Boosting the Efficiency of Photoelectrolysis by the Addition of Non-Noble Plasmonic Metals: Al & Cu
Abstract
1. Introduction
2. Plasmonic Effects
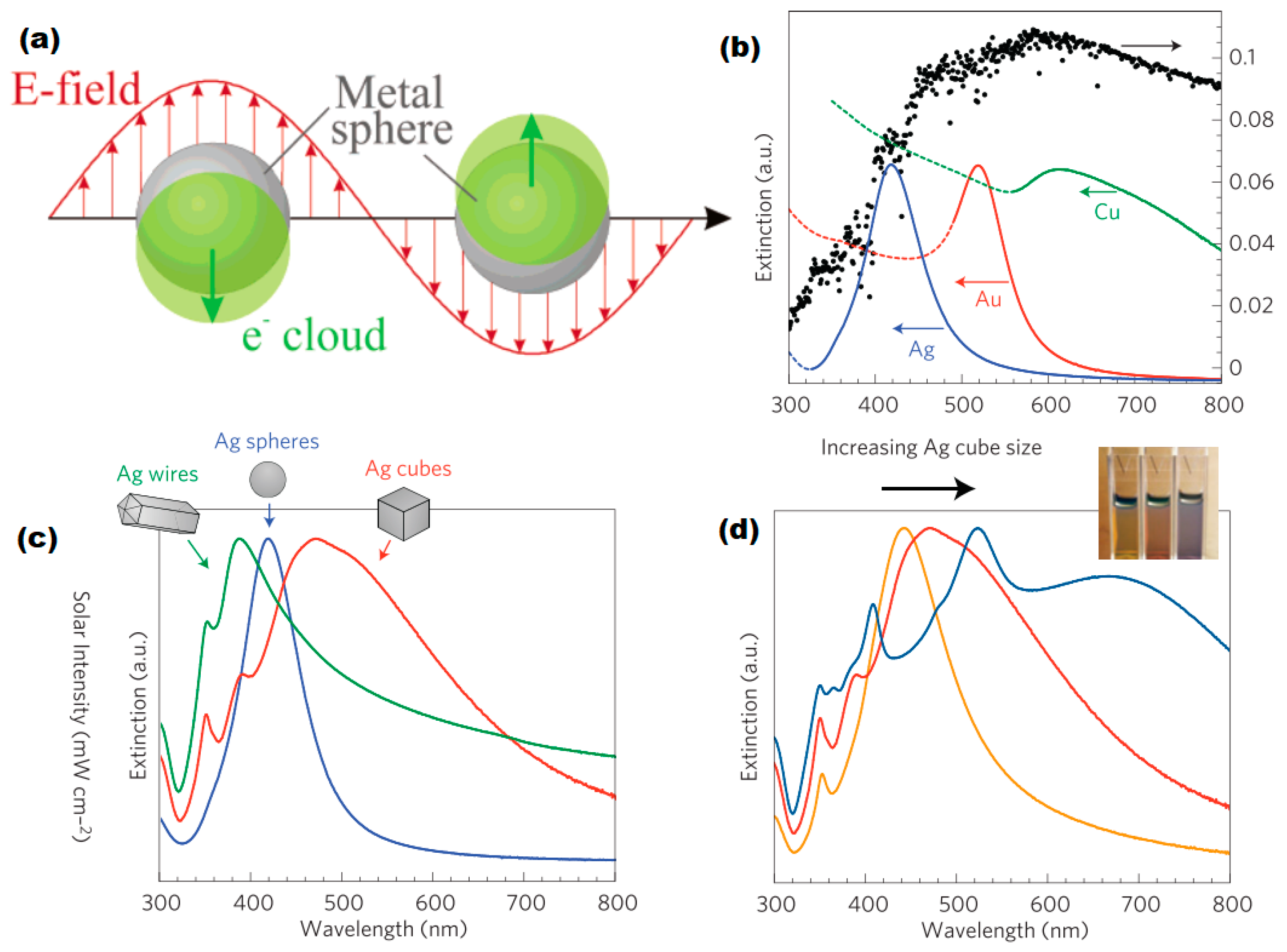
2.1. Optical Properties of Plasmonic Metal Nanoparticles
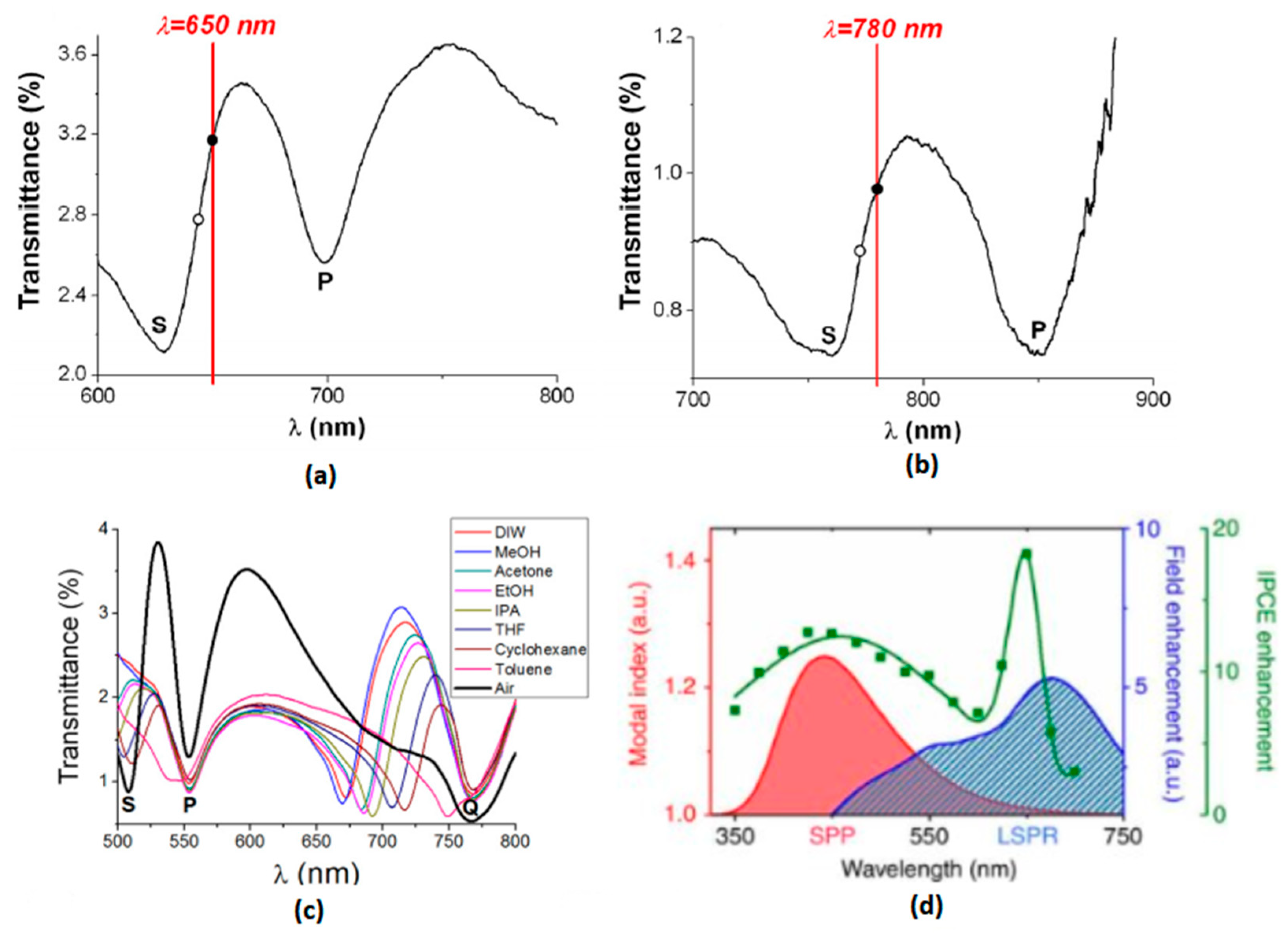
2.2. Surface Plasmon Polaritons at Dielectric/Plasmonic Metal Interfaces
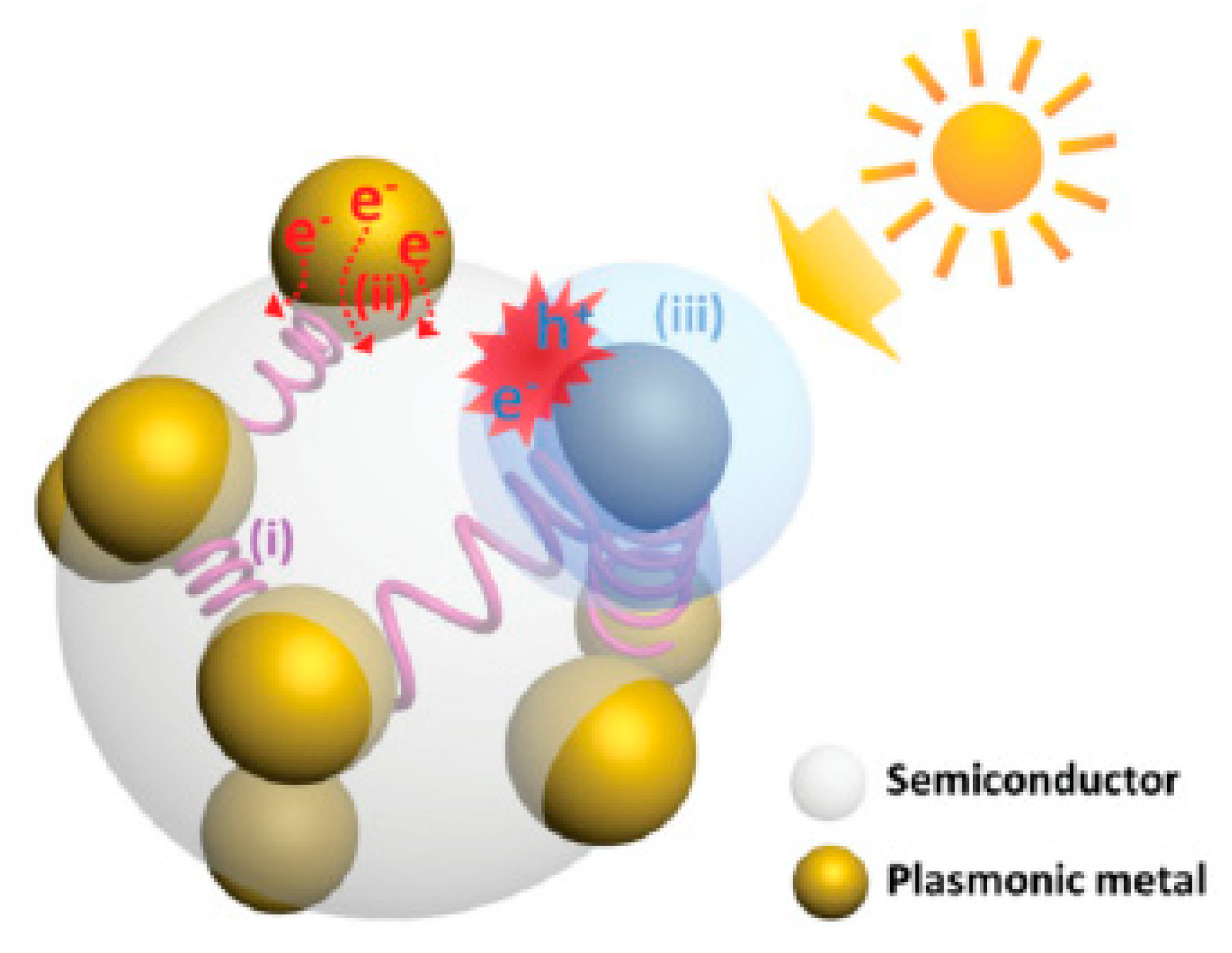
3. Mechanisms of Plasmonic-Enhanced Photoelectrolysis
3.1. Near–Field Excitation Enhancement
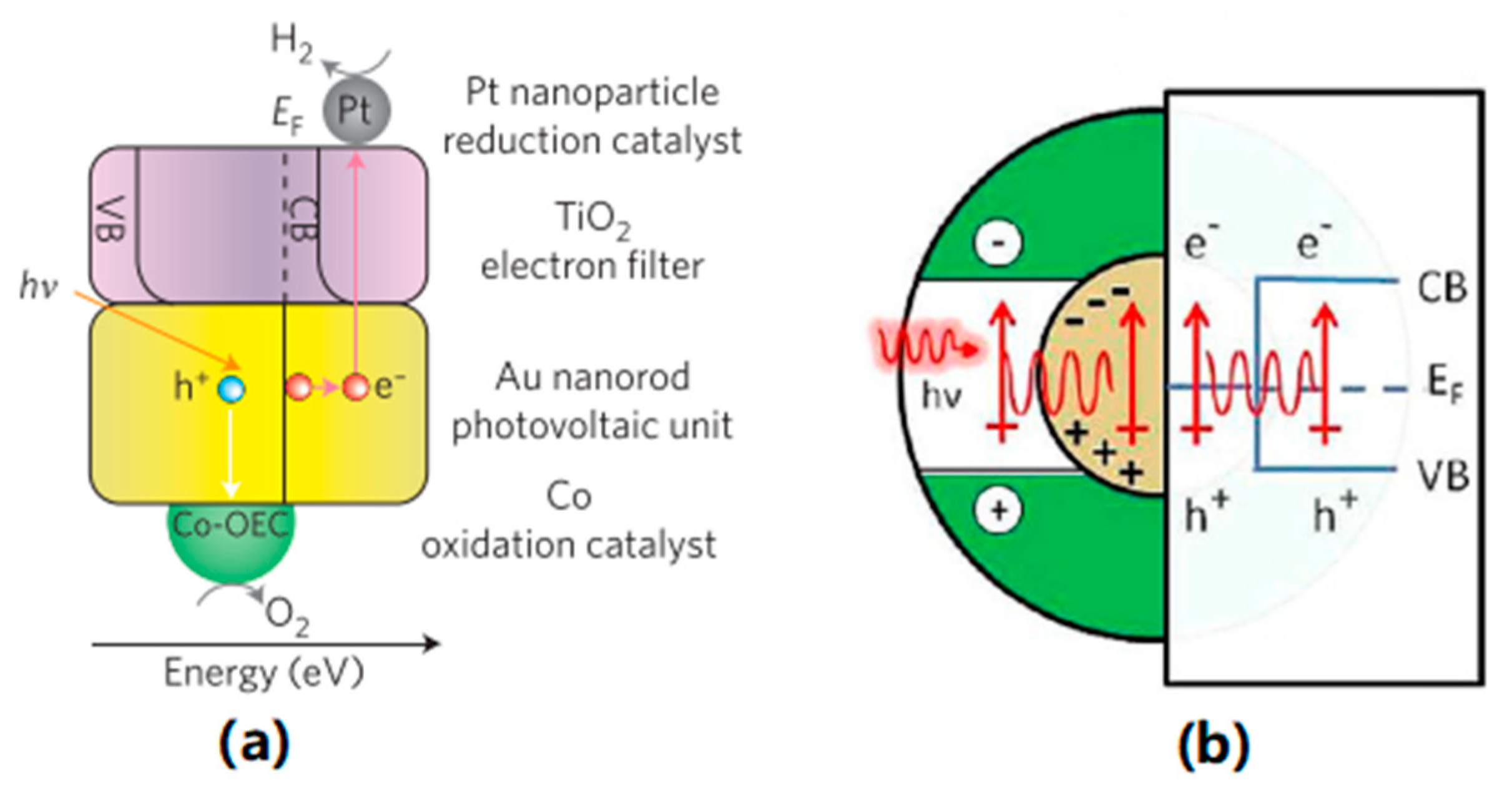
3.2. Hot Electron Injection (HEI)
3.3. Plasmon Induced Resonance Energy Transfer (PIRET)
3.4. Photothermal Effect
4. Enhancement of Photoelectrolysis Using Aluminum Plasmonic Systems
5. Enhancement of Photoelectrolysis Using Copper Plasmonic Systems
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IIASA. Global Energy Assessment: Summary for Policy Makers and Technical Summary. In Global Energy Assessment Toward a Sustainable Future; Cambridge University Press: Cambridge, UK, 2012; pp. 3–93. [Google Scholar]
- Wu, X.; Scott, K.; Xie, F.; Alford, N. A reversible water electrolyser with porous PTFE based OH-conductive membrane as energy storage cells. J. Power Sources 2014, 246, 225–231. [Google Scholar] [CrossRef]
- Chen, Z.; Dinh, H.N.; Miller, E. Photoelectrochemical Water Splitting; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-8297-0. [Google Scholar]
- Barrios, C.A.; Canalejas-Tejero, V.; Herranz, S.; Urraca, J.; Moreno-Bondi, M.C.; Avella-Oliver, M.; Maquieira, Á.; Puchades, R. Aluminum nanoholes for optical biosensing. Biosensors 2015, 5, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Valsecchi, C.; Brolo, A.G. Periodic metallic nanostructures as plasmonic chemical sensors. Langmuir 2013, 29, 5638–5649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Centeno, A.; Ryan, M.P.; Alford, N.M.; Riley, D.J.; Xie, F. Significant broadband photocurrent enhancement by Au-CZTS core-shell nanostructured photocathodes. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Pang, J.; Theodorou, I.G.; Centeno, A.; Petrov, P.K.; Alford, N.M.; Ryan, M.P.; Xie, F. Gold nanodisc arrays as near infrared metal-enhanced fluorescence platforms with tuneable enhancement factors. J. Mater. Chem. C 2017, 5, 917–925. [Google Scholar] [CrossRef]
- Theodorou, I.G.; Jawad, Z.A.R.; Qin, H.; Aboagye, E.O.; Porter, A.E.; Ryan, M.P.; Xie, F. Significant metal enhanced fluorescence of Ag2S quantum dots in the second near-infrared window. Nanoscale 2016, 8, 12869–12873. [Google Scholar] [CrossRef] [PubMed]
