Hexagonal Boron Nitride Functionalized with Au Nanoparticles—Properties and Potential Biological Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Exfoliation of h-BN
2.2.2. Hexagonal Boron Nitride Au Functionalization
2.3. Characterization of Synthesized Nanomaterial
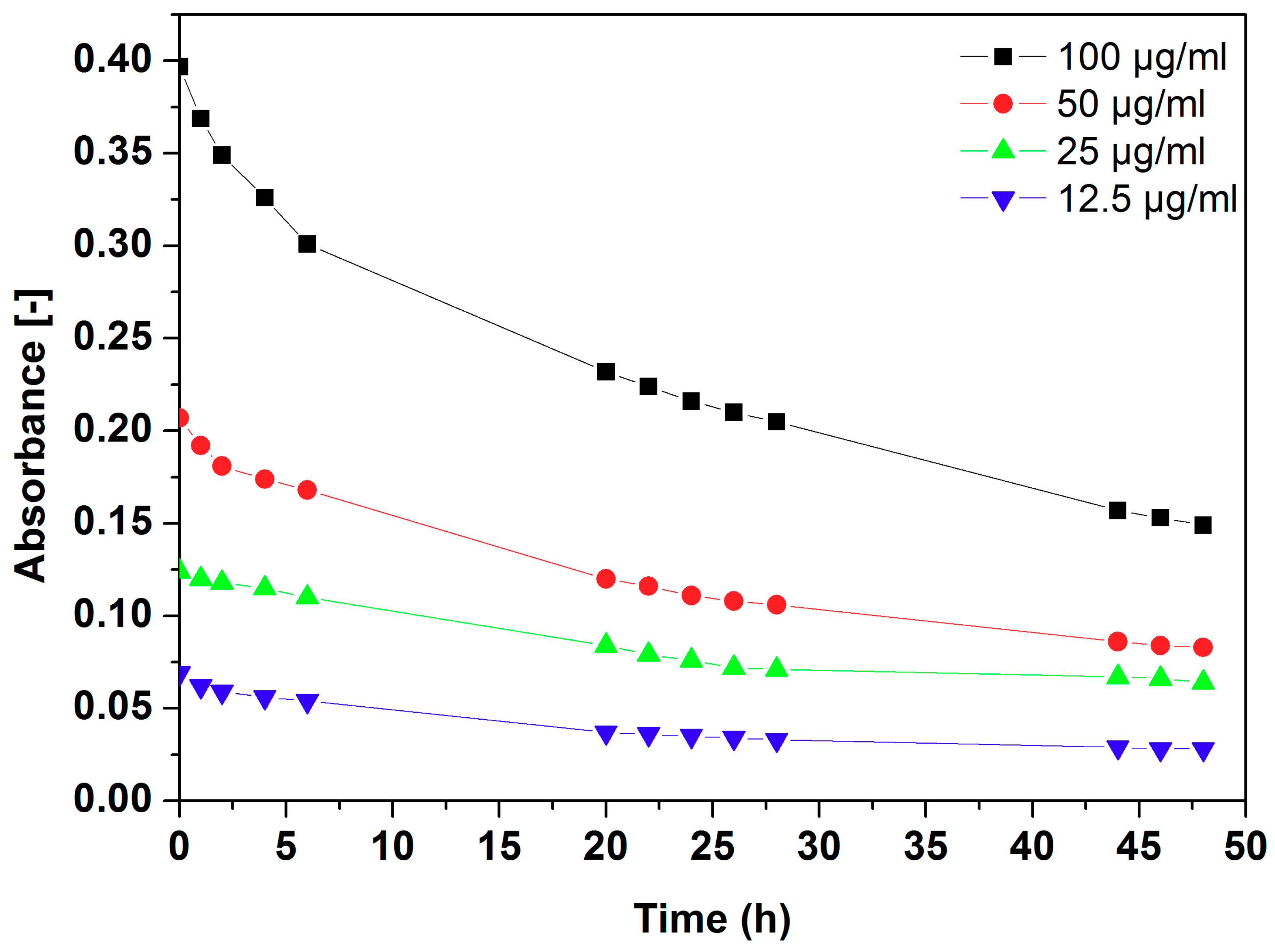
2.4. Dispersion Stability of Au Functionalized h-BN
