Surface-Limited Electrodeposition of Continuous Platinum Networks on Highly Ordered Pyrolytic Graphite
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. TM-AFM Analysis
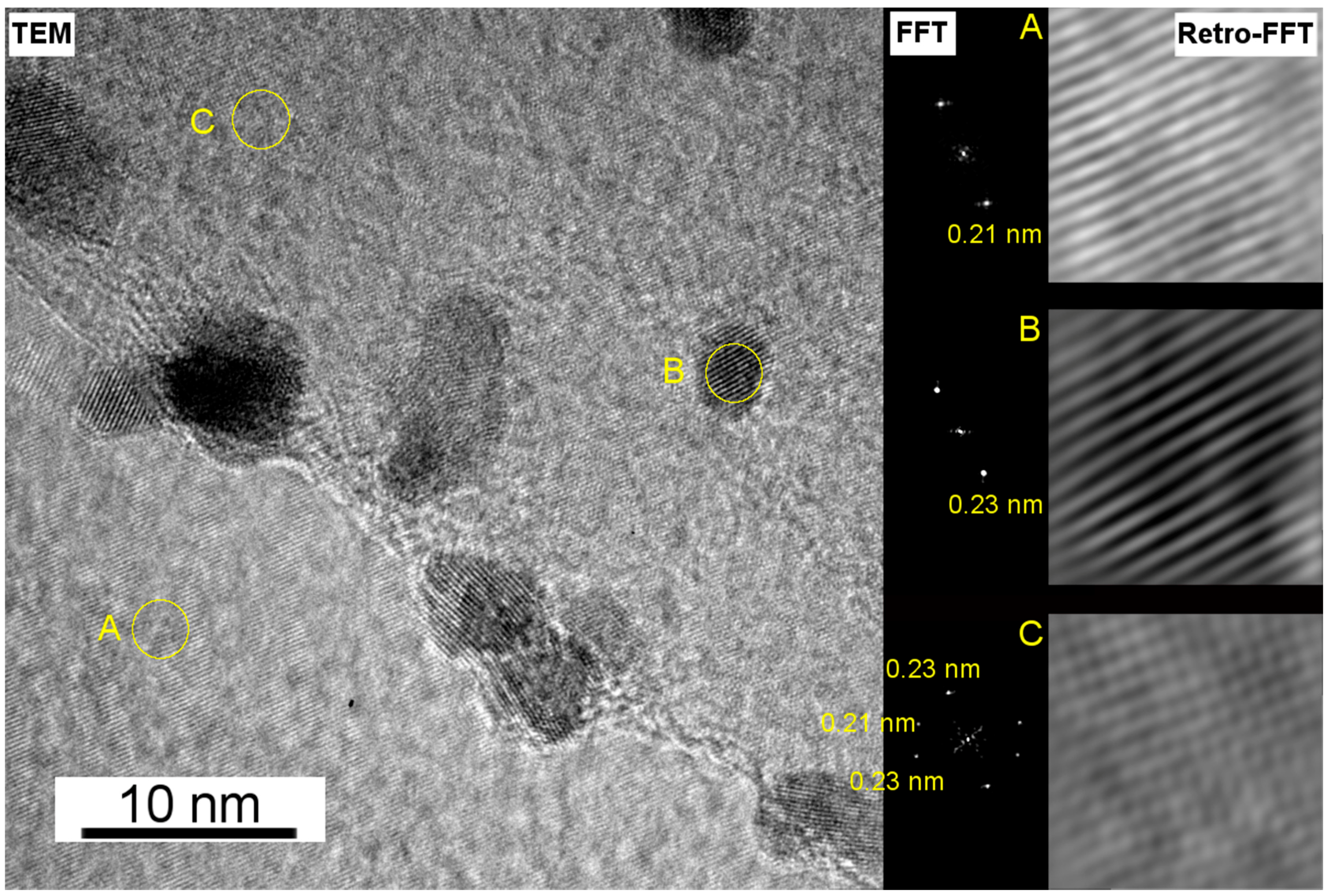
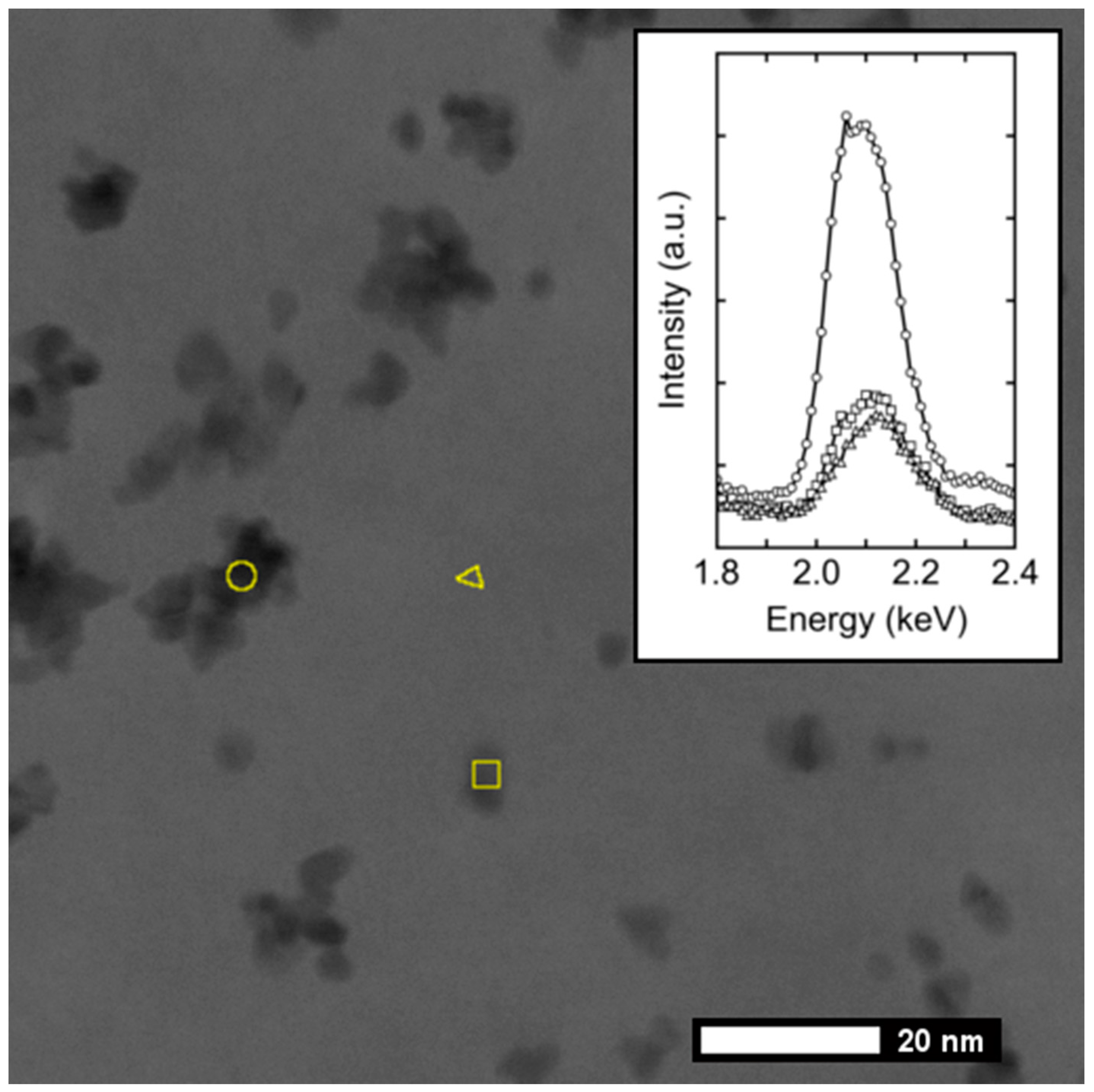
3.2. Electron Microscopy Analysis
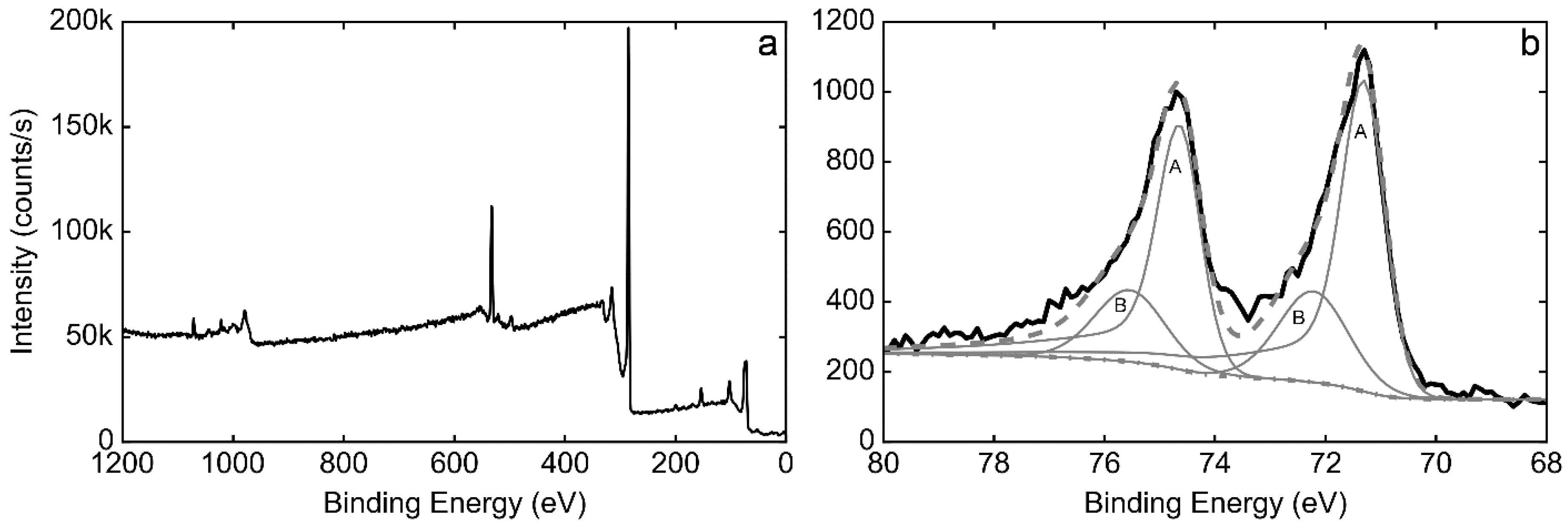
3.3. XPS Characterization
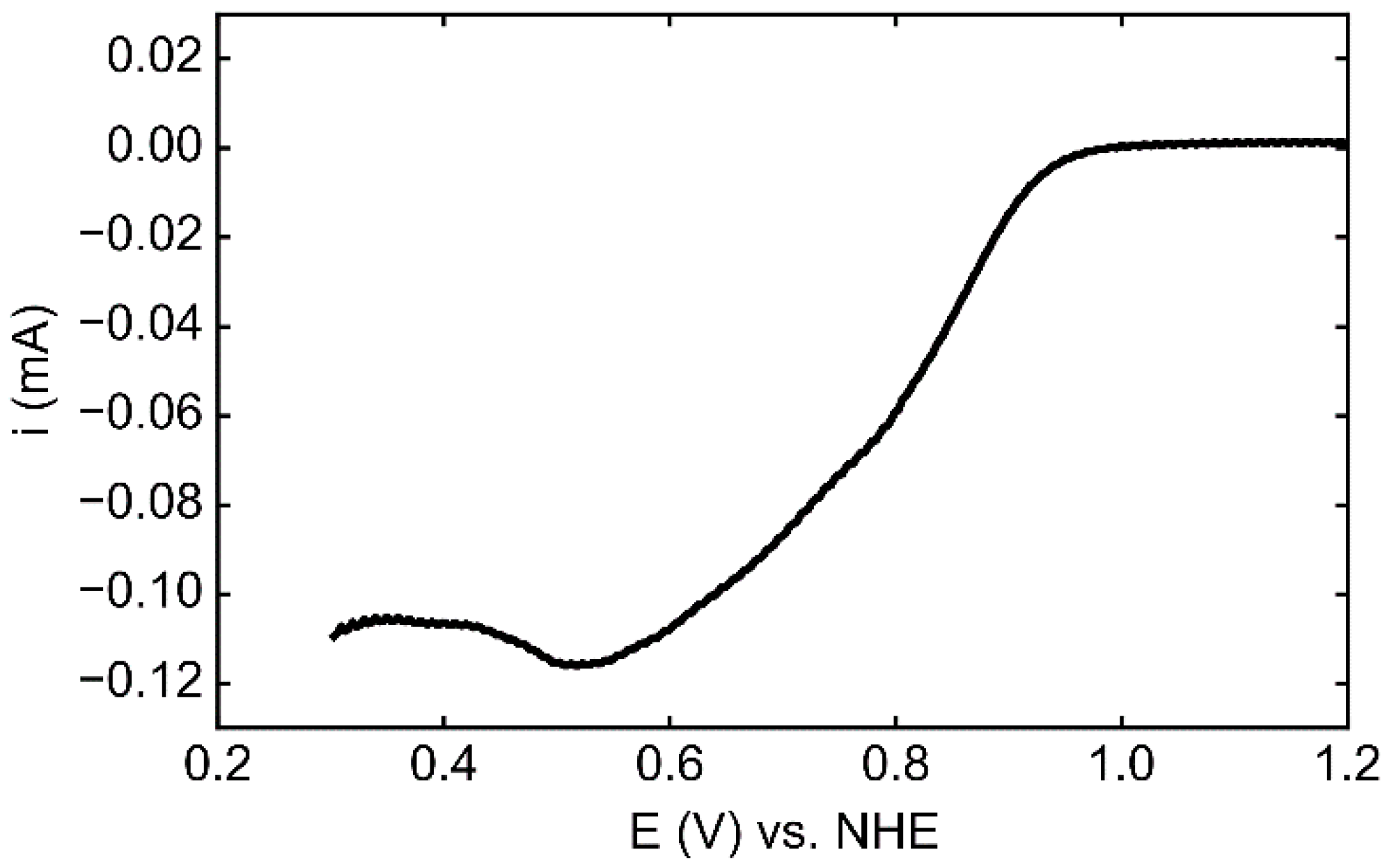
3.4. Electrocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guerrero Moreno, N.; Cisneros Molina, M.; Gervasio, D.; Pérez Robles, J.F. Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustain. Energy Rev. 2015, 52, 897–906. [Google Scholar] [CrossRef]
- Ercolano, G.; Cavaliere, S.; Rozière, J.; Jones, D.J. Recent developments in electrocatalyst design thrifting noble metals in fuel cells. Curr. Opin. Electrochem. 2018, 9, 271–277. [Google Scholar] [CrossRef]
- Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Core−shell compositional fine structures of dealloyed Pt. Nano Lett. 2012, 12, 5423–5430. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Du, D.; Eychmüller, A.; Lin, Y. Engineering ordered and nonordered porous noble metal nanostructures: Synthesis, assembly, and their applications in electrochemistry. Chem. Rev. 2015, 115, 8896–8943. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Yeom, Y.S.; Seo, H.Y.; Kumar, P.; Baek, K.