- Mateo, D.; Esteve-Adell, I.; Albero, J.; Royo, J.F.S.; Primo, A.; Garcia, H. 111 oriented gold nanoplatelets on multilayer graphene as visible light photocatalyst for overall water splitting. Nat. Commun. 2016, 7, 11819. [Google Scholar] [CrossRef]
- Liu, T.; Chen, W.; Huang, T.; Duan, G.; Yang, X.; Liu, X. Titania-on-gold nanoarchitectures for visible-light-driven hydrogen evolution from water splitting. J. Mater. Sci. 2016, 51, 6987–6997. [Google Scholar] [CrossRef]
- Theodorou, I.G.; Jawad, Z.A.R.; Jiang, Q.; Aboagye, E.O.; Porter, A.E.; Ryan, M.P.; Xie, F. Gold nanostar substrates for metal-enhanced fluorescence through the first and eecond near-infrared windows. Chem. Mater. 2017, 29, 6916–6926. [Google Scholar] [CrossRef]
- Centeno, A.; Xie, F.; Alford, N. Light absorption and field enhancement in two-dimensional arrays of closely spaced silver nanoparticles. J. Opt. Soc. Am. B 2011, 28, 325–330. [Google Scholar] [CrossRef]
- Ritchie, R.H. Plasma Losses by Fast Electrons in Thin Films. Phys. Rev. 1967, 106, 874. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Chang, S.-H.; Gray, S.K.; Schatz, G.C. Surface plasmon generation and light transmission by isolated nanoholes and arrays of nanoholes in thin metal films. Opt. Express 2005, 13, 3150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cushing, S.K.; Zheng, P.; Meng, F.; Chu, D.; Wu, N. Plasmon-induced photonic and energy-transfer enhancement of solar water splitting by a hematite nanorod array. Nat. Commun. 2013, 4, 2651. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Gong, J. Mechanistic understanding of the plasmonic enhancement for solar water splitting. Adv. Mater. 2015, 27, 5328–5342. [Google Scholar] [CrossRef] [PubMed]
- Hogan, N.J.; Urban, A.S.; Ayala-Orozco, C.; Pimpinelli, A.; Nordlander, P.; Halas, N.J. Nanoparticles heat through light localization. Nano Lett. 2014, 14, 4640–4645. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, P.; Deutsch, B.; Novotny, L. Optical Antennas. Adv. Opt. Photonics 2009, 1, 438. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef]
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Lee, J.; Singh, N.; Krämer, S.; Stucky, G.D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Ahmadivand, A.; Pala, N.; Güney, D.Ö. Enhancement of photothermal heat generation by metallodielectric nanoplasmonic clusters. Opt. Express 2015, 23, A682. [Google Scholar] [CrossRef] [PubMed]
