrogen-doped TiO2 nanopowder. Catal. Today 2009, 144, 7–12. [Google Scholar] [CrossRef]
- Liu, X.; Xing, X.; Li, Y.; Chen, N.; Djerdj, I.; Wang, Y. Controllable synthesis and change of emission color from green to orange of ZnO quantum dots using different solvents. New J. Chem. 2015, 39, 2882–2888. [Google Scholar] [CrossRef]
- Pepp, S.; Erdem, E. Controlling the exciton energy of zinc oxide (ZnO) quantum dots by changing the confinement conditions. Spectrochim. Acta A 2015, 152, 637–644. [Google Scholar] [CrossRef]
- Brus, L.E. Electron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J. Chem. Phys. 1984, 80, 4403–4407. [Google Scholar] [CrossRef]
- Brus, L. Electronic wave functions in semiconductor clusters: Experiment and theory. J. Phys. Chem. 1986, 90, 2555–2560. [Google Scholar] [CrossRef]
- Ruf, T.; Repp, S.; Urban, J.; Thomann, R.; Erdem, E. Competing effects between intrinsic and extrinsic defectsin pure and Mn-doped ZnO nanocrystals. J. Nanopart. Res. 2016, 18, 109. [Google Scholar] [CrossRef]
- Han, X.; Wahi, S.; Russo, P.A.; Pinna, N. Cobalt-assisted morphology and assembly control of Co-doped ZnO nanoparticles. Nanomaterials 2018, 8, 249. [Google Scholar] [CrossRef]
- Xu, H.; Gan, Z. Modulation of nonradiative processes of single colloidal quantum dots by glycerol passivation. J. Phys. D Appl. Phys. 2017, 50, 435103. [Google Scholar] [CrossRef]
- Caigas, S.P.; Santiago, S.R.M.; Lin, T.N.; Lin, C.A.J.; Yuan, C.T.; Shen, J.L.; Lin, T.Y. Origins of excitation-wavelength-dependent photoluminescence in WS2 quantum dots. Appl. Phys. Lett. 2018, 112, 092106. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, B.; Wang, X. Investigations on photoluminescence and cathodoluminescence properties of Ca3La6(SiO4)6:Tb3+, Mn2+. Spectrochim. Acta A 2016, 165, 85–89. [Google Scholar] [CrossRef]
- Isarov, A.V.; Chrysochoos, J. Optical and photochemical properties of nonstoichiometric cadmium sulfide nanoparticles: Surface modification with copper(II) ions. Langmuir 1997, 13, 3142–3149. [Google Scholar] [CrossRef]
- Sanz, M.; Coffea-Duarte, M.A.; Liz-Marzan, L.M.; Douhal, A. Femtosecond dynamics of CdTe quantum dots in water. J. Photochem. Photobiol. A 2008, 196, 51–58. [Google Scholar] [CrossRef]
- Jagadeeswari, S.; Jhonsi, M.A.; Kathiravan, A.; Renganathan, R. Photoinduced interaction between MPA capped CdTe QDs and certain anthraquinone dyes. J. Lumin. 2011, 131, 297–602. [Google Scholar] [CrossRef]