-Y.; Yoon, H.G. A facile synthetic route for highly durable mesoporous platinum thin film electrocatalysts based on graphene: morphological and support effects on the oxygen reduction reaction. J. Mater. Chem. A 2017, 5, 3129–3135. [Google Scholar] [CrossRef]
- Lee, H.B.R.; Baeck, S.H.; Jaramillo, T.F.; Bent, S.F. Growth of Pt nanowires by atomic layer deposition on highly ordered pyrolytic graphite. Nano Lett. 2013, 13, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Aaltonen, T.; Ritala, M.; Sajavaara, T.; Keinonen, J.; Leskelä, M. Atomic layer deposition of platinum thin films. Chem. Mater. 2003, 15, 1924–1928. [Google Scholar] [CrossRef]
- Dolbec, R.; El Khakani, M.A. Sub-ppm sensitivity towards carbon monoxide by means of pulsed laser deposited SnO2:Pt based sensors. Appl. Phys. Lett. 2007, 90, 173114. [Google Scholar] [CrossRef]
- Achari, I.; Ambrozik, S.; Dimitrov, N. Electrochemical atomic layer deposition of Pd ultrathin films by surface limited redox replacement of underpotentially deposited H in a single cell. J. Phys. Chem. C 2017, 121, 4404–4411. [Google Scholar] [CrossRef]
- Gruber, D.; Ponath, N.; Müller, J.; Lindstaedt, F. Sputter-deposited ultra-low catalyst loadings for PEM fuel cells. J. Power Sources 2005, 150, 67–72. [Google Scholar] [CrossRef]