- Toroghi, S.; Kik, P.G. Photothermal response enhancement in heterogeneous plasmon-resonant nanoparticle trimers. Phys. Rev. B Condens. Matter Mater. Phys. 2014, 90, 1–6. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, X.; Meng, M.; Zhu, X.; Yang, L.; Chu, P.K. Photothermal contribution to enhanced photocatalytic performance of graphene-based nanocomposites. ACS Nano 2014, 8, 9304–9310. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, J.U.; Lee, K.J.; Ahn, S.; Shin, Y.B.; Shin, J.; Park, C.B. Aluminum nanoarrays for plasmon-enhanced light harvesting. ACS Nano 2015, 9, 6206–6213. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, C.; Huang, H.; Li, W.; Du, D.; Han, D.; Qiu, T.; Chu, P.K. Aluminum plasmonic photocatalysis. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Zheng, B.Y.; Wang, Y.; Nordlander, P.; Halas, N.J. Color-selective and CMOS-compatible photodetection based on aluminum plasmonics. Adv. Mater. 2014, 26, 6318–6323. [Google Scholar] [CrossRef]
- McClain, M.J.; Schlather, A.E.; Ringe, E.; King, N.S.; Liu, L.; Manjavacas, A.; Knight, M.W.; Kumar, I.; Whitmire, K.H.; Everitt, H.O.; et al. Aluminum nanocrystals. Nano Lett. 2015, 15, 2751–2755. [Google Scholar] [CrossRef]
- Lecarme, O.; Sun, Q.; Ueno, K.; Misawa, H. Robust and versatile light absorption at near-infrared wavelengths by plasmonic aluminum nanorods. ACS Photonics 2014, 1, 538–546. [Google Scholar] [CrossRef]
- Schmidt, T.M.; Bochenkov, V.E.; Espinoza, J.D.A.; Smits, E.C.P.; Muzafarov, A.M.; Kononevich, Y.N.; Sutherland, D.S. Plasmonic fluorescence enhancement of DBMBF2monomers and DBMBF2-toluene exciplexes using al-hole arrays. J. Phys. Chem. C 2014, 118, 2138–2145. [Google Scholar] [CrossRef]
- Mahendiran, C.; Ganesan, R.; Gedanken, A. Sonoelectrochemical synthesis of metallic aluminum nanoparticles chinnathambi mahendiran. Quantum 2009, 2050–2053. [Google Scholar]
- Lee, S.; Jung, H.J.; Shin, J.H.; Choi, M.Y. Production of Size Controlled aluminum and alumina nanoparticles via pulsed laser ablation in water. J. Nanosci. Nanotechnol. 2012, 12, 8900–8903. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.S.; Hu, J.S.; Liang, H.P.; Cao, A.M.; Song, W.G.; Wan, L.J. Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Maidecchi, G.; Gonella, G.; Proietti Zaccaria, R.; Moroni, R.; Anghinolfi, L.; Giglia, A.; Nannarone, S.; Mattera, L.; Dai, H.L.; Canepa, M.; et al. Deep ultraviolet plasmon resonance in aluminum nanoparticle arrays. ACS Nano 2013, 7, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Ramadurgam, S.; Lin, T.G.; Yang, C. Aluminum plasmonics for enhanced visible light absorption and high efficiency water splitting in core-multishell nanowire photoelectrodes with ultrathin hematite shells. Nano Lett. 2014, 14, 4517–4522. [Google Scholar] [CrossRef]
- Zhou, X.; Gossage, Z.T.; Simpson, B.H.; Hui, J.; Barton, Z.J.; Rodríguez-López, J. Electrochemical imaging of photoanodic water oxidation enhancements on TiO2 thin films modified by subsurface aluminum nanodimers. ACS Nano 2016, 10, 9346–9352. [Google Scholar] [CrossRef] [PubMed]