2.5. Cell Lines and Cell Culture Conditions
2.6. Experimental Treatment
2.7. Microscopic Analyses
2.8. Cell Counting Kit-8 Analysis
2.9. Lactate Dehydrogenase Leaking Assay
2.10. Neutral Red Uptake Assay
2.11. Cellular Uptake and Confocal Microscope Imaging
2.12. Statistical Analysis
3. Results
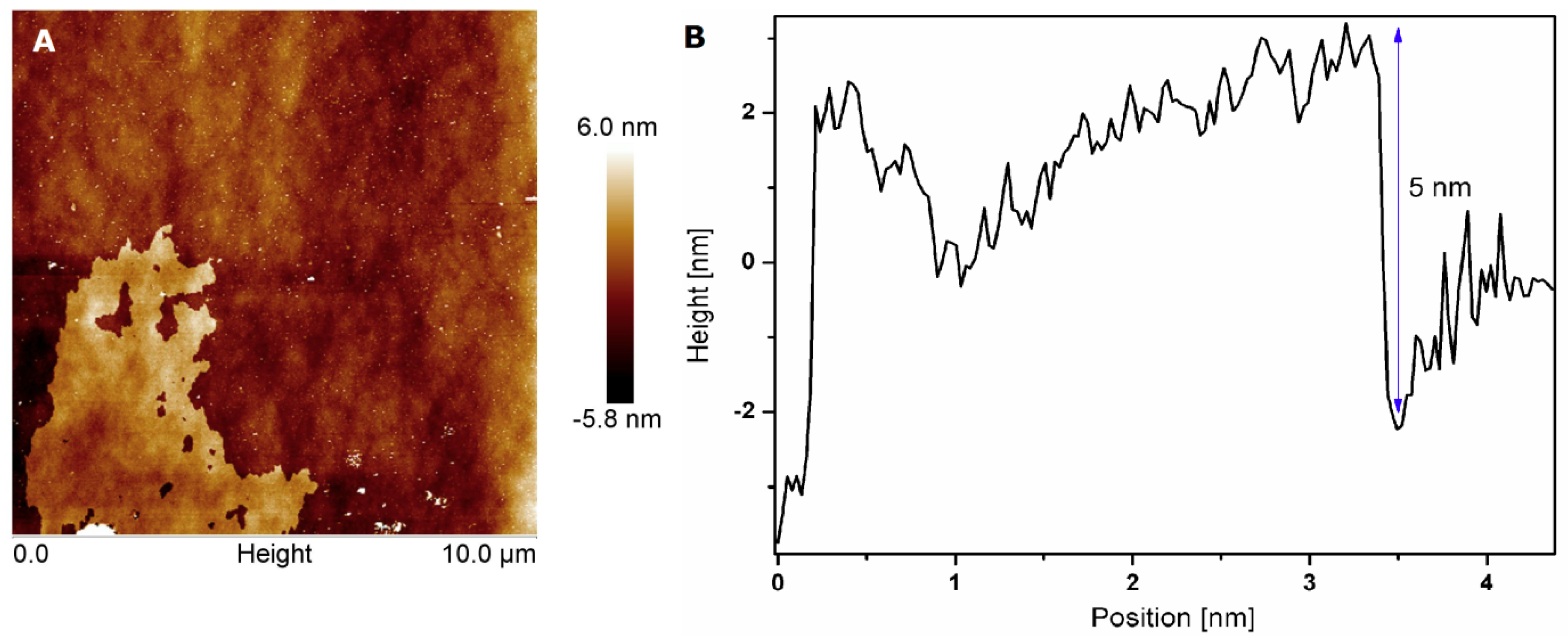
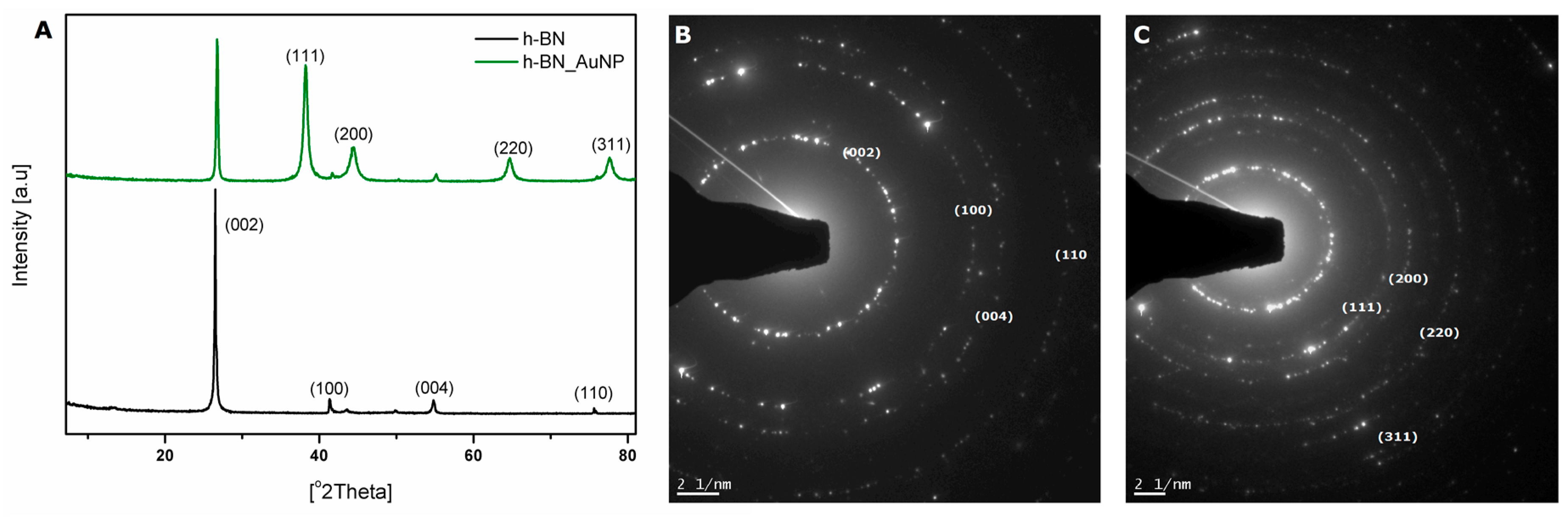
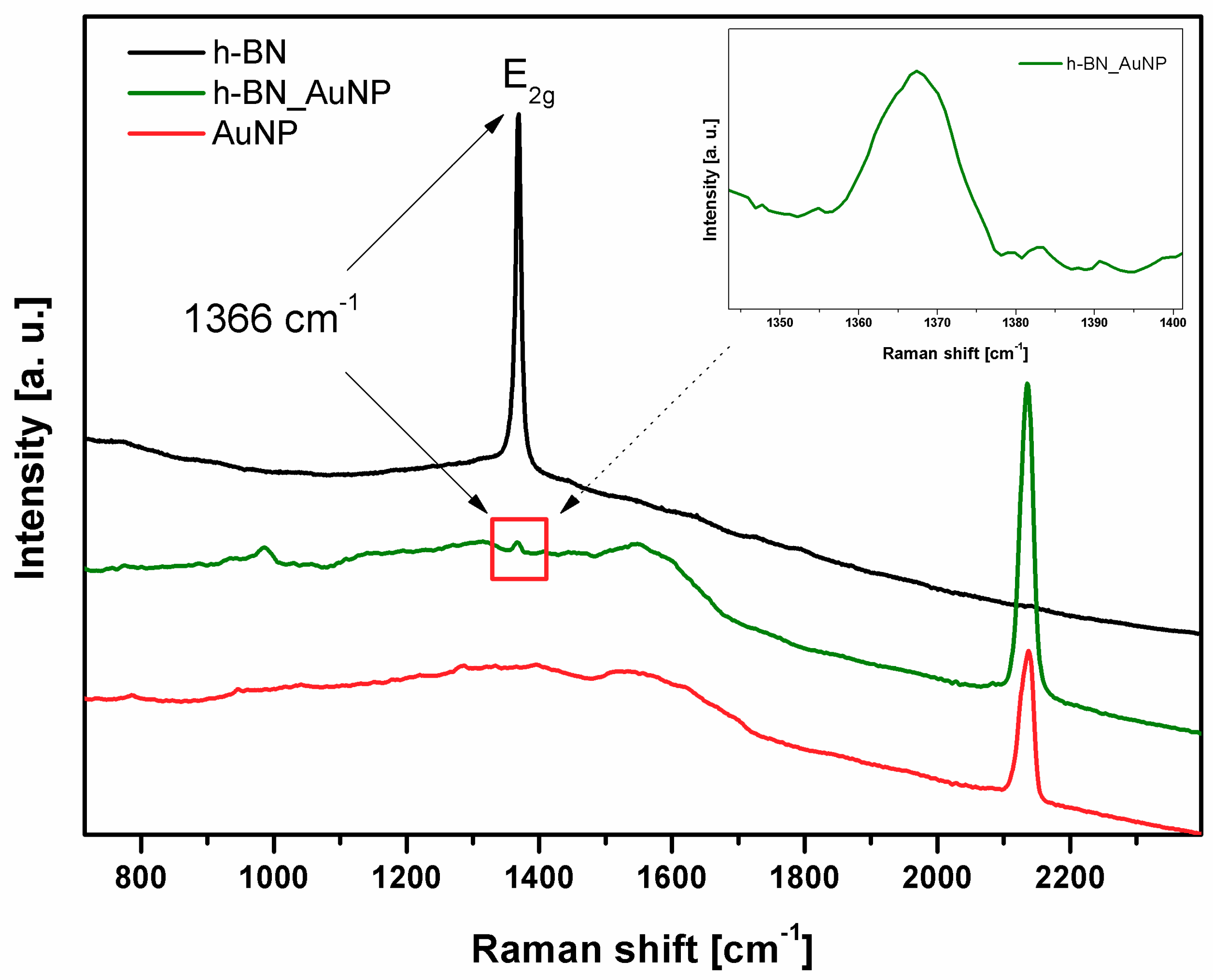
3.1. Characterization of h-BN and h-BN_AuNP
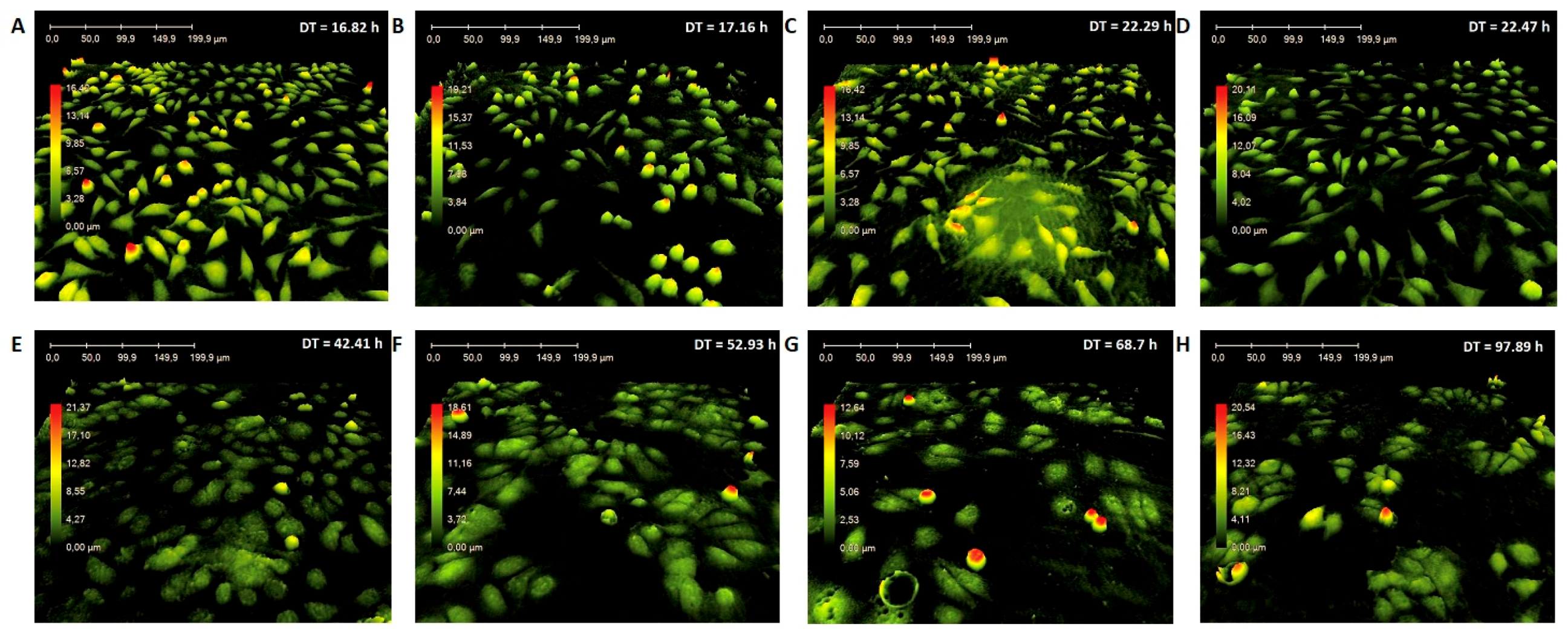
3.2. In Vitro Microscopic Analyses
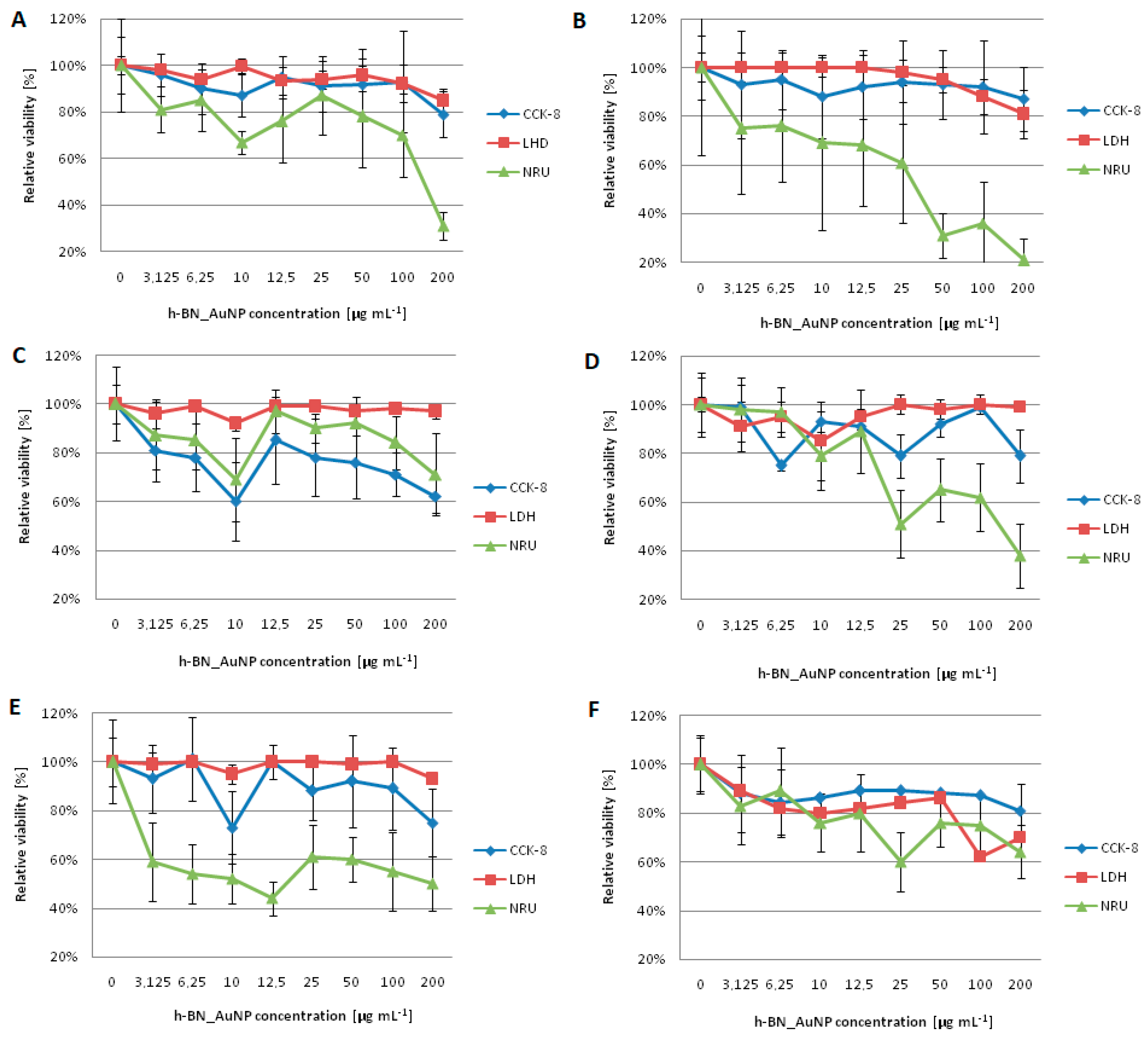
3.3. Analysis of Cytotoxicity Results
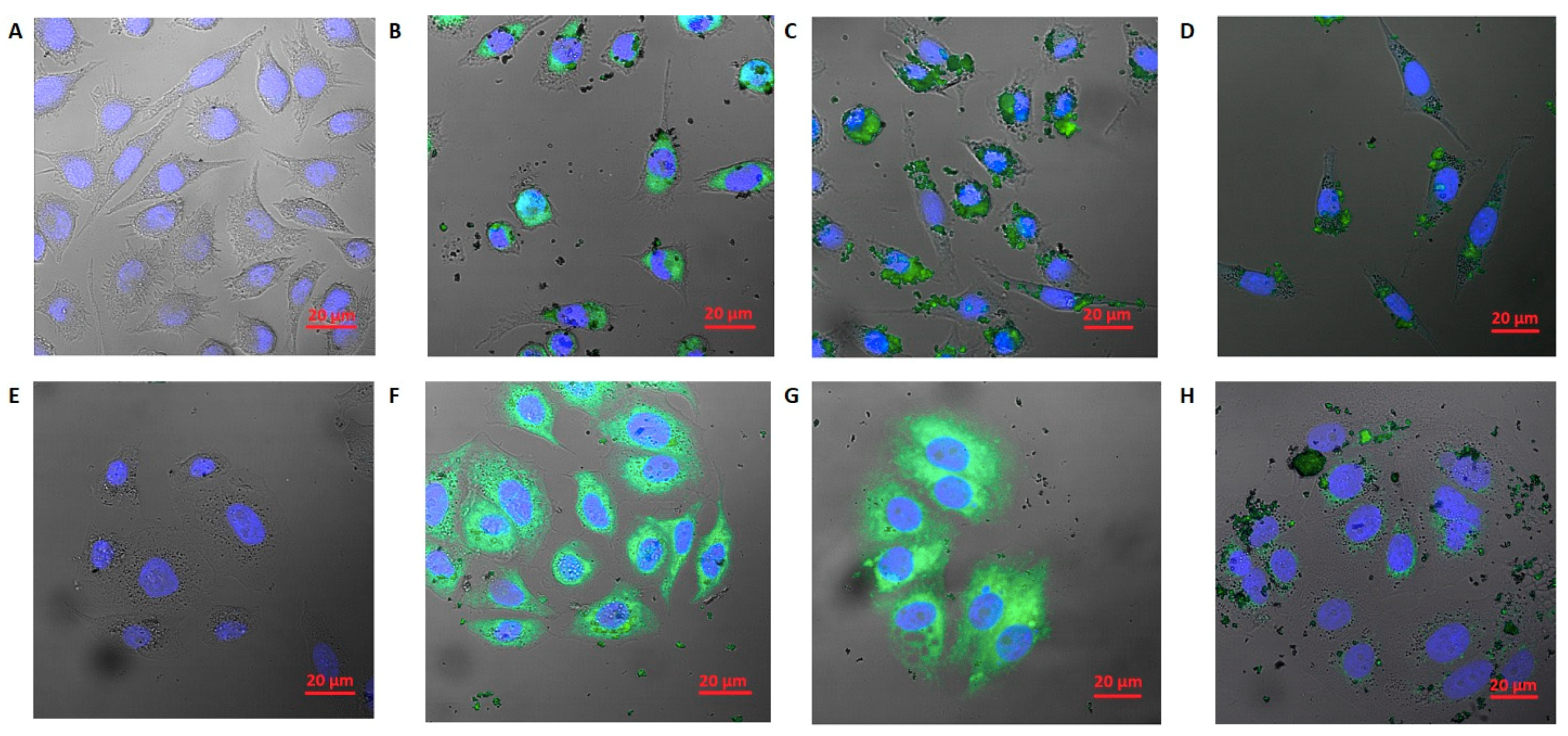
3.4. Cellular Uptake Results
4. Discussion
4.1. The Cytotoxicity of Hexagonal Boron Nitride
4.2. Presence of Au Nanoparticles on h-BN Nanoplates and Their Impact on Cell Activity and Proliferation Rate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, X.; Wang, X.; Bando, Y.; Golberg, D. Functionalized hexagonal boron nitride nanomaterials: Emerging properties and applications. Chem. Soc. Rev. 2016, 45, 3989–4012. [Google Scholar] [CrossRef] [PubMed]
- Dobrzhinetskaya, L.F.; Wirth, R.; Yang, J.; Green, H.W.; Hutcheon, I.D.; Weber, P.K.; Grew, E.S. Qingsongite, natural cubic boron nitride: The first boron mineral from the Earth’s mantle. Am. Miner. 2014, 99, 764–772. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terano, T.; Mitome, M.; Tang, C.; Zhi, C. Boron nitride nanotubes and nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Safety Assessment of Boron Nitride as Used in Cosmetics; Scientific Literature Review for Public Comment; Cosmetics Ingredient Review: Washington, DC, USA, 2012.