- Su, X.; Zhan, X.; Hinds, B.J. Pt monolayer deposition onto carbon nanotube mattes with high electrochemical activity. J. Mater. Chem. 2012, 22, 7979. [Google Scholar] [CrossRef]
- Whalen, J.J.; Weiland, J.D.; Searson, P.C. Electrochemical deposition of platinum from aqueous ammonium hexachloroplatinate solution. J. Electrochem. Soc. 2005, 152, C738. [Google Scholar] [CrossRef]
- Chen, X.; Li, N.; Eckhard, K.; Stoica, L.; Xia, W.; Assmann, J.; Muhler, M.; Schuhmann, W. Pulsed electrodeposition of Pt nanoclusters on carbon nanotubes modified carbon materials using diffusion restricting viscous electrolytes. Electrochem. Commun. 2007, 9, 1348–1354. [Google Scholar] [CrossRef]
- Ye, F.; Chen, L.; Li, J.; Li, J.; Wang, X. Shape-controlled fabrication of platinum electrocatalyst by pulse electrodeposition. Electrochem. Commun. 2008, 10, 476–479. [Google Scholar] [CrossRef]
- Ercolano, G.; Farina, F.; Cavaliere, S.; Jones, D.J.; Rozière, J. Towards ultrathin Pt films on nanofibres by surface-limited electrodeposition for electrocatalytic applications. J. Mater. Chem. A 2017, 5, 3974–3980. [Google Scholar] [CrossRef]
- Ercolano, G.; Farina, F.; Cavaliere, S.; Jones, D.J.; Rozière, J. Multilayer hierarchical nanofibrillar electrodes with tuneable lacunarity with 2D like Pt deposits for PEMFC. ECS Trans. 2017, 80, 757–762. [Google Scholar] [CrossRef]