- Kakavelakis, G. Aluminum nanoparticles for efficient and stable organic photovoltaics. J. Anal. At. Spectrom. 2013, 7, 155–164. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, W.; Shao, W.; Huang, J.; Guan, T.; Wen, P.; Cao, G.; Jiang, L. Fabrication of tunable aluminum nanodisk arrays: Via a self-assembly nanoparticle template method and their applications for performance enhancement in organic photovoltaics. J. Mater. Chem. A 2018, 6, 3649–3658. [Google Scholar] [CrossRef]
- Hylton, N.P.; Li, X.F.; Giannini, V.; Lee, K.H.; Ekins-Daukes, N.J.; Loo, J.; Vercruysse, D.; Van Dorpe, P.; Sodabanlu, H.; Sugiyama, M.; et al. Loss mitigation in plasmonic solar cells: Aluminium nanoparticles for broadband photocurrent enhancements in gaas photodiodes. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; McClain, M.J.; Manjavacas, A.; Krauter, C.M.; Tian, S.; Berg, F.; Everitt, H.O.; Carter, E.A.; Nordlander, P.; et al. Aluminum nanocrystals as a plasmonic photocatalyst for hydrogen dissociation. Nano Lett. 2016, 16, 1478–1484. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, H.; Zhou, L.; Schlather, A.E.; Dong, L.; McClain, M.J.; Swearer, D.F.; Nordlander, P.; Halas, N.J. Al-Pd Nanodisk heterodimers as antenna-reactor photocatalysts. Nano Lett. 2016, 16, 6677–6682. [Google Scholar] [CrossRef] [PubMed]
- Ramadurgam, S.; Lin, T.G.; Yang, C. Tailoring optical and plasmon resonances in core-shell and core-multishell nanowires for visible range negative refraction and plasmonic light harvesting: A review. J. Mater. Sci. Technol. 2015, 31, 533–541. [Google Scholar] [CrossRef]
- Mahani, F.F.; Mokhtari, A. Polarization-tuned chromatic electrodes using hybrid design of graphene-aluminum nanocross arrays for efficient organic solar cells. Opt. Mater. (Amst). 2018, 84, 158–165. [Google Scholar] [CrossRef]
- Di Martino, G.; Turek, V.A.; Braeuninger-Weimer, P.; Hofmann, S.; Baumberg, J.J. Laser-induced reduction and in-situ optical spectroscopy of individual plasmonic copper nanoparticles for catalytic reactions. Appl. Phys. Lett. 2017, 110, 71111. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-M.; Park, J.-E.; Nam, J.-M. Nonnoble-metal-based plasmonic nanomaterials: Recent advances and future perspectives. Adv. Mater. 2018, 30, 1704528. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-based nanoparticles: Synthesis and applications in catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Din, M.I.; Rehan, R. Synthesis, characterization, and applications of copper nanoparticles. Anal. Lett. 2017, 50, 50–62. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chen, D.-H. Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J. Colloid Interface Sci. 2004, 273, 165–169. [Google Scholar] [CrossRef]