- Sediri, H.; Pierucci, D.; Hajlaoui, M.; Henck, H.; Patriarche, G.; Dappe, Y.J.; Yuan, S.; Toury, B.; Belkhou, R.; Silly, M.G.; et al. Atomically sharp interface in an h-BN-epitaxial graphene van der Waals heterostructure. Sci. Rep. 2015, 5, 16465. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.L.; Paine, R.T. Aerosol synthesis of hollow spherical morphology boron nitride particles. Chem. Mater. 2006, 18, 4716–4718. [Google Scholar] [CrossRef]
- Cai, Q.; Mateti, S.; Yang, W.; Jones, R.; Watanabe, K.; Taniguchi, T.; Huang, S.; Chen, Y.; Li, L.H. Boron nitride nanosheets improve sensitivity and reusability of surface enhanced Raman spectroscopy. Angew. Chem. Int. Ed. 2016, 55, 8405–8409. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, F.; Liang, W.; Sun, M. Electrical properties and applications of graphene, hexagonal boron nitride (h-BN), and graphene/h-BN heterostructures. Mater. Today Phys. 2017, 2, 6–34. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.; Santos, E.J.G.; Scullion, D.; Qian, D.; Zhang, R.; Yang, Z.; Huang, S.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Commun. 2017, 8, 15815. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wang, L.; Sun, J.; Long, Y.; Hu, P.; Liu, F.; He, X. Production methods of van der Waals heterostructures based on transition metal dichalcogenides. Crystals 2018, 8, 35. [Google Scholar] [CrossRef]
- Jariwala, D.; Marks, T.J.; Hersam, M. Mixed-dimensional van der Waals heterostructures. Nat. Mater. 2017, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.F.; Elbaz, G.A.; Bediako, D.K.; Yu, C.; Efetov, D.K.; Guo, Y.; Rivichandran, J.; Min, K.A.; Hong, S.; Taniguchi, T.; et al. Controlled electrochemical intercalation of graphene/h-BN van der Waals heterostructures. Nano Lett. 2018, 18, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Schiros, T.; Santos, E.J.G.; Kim, B.; Yager, K.G.; Kang, S.J.; Lee, S.; Yu, J.; Watanabe, K.; Taniguchi, T.; et al. Epitaxial growth of molecular crystals on van der Waals substrates for high-performance organic electronics. Adv. Mater. 2014, 26, 2812–2817. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-H.; Lee, C.-H.; van der Zande, A.M.; Han, M.; Cui, X.; Arefe, G.; Nuckolls, C.; Heinz, T.F.; Hone, J.; Kim, P. Heterostructures based on inorganic and organic van der Waals systems. APL Mater. 2014, 2, 092511. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kumakura, K.; Akasaka, T.; Makimoto, T. Layered boron nitride as a release layer for mechanical transfer of GaN-based devices. Nature 2012, 484, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Lee, C.-H.; Yi, G.-C. Transferable GaN layers grown on ZnO-coated graphene layers for optoelectronic devices. Science 2010, 330, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Chen, C.H.; Shi, Y.; Li, L.J. Heterostructures based on two-dimensional layered materials and their potential applications. Mater. Today 2016, 19, 322–335. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, B.; Wang, X.; Hanagata, N.; Li, X.; Liu, D.; Wang, X.; Jiang, X.; Bando, Y.; Golberg, D. Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano 2014, 8, 6123–6130. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Noncovalent functionalization of boron nitride nanotubes in aqueous media opens application roads in nanomedicine. Nanomedicine 2014, 1, 7. [Google Scholar]
- Gottschalck, T.E.; Breslawec, H. International Cosmetic Ingredient Dictionary and Handbook; Personal Care Products Council: Washington, DC, USA, 2012. [Google Scholar]
- Rasel, A.I.; Li, T.; Nguyen, T.D.; Singh, S.; Zhou, Y.; Xiao, Y.; Gu, Y.T. Biophysical response of living cells to boron nitride nanoparticles: Uptake mechanism and bio-mechanical characterization. J. Nanopart. Res. 2015, 17, 441. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C.R. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef] [PubMed]
- Horváth, L.; Magrez, A.; Golberg, D.; Zhi, C.; Bando, Y.; Smajda, R.; Horváth, E.; Forró, L.; Schwaller, B. In vitro investigation of the cellular toxicity of boron nitride nanotubes. ACS Nano 2011, 5, 3800–3810. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.F.; Randviir, E.P.; Brownson, D.A.; Ji, X.; Smith, G.C.; Banks, C.E. 2D hexagonal boron nitride (2D-hBN) explored as a potential electrocatalyst for the oxygen reduction reaction. Electrocatalysis 2017, 29, 622–634. [Google Scholar] [CrossRef]
- Xu, X.; Hao, H.; Khanaki, A.; Zheng, R.; Suja, M.; Liu, J. Large-area growth of multi-layer hexagonal boron nitride on polished cobalt foils by plasma-assisted molecular beam epitaxy. Sci. Rep. 2017, 7, 43100. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, P.M.; Vinod, S.; Ozden, S.; Sruthi, R.; Kukovecz, A.; Konya, Z.; Vajtai, R.; Anantharaman, M.R.; Ajayan, P.M.; Narayanan, T.N. Functionalized boron nitride porous solids. RSC Adv. 2015, 5, 93964–93968. [Google Scholar] [CrossRef]