- Yivlialin, R.; Bussetti, G.; Brambilla, L.; Castiglioni, C.; Tommasini, M.; Duò, L.; Passoni, M.; Ghidelli, M.; Casari, C.S.; Li Bassi, A. Microscopic analysis of the different perchlorate anions intercalation stages of graphite. J. Phys. Chem. C 2017, 121, 14246–14253. [Google Scholar] [CrossRef]
- Yasin, H.M.; Denuault, G.; Pletcher, D. Studies of the electrodeposition of platinum metal from a hexachloroplatinic acid bath. J. Electroanal. Chem. 2009, 633, 327–332. [Google Scholar] [CrossRef]
- Pang, L.; Zhang, Y.; Liu, S. Monolayer-by-monolayer growth of platinum films on complex carbon fiber paper structure. Appl. Surf. Sci. 2017, 407, 386–390. [Google Scholar] [CrossRef]
- Duarte, M.M.E.; Pilla, A.S.; Sieben, J.M.; Mayer, C.E. Platinum particles electrodeposition on carbon substrates. Electrochem. Commun. 2006, 8, 159–164. [Google Scholar] [CrossRef]
- Lee, I.; Chan, K.-Y.; Lee Phillips, D. Atomic force microscopy of platinum nanoparticles prepared on highly oriented pyrolytic graphite. Ultramicroscopy 1998, 75, 69–76. [Google Scholar] [CrossRef]
- Lee, I.; Chan, K.-Y.; Phillips, D.L. Growth of electrodeposited platinum nanocrystals studied by atomic force microscopy. Appl. Surf. Sci. 1998, 136, 321–330. [Google Scholar] [CrossRef]
- Liu, Y.H.; Gokcen, D.; Bertocci, U.; Moffat, T.P. Self-terminating growth of platinum films by electrochemical deposition. Science 2012, 338, 1327–1330. [Google Scholar] [CrossRef] [PubMed]