- Liu, H.; Wang, T.; Zeng, H. CuNPs for efficient photocatalytic hydrogen evolution. Part. Part. Syst. Char. 2015, 32, 869–873. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L.; Vishwanatha, U.; Ravishankar, B.; Gururaj, H.; Niranjana, P.; Hungund, B.S. Phytochemically functionalized Cu and Ag nanoparticles embedded in MWCNTs for enhanced antimicrobial and anticancer properties. Nano-Micro Lett. 2016, 8, 120–130. [Google Scholar] [CrossRef]
- Kaur, R.; Giordano, C.; Gradzielski, M.; Mehta, S.K. Synthesis of highly stable, water-dispersible copper nanoparticles as catalysts for nitrobenzene reduction. Chem.-An Asian J. 2014, 9, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Raspolli Galletti, A.M.; Antonetti, C.; Marracci, M.; Piccinelli, F.; Tellini, B. Novel microwave-synthesis of Cu nanoparticles in the absence of any stabilizing agent and their antibacterial and antistatic applications. Appl. Surf. Sci. 2013, 280, 610–618. [Google Scholar] [CrossRef]
- Vázquez-Vázquez, C.; Bañobre-López, M.; Mitra, A.; López-Quintela, M.A.; Rivas, J. Synthesis of small atomic copper clusters in microemulsions. Langmuir 2009, 25, 8208–8216. [Google Scholar] [CrossRef]
- Kim, J.; Kang, S.W.; Mun, S.H.; Kang, Y.S. Facile Synthesis of Copper Nanoparticles by ionic liquids and its application to facilitated olefin transport membranes. Ind. Eng. Chem. Res. 2009, 48, 7437–7441. [Google Scholar] [CrossRef]
- Jin, M.; He, G.; Zhang, H.; Zeng, J.; Xie, Z.; Xia, Y. Shape-controlled synthesis of copper nanocrystals in an aqueous solution with glucose as a reducing agent and hexadecylamine as a capping agent. Angew. Chem. Int. Ed. 2011, 50, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza-Santos, I.; Sánchez-Iglesias, A.; Rodríguez-González, B.; Liz-Marzán, L.M. Aerobic synthesis of Cu nanoplates with intense plasmon resonances. Small 2009, 5, 440–443. [Google Scholar] [CrossRef]
- Zhang, P.; Song, T.; Wang, T.; Zeng, H. Plasmonic Cu nanoparticle on reduced graphene oxide nanosheet support: An efficient photocatalyst for improvement of near-infrared photocatalytic H2 evolution. Appl. Catal. B-Environ. 2018, 225, 172–179. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Dong, W.; Xi, J.; Du, G.; Ji, Z. Cu nanoparticles hybridized with ZnO thin film for enhanced photoelectrochemical oxygen evolution. J. Alloys. Compd. 2018, 768, 830–837. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kazuma, E.; Sakai, N.; Tatsuma, T. Photoelectrochemical responses from polymer-coated plasmonic copper nanoparticles on TiO2. Chem. Lett.. 2012, 41, 1340–1342. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, Y.; Cheng, L.; Chen, J.; Zhao, Y. A stable plasmonic Cu@Cu2O/ZnO heterojunction for enhanced photocatalytic hydrogen generation. ChemSusChem 2018, 11, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, P.; Johans, C.; Merta, J.; Kontturi, K. Influence of ligand structure on the stability and oxidation of copper nanoparticles. J. Colloid Interface Sci. 2008, 318, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Sakuraba, T. Silica-coating of metallic copper nanoparticles in aqueous solution. Colloids Surf. A 2008, 317, 756–759. [Google Scholar] [CrossRef]
- Susman, M.D.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Chemical deposition and stabilization of plasmonic copper nanoparticle films on transparent substrates. Chem. Mater. 2012, 24, 2501–2508. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Gold and copper nanoparticles supported on Cerium(IV) Oxide-A photocatalyst mineralizing organic acids under red light irradiation. ChemCatChem 2011, 3, 1619–1623. [Google Scholar] [CrossRef]
- Du, J.; Chen, Z.; Ye, S.; Wiley, B.J.; Meyer, T.J. Copper as a robust and transparent electrocatalyst for water oxidation. Angew. Chem. 2018, 54, 2073–2078. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef]
- Ranno, L.; Forno, S.D.; Lischner, J. Computational design of bimetallic core-shell nanoparticles for hot-carrier photocatalysis. Comput. Mater. Sci. 2018, 4, 31. [Google Scholar] [CrossRef]
- Cheng, N.; Xue, Y.; Liu, Q.; Tian, J.; Zhang, L.; Asiri, A.M.; Sun, X. Cu/(Cu(OH)2-CuO) core/shell nanorods array: in-situ growth and application as an efficient 3D oxygen evolution anode. Electrochim. Acta 2015, 163, 102–106. [Google Scholar] [CrossRef]
- Xu, S.; Du, A.J.; Liu, J.; Ng, J.; Sun, D.D. Highly efficient CuO incorporated TiO2 nanotube photocatalyst for hydrogen production from water. Int. J. Hydrogen Energy 2011, 36, 6560–6568. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, X.; Li, Y.; Li, S.; Lu, G. 5.1% Apparent quantum efficiency for stable hydrogen generation over eosin-sensitized CuO/TiO2 photocatalyst under visible light irradiation. Catal. Commun. 2007, 8, 1267–1273. [Google Scholar] [CrossRef]
- Yu, J.; Ran, J. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364. [Google Scholar] [CrossRef]