- Uchihara, T.; Kato, H.; Miyagi, E. Subpicosecond time-resolved photoluminescence of thioglycerol-capped CdS nanoparticles in water. J. Photochem. Photobiol. A 2006, 181, 86–93. [Google Scholar] [CrossRef]
- Uppa, Y.; Kulchat, S.; Ngamdee, K.; Pradublai, K.; Tuntulani, T.; Ngeontae, W. Silver ion modulated CdS quantum dots for highly selective detection of trace Hg2+. J. Lumin. 2016, 178, 437–445. [Google Scholar] [CrossRef]




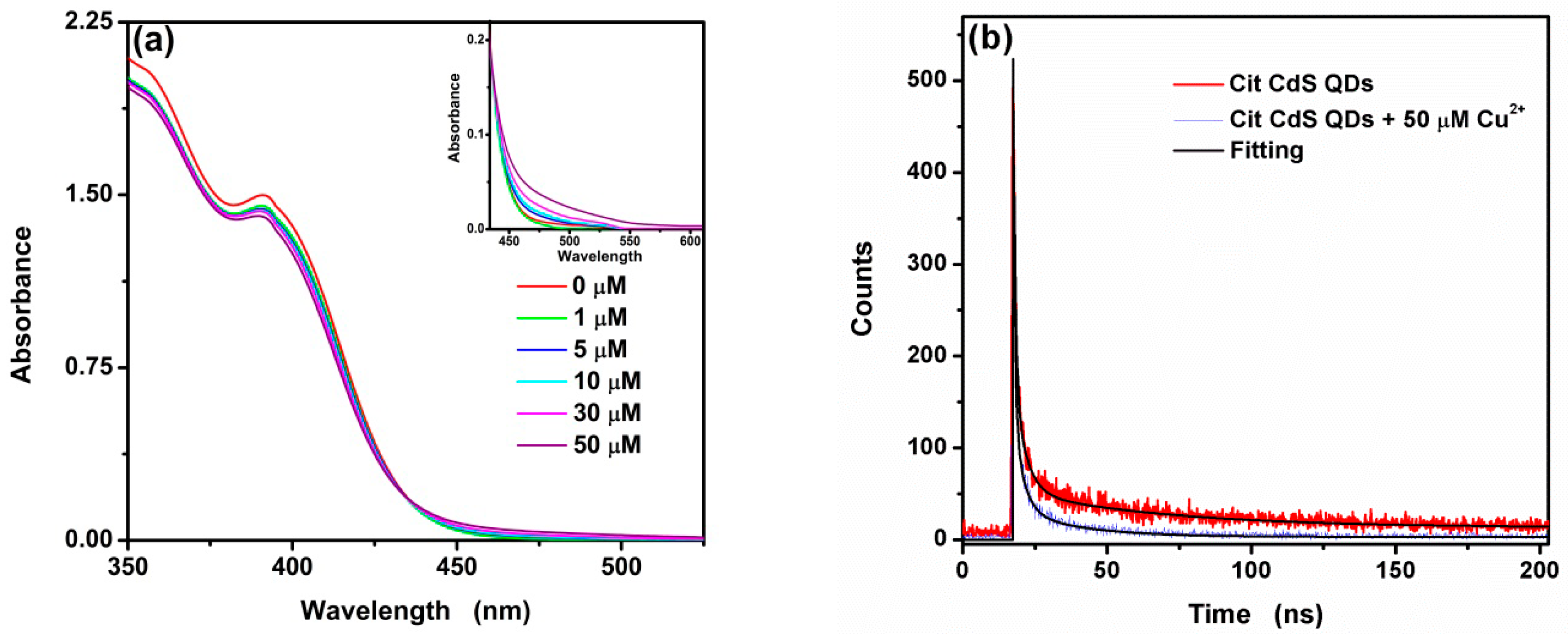

| Sample | τ | A1 | τ1 | A2 | τ2 | A3 | τ3 |
|---|---|---|---|---|---|---|---|
| CA-CdS QDs | 38.6 | 432.267 | 0.711 | 137.739 | 3.958 | 39.992 | 53.070 |
| CA-CdS QDs with Cu2+ | 12.1 | 541.551 | 0.552 | 118.948 | 3.272 | 30.542 | 22.198 |
| Fluorescent Probe | Linear range (μM) | Detection Limit (μM) | Sensitivity (μM) | Ref. |
|---|---|---|---|---|
| zinc-dopedAgInS2 QDs | 0–340 | 0.0273 | 0.004 | [2] |
| ZnSe QDs | 1–6 | 0.47 | 0.22 | [29] |
| PEG@ZnO QDs | 0.01–0.2/2–10 | 0.0033 | 0.1768 | [30] |
| CdTe-L QDs | 0.515–15 | 0.015 | 0.4626 | [31] |
| Cys-CdS QDs | 2–10 | 1.5 | 0.001 | [32] |
| CA-CdS QDs | 0.01–5 | 0.0092 | 0.06 | This work |
| Samples | Spiked (μM) | Found (μM) | Recovery (%, n = 5) | RSD (%, n = 5) |
|---|---|---|---|---|
| Tap water | 10 | 9.97 | 99.7 | 2.9 |
| 20 | 19.16 | 95.8 | 3.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xiao, X.; Zou, T.; Yang, Y.; Xing, X.; Zhao, R.; Wang, Z.; Wang, Y. Citric Acid Capped CdS Quantum Dots for Fluorescence Detection of Copper Ions (II) in Aqueous Solution. Nanomaterials 2019, 9, 32. https://doi.org/10.3390/nano9010032
Wang Z, Xiao X, Zou T, Yang Y, Xing X, Zhao R, Wang Z, Wang Y. Citric Acid Capped CdS Quantum Dots for Fluorescence Detection of Copper Ions (II) in Aqueous Solution. Nanomaterials. 2019; 9(1):32. https://doi.org/10.3390/nano9010032
Chicago/Turabian StyleWang, Zhezhe, Xuechun Xiao, Tong Zou, Yue Yang, Xinxin Xing, Rongjun Zhao, Zidong Wang, and Yude Wang. 2019. "Citric Acid Capped CdS Quantum Dots for Fluorescence Detection of Copper Ions (II) in Aqueous Solution" Nanomaterials 9, no. 1: 32. https://doi.org/10.3390/nano9010032
APA StyleWang, Z., Xiao, X., Zou, T., Yang, Y., Xing, X., Zhao, R., Wang, Z., & Wang, Y. (2019). Citric Acid Capped CdS Quantum Dots for Fluorescence Detection of Copper Ions (II) in Aqueous Solution. Nanomaterials, 9(1), 32. https://doi.org/10.3390/nano9010032