- Zoval, J.V.; Lee, J.; Gorer, S.; Penner, R.M. Electrochemical preparation of platinum nanocrystallites with size selectivity on basal plane oriented graphite surfaces. J. Phys. Chem. B 1998, 5647, 1166–1175. [Google Scholar] [CrossRef]
- Shen, P.; Chi, N.; Chan, K.Y.; Phillips, D.L. Platinum nanoparticles spontaneously formed on HOPG. Appl. Surf. Sci. 2001, 172, 159–166. [Google Scholar] [CrossRef]
- Arroyo Gõmez, J.J.; García, S.G. Spontaneous deposition of Pt-nanoparticles on HOPG surfaces. Surf. Interface Anal. 2015, 47, 1127–1131. [Google Scholar] [CrossRef]
- Juarez, M.F.; Fuentes, S.; Soldano, G.J.; Avalle, L.; Santos, E. Spontaneous formation of metallic nanostructures on highly oriented pyrolytic graphite (HOPG): An ab initio and experimental study. Faraday Discuss. 2014, 172, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Alpuche-Aviles, M.A.; Farina, F.; Ercolano, G.; Subedi, P.; Cavaliere, S.; Jones, D.J.; Rozière, J. Electrodeposition of two-dimensional Pt nanostructures on highly oriented pyrolytic graphite (HOPG): the effect of evolved hydrogen and chloride ions. Nanomaterials 2018, 8, 668. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78. [Google Scholar] [CrossRef] [PubMed]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Lu, G.; Zangari, G. Electrodeposition of platinum nanoparticles on highly oriented pyrolitic graphite: Part II: Morphological characterization by atomic force microscopy. Electrochim. Acta 2006, 51, 2531–2538. [Google Scholar] [CrossRef]
- Ustarroz, J.; Altantzis, T.; Hammons, J.A.; Hubin, A.; Bals, S.; Terryn, H. The role of nanocluster aggregation, coalescence, and recrystallization in the electrochemical deposition of platinum nanostructures. Chem. Mater. 2014, 26, 2396–2406. [Google Scholar] [CrossRef]
- Lu, G.J.; Zangari, G. Electrodeposition of platinum on highly oriented pyrolytic graphite. Part 1: Electrochemical characterization. J. Phys. Chem. B 2005, 109, 7998–8007. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lipkowski, J. Chronocoulometric studies of chloride adsorption at the Pt(111) electrode surface. J. Electroanal. Chem. 2000, 491, 95–102. [Google Scholar] [CrossRef]
- Horanyi, G.; Rizmayer, E.Z. A coupled voltammetric and radiometric (voltradiometric) study of the simultaneous adsorption of hydrogen and anions at platinized platinum electrodes. J. Electroanal. Chem. 1987, 218, 337–340. [Google Scholar] [CrossRef]
- Gossenberger, F.; Roman, T.; Groß, A. Hydrogen and halide co-adsorption on Pt(111) in an electrochemical environment: A computational perspective. Electrochim. Acta 2016, 216, 152–159. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Beard, B.C. Agglomeration of Pt particles in the presence of chlorides. Appl. Catal. A Gen. 1999, 188, 229–240. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, W.; Ding, H.; He, B. Hydrogenolysis of glycerol over Pt/C catalyst in combination with alkali metal hydroxides. Open Chem. 2016, 14, 279–286. [Google Scholar] [CrossRef]
- Raynal, F.; Etcheberry, A.; Cavaliere, S.; Noël, V.; Perez, H. Characterization of the unstability of 4-mercaptoaniline capped platinum nanoparticles solution by combining LB technique and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2006, 252, 2422–2431. [Google Scholar] [CrossRef]
- Spataru, T.; Osiceanu, P.; Marcu, M.; Lete, C.; Munteanu, C.; Spa, N. Functional effects of the deposition substrate on the electrochemical behavior of platinum particles. Jpn. J. Appl. Phys. 2012, 51, 090119. [Google Scholar] [CrossRef]
- Marcus, P.; Hinnen, C. XPS study of the early stages of deposition of Ni, Cu and Pt on HOPG. Surf. Sci. 1997, 392, 134–142. [Google Scholar] [CrossRef]
- Motin, A.M.; Haunold, T.; Bukhtiyarov, A.V.; Bera, A.; Rameshan, C.; Rupprechter, G. Surface science approach to Pt/carbon model catalysts: XPS, STM and microreactor studies. Appl. Surf. Sci. 2018, 440, 680–687. [Google Scholar] [CrossRef]
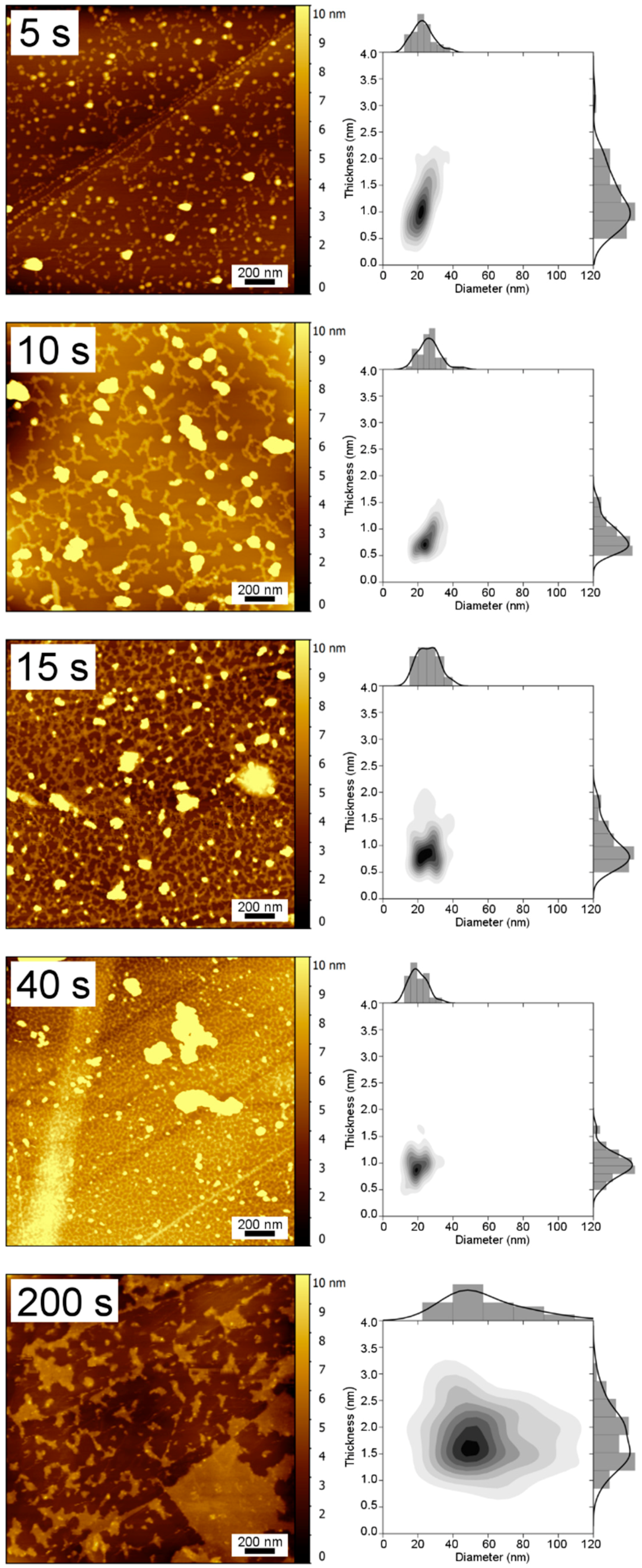
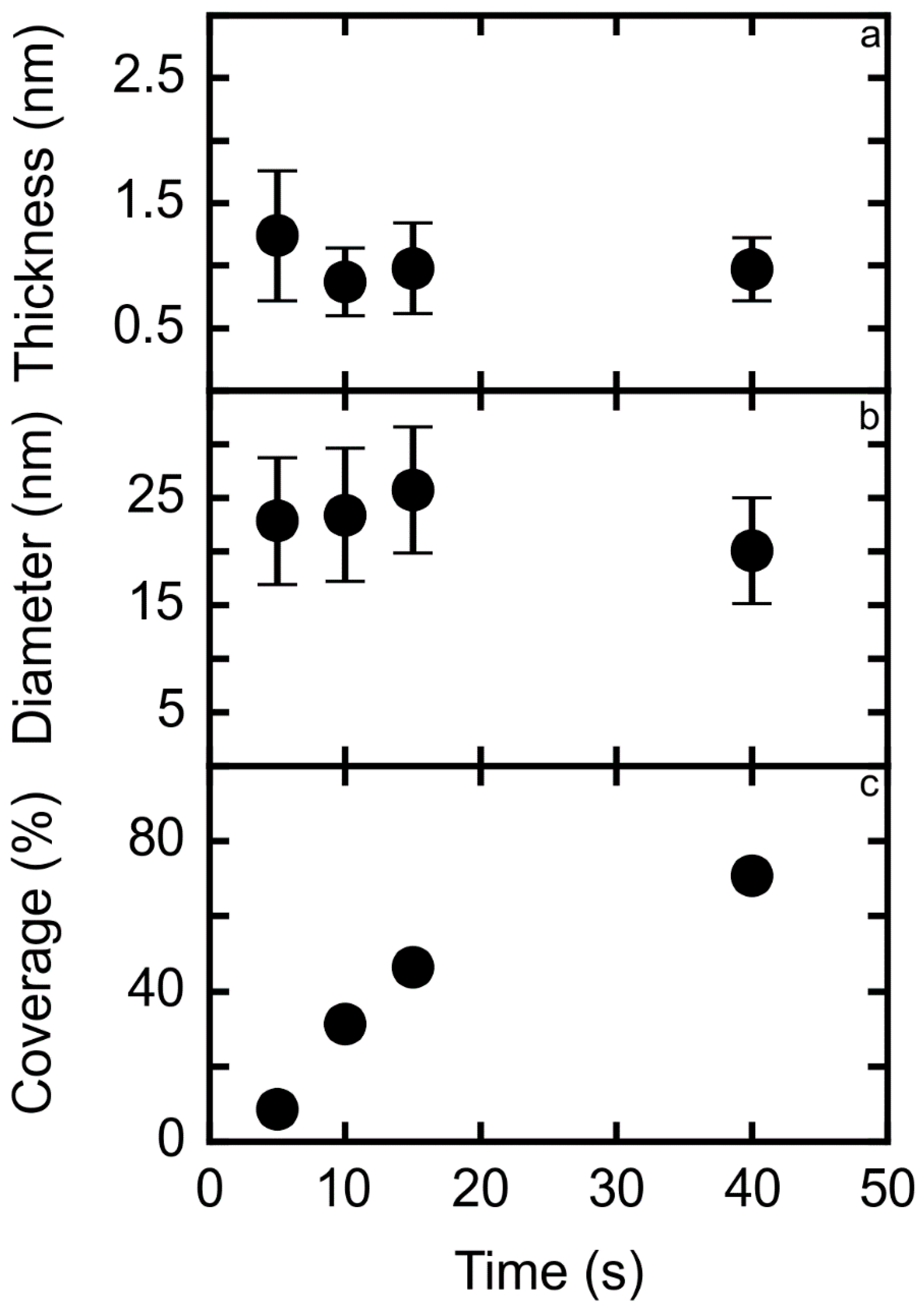