- Marimuthu, A.; Zhang, J.; Linic, S. Tuning selectivity in propylene epoxidation by plasmon mediated photo-switching of Cu oxidation state. Science 2013, 339, 1590–1593. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Qi, L.; Bian, J.; Chen, Y.; Hu, X.; Fan, J.; Liu, H.; Zhu, C.; Wang, Q. A facile strategy to fabricate plasmonic Cu modified TiO2 nano-flower films for photocatalytic reduction of CO2 to methanol. Mater. Res. Bull. 2015, 68, 203–209. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Li, J.; Li, Y.; Han, J.; Wang, Y.; Liu, Z.; Ya, J. Cu-doping ZnO/ZnS nanorods serve as the photoanode to enhance photocurrent and conversion efficiency. Microelectron. Eng. 2013, 103, 12–16. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal activity of copper-deposited TiO2 thin film under weak UV light illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
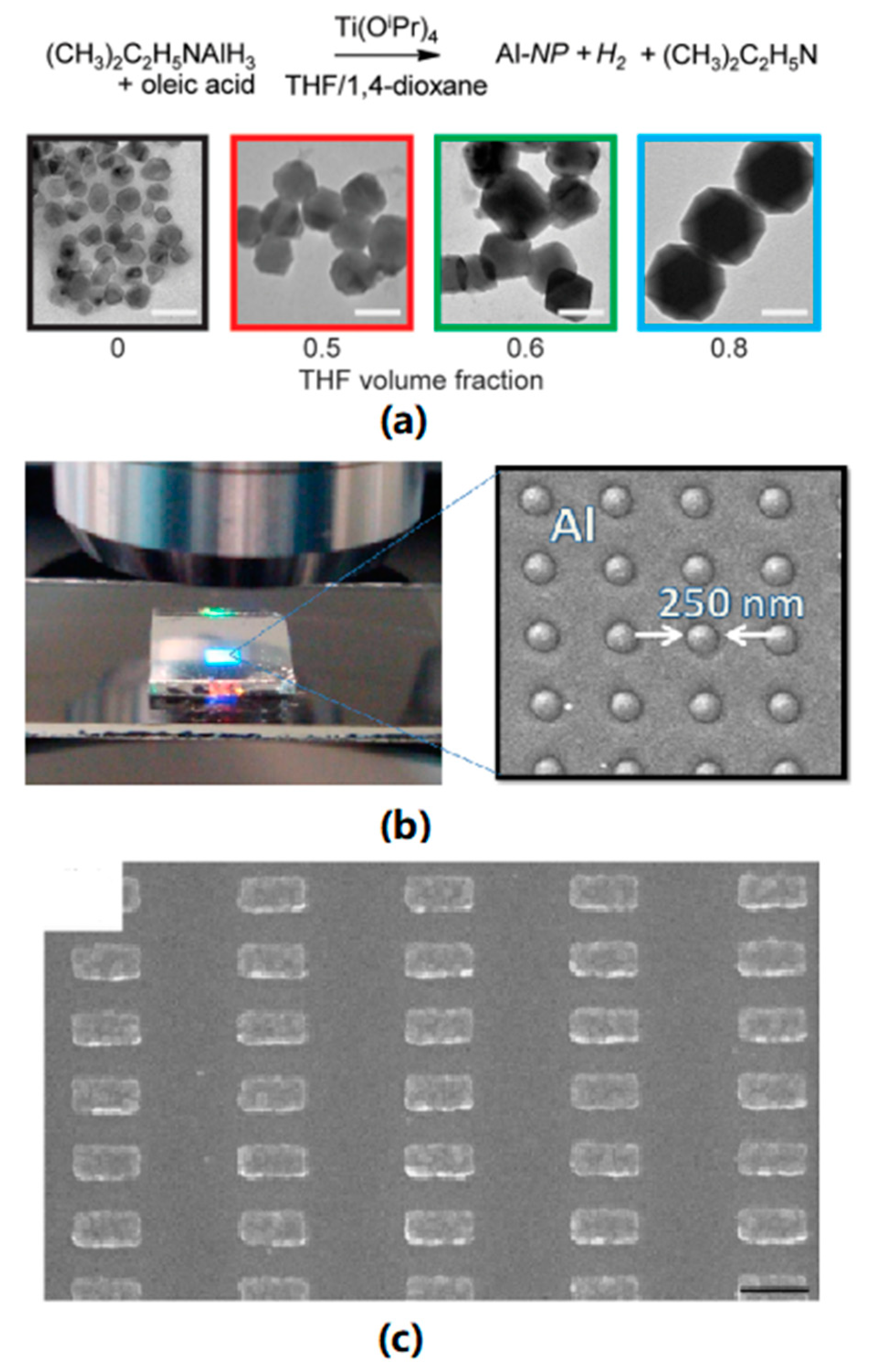

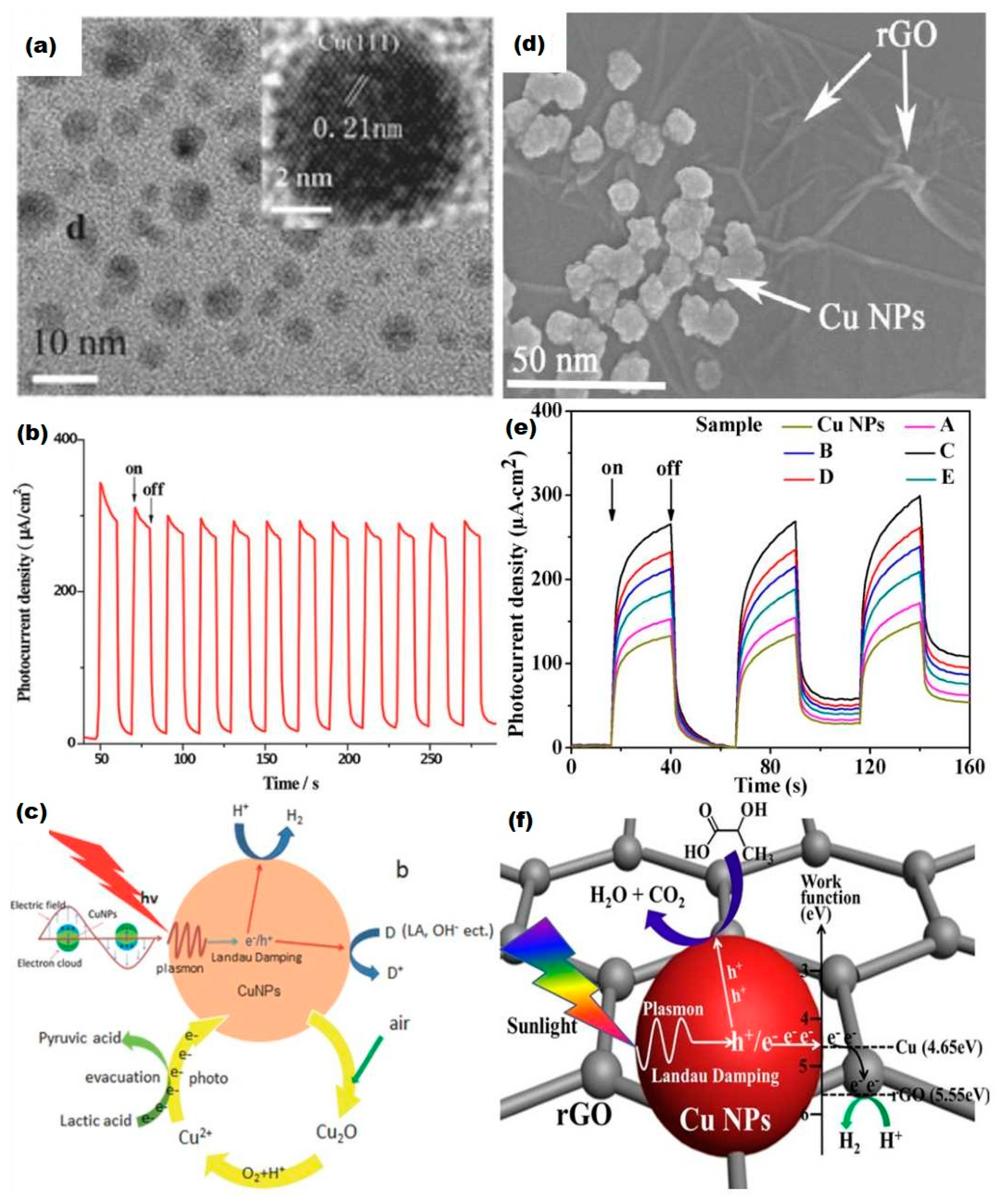

| Method | Precursor | Morphology | Size | References |
|---|---|---|---|---|
| Chemical reduction | (CH3)2C2H5NAlH3 | Nanocrystal | 70–200 nm in diameter | [30] |
| E-beam lithography | Al | Nanorod arrays1 | 130 × 65 nm | [31] |
| E-beam lithography | Al | Nanohole arrays | 200–800 nm in diameter | [4] |
| Colloidal lithography | Al | Nanoholes | 67 nm in diameter | [32] |
| Sonoelectrochemical | AlCl3, LiAlH4 | Nanoparticles | 10–20 nm in diameter | [33] |
| Laser ablation | Al | Nanoparticles | 20–50 nm in diameter | [34] |
| Anodic oxidation | Al | Porous Al | Porous film | [35] |
| Deposition/Dewetting | Al | Al/Al2O3 nanoparticle arrays | 12–25 nm | [36] |
| Application | Structure | Performance | Reference |
|---|---|---|---|
| Photocatalyst | Si-Al-Fe2O3 core-shell nanowires | (theoretical) 14.5% solar to hydrogen efficiency | [44] |
| Photoelectrochemical | Al nanodimer and TiO2 thin film | 27.8% increase in the local oxygen evolution rate | [38] |
| Organic photovoltaics | Aluminum nanoparticles | 30% efficiency enhancement | [39] |
| Organic photovoltaics | Aluminum nanodisk array | 7.28% to 8.04% enhancement in PCE | [40] |
| Solar cells | Aluminum nanodisk array | 38% external quantum efficiency at 530 nm | [41] |
| Photocatalyst | Aluminum nanocrystals and GaAs | 1.5e5 c/s HD Rate under 4 kW/cm2 laser illumination | [42] |
| Photocatalyst | Al-Pd nanodisk heterodimers | 28 nmol/s HD Rate at 450 nm | [43] |
| Organic solar cells | Graphene-aluminum nanocross arrays | 11.82 to 17.05 mA/cm2 photocurrent density | [45] |
| Method | Precursor | Reducing Agent | Stabilizer | Size | Product | References |
|---|---|---|---|---|---|---|
| Wet-chemical | Cupric chloride | Hydrazinium hydroxide | CTAB | 5–15 nm | CuNPs | [50] |
| Photoreduction | Cu(OAc)2 | Irradiated with a xenon lamp | - | 10 nm | CuNPs | [51] |