| Sample | Thickness (nm) | Diameter (nm) | Surface Coverage (%) |
|---|---|---|---|
| 5 | 1.24 ± 0.52 | 22.8 ± 6 | 8.7 |
| 10 | 0.87 ± 0.27 | 23.4 ± 6 | 31.4 |
| 15 | 0.98 ± 0.36 | 25.8 ± 6 | 46.5 |
| 40 | 0.97 ± 0.25 | 20.1 ± 5 | 70.7 |
| 200 | 1.82 ± 0.52 | 72.0 ± 69 | 44.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farina, F.; Ercolano, G.; Cavaliere, S.; Jones, D.J.; Rozière, J. Surface-Limited Electrodeposition of Continuous Platinum Networks on Highly Ordered Pyrolytic Graphite. Nanomaterials 2018, 8, 721. https://doi.org/10.3390/nano8090721
Farina F, Ercolano G, Cavaliere S, Jones DJ, Rozière J. Surface-Limited Electrodeposition of Continuous Platinum Networks on Highly Ordered Pyrolytic Graphite. Nanomaterials. 2018; 8(9):721. https://doi.org/10.3390/nano8090721
Chicago/Turabian StyleFarina, Filippo, Giorgio Ercolano, Sara Cavaliere, Deborah J. Jones, and Jacques Rozière. 2018. "Surface-Limited Electrodeposition of Continuous Platinum Networks on Highly Ordered Pyrolytic Graphite" Nanomaterials 8, no. 9: 721. https://doi.org/10.3390/nano8090721
APA StyleFarina, F., Ercolano, G., Cavaliere, S., Jones, D. J., & Rozière, J. (2018). Surface-Limited Electrodeposition of Continuous Platinum Networks on Highly Ordered Pyrolytic Graphite. Nanomaterials, 8(9), 721. https://doi.org/10.3390/nano8090721