| Wet-chemical & MW-assisted | Cupric nitrate | Terminalia arjuna bark extract | Terminalia arjuna bark extract | 23 nm | Cu-MWCNTs | [52] |
| Wet-chemical | Copper-surfactant complex | Hydrazine hydrate; | Deprotonated polyacrylic acid | 40–85 nm | CuNPs | [53] |
| Wet-chemical | Copper-surfactant complex | Sodium borohydrate | Deprotonated polyacrylic acid | 50–54 nm | CuNPs | [53] |
| MW-assisted | copper acetate | Sodium hydroxide | - | 7 nm | CuNPs | [54] |
| MW-assisted | copper nitrate | L-Ascorbic acid | - | 9 nm | CuNPs | [54] |
| Micelle method | copper(II) sulfate pentahydrate | Sodium borohydrate | sodium dodecyl sulfate | ~2 nm | Cu nanoclusters | [55] |
| Ionic-liquid (IL)-assisted synthesis | Microsized copper particles (1–5 µm) | 1-butyl-3-methylimidazolium tetrafluoroborate | - | 20–200 nm | CuNPs | [56] |
| Structure | Reaction | Performance | References |
|---|---|---|---|
| CuNP | PC-HER | Hydrogen evolution rate of 35 mmol g−1 h−1 | [51] |
| CuNP-ZnO composite | PEC-OER | 0.017 mA cm−2 at 0.5 V (vs. SCE) under visible light illumination | [60] |
| CuNP/rGO | PEC-HER | Hydrogen evolution rate of ~59 mmol/h/g | [59] |
| CuNP@Cu2O/ZnO | PC-HER | Hydrogen evolution rate of ~1.47 mmol/h/g | [62] |
| CuO/Cu(OH)2@Cu substrate film | PEC-OER | 10 mA cm−2 at an overpotential of 580 mV | [67] |
| Nanorods with Cu(OH)2-CuO@Cu core-shell structure | PEC-OER | 10 mA cm−2 at an overpotential of 417 mV | [70] |
| CuO-modifed TiO2 | PC-HER | Hydrogen evolution rate of 64.2–71.6 mmol/h/g | [71] |
| CuO-TiO2 | PC-HER | Apparent quantum yield of 5.1% | [72] |
| Cu(OH)2-nanocluster-modified TiO2 | PC-HER | Hydrogen evolution rate of ~3.4 mmol/h/g, quantum efficiency (QE) ~13.9% | [73] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Ji, C.; Riley, D.J.; Xie, F. Boosting the Efficiency of Photoelectrolysis by the Addition of Non-Noble Plasmonic Metals: Al & Cu. Nanomaterials 2019, 9, 1. https://doi.org/10.3390/nano9010001
Jiang Q, Ji C, Riley DJ, Xie F. Boosting the Efficiency of Photoelectrolysis by the Addition of Non-Noble Plasmonic Metals: Al & Cu. Nanomaterials. 2019; 9(1):1. https://doi.org/10.3390/nano9010001
Chicago/Turabian StyleJiang, Qianfan, Chengyu Ji, D. Jason Riley, and Fang Xie. 2019. "Boosting the Efficiency of Photoelectrolysis by the Addition of Non-Noble Plasmonic Metals: Al & Cu" Nanomaterials 9, no. 1: 1. https://doi.org/10.3390/nano9010001
APA StyleJiang, Q., Ji, C., Riley, D. J., & Xie, F. (2019). Boosting the Efficiency of Photoelectrolysis by the Addition of Non-Noble Plasmonic Metals: Al & Cu. Nanomaterials, 9(1), 1. https://doi.org/10.3390/nano9010001