- Meunier, F.C. In situ FT-IR spectroscopy investigations of dimethyl carbonate synthesis: On the contribution of gas-phase species. RSC Adv. 2016, 6, 17288–17289. [Google Scholar] [CrossRef]
- Esumi, K.; Hosoya, T.; Suzuki, A.; Torigoe, K. Spontaneous formation of gold nanoparticles in aqueous solution of sugar-persubstituted poly(amidoamine)dendrimers. Langmuir 2000, 16, 2978–2980. [Google Scholar] [CrossRef]
- Korsaks, V. Hexagonal boron nitride luminescence dependent on vacuum level and surrounding gases. Mater. Res. Bull. 2015, 70, 976–979. [Google Scholar] [CrossRef]
- Vijayan, S.R.; Santhiyagu, P.; Singamuthu, M.; Ahila, N.K.; Jayaraman, R.; Ethiraj, K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014, 2014, 938272. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Du, A.; Gao, G.; Mateti, S.; Cowie, B.C.; Qian, D.; Zhang, S.; Lu, Y.; Fu, L.; Taniguchi, T.; et al. Molecule-induced conformational change in boron nitride nanosheets with enhanced surface adsorption. Adv. Funct. Mater. 2016, 26, 8202–8210. [Google Scholar] [CrossRef]
- Griffin, A.; Harvey, A.; Cunningham, B.; Scullion, D.; Tian, T.; Shih, C.J.; Gruening, M.; Donegan, J.F.; Santos, E.J.; Backes, C.; et al. Spectroscopic size and thickness metrics for liquid-exfoliated h-BN. Chem. Mater. 2018, 30, 1998–2005. [Google Scholar] [CrossRef]
- Draz, M.S.; Lu, X. Development of a loop mediated isothermal amplification (LAMP)—Surface Enhanced Raman spectroscopy (SERS) assay for the detection of Salmonella enterica serotype Enteritidis. Theranostics 2016, 6, 522. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.H.; Hollanda, L.M.; Lancellotti, M.; de Sous, E.M.B. Boron nitride nanotubes chemically functionalized with glycol chitosan for gene transfection in eukaryotic cell lines. J. Biomed. Mater. Res. A 2015, 103, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.Y.; Bando, Y.; Tang, C.C.; Xie, R.G.; Sekiguchi, T.; Golberg, D. Perfectly dissolved boron nitride nanotubes due to polymer wrapping. J. Am. Chem. Soc. 2005, 127, 15996–15997. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Bando, Y.; Zhi, C.Y.; Fu, W.Y.; Wang, E.G.; Golberg, D. Aqueous noncovalent functionalization and controlled near-surface carbon doping of multiwalled boron nitride nanotubes. J. Am. Chem. Soc. 2008, 130, 8144–8145. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Wang, L.; Jiang, Y.; Iiu, Q.; Huang, C. Hexagonal boron nitride nanoplates as emerging biological nanovectors and their potential applications in biomedicine. J. Mater. Chem. B 2016, 4, 6103–6110. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Nafiujjaman, M.; Lee, S.J.; Park, I.K.; Huh, K.M.; Lee, Y. Preparation of ultra-thin hexagonal boron nitride nanoplates for cancer cell imaging and neurotransmitter sensing. Chem. Commun. 2016, 52, 6146–6149. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhang, J.; Hanagata, N.; Wang, X.; Weng, Q.; Ito, A.; Bando, Y.; Golberg, D. Hollow boron nitride nanospheres as boron reservoir for prostate cancer treatment. Nat. Commun. 2017, 8, 13936. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Polypropylene biocomposites with boron nitride and nano-hydroxyapatite reinforcements. Materials 2015, 8, 992–1008. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Yague, M.A.; Larranaga, A.; Gladkovskaya, O.; Stanley, A.; Tadayyon, G.; Guo, Y.; Sarasua, J.R.; Tofail, S.A.M.; Zeugolis, D.I.; Pandit, A.; et al. Effects of polydopamine functionalization on boron nitride nanotube dispersion and cytocompatibility. Bioconjug. Chem. 2015, 26, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, A.S.; Menciassi, A. Assessing cytotoxicity of boron nitride nanotubes: Interference with the MTT assay. Biochem. Biophys. Res. Commun. 2010, 394, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; Genchi, G.G.; Mazzolai, B.; Mattoli, V. Boron nitride nanotubes: Biocompatibility and potential spill-over in nanomedicine. Small 2013, 9, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Ricotti, L.; Danti, S.; Moscato, S.; Nesti, C.; D’Alessandro, D.; Dinucci, D.; Chiellini, F.; Pietrabissa, A.; Petrini, M.; et al. Investigation of interactions between poly-l-lysine-coated boron nitride nanotubes and C2C12 cells: Up-take, cytocompatibility, and differentiation. Int. J. Nanomed. 2010, 5, 285. [Google Scholar] [CrossRef]
- Ciofani, G.; del Turco, S.; Rocca, A.; de Vito, G.; Cappello, V.; Yamaguchi, M.; Li, X.; Mazzolai, B.; Basta, G.; Gemmi, M.; et al. Cytocompatibility evaluation of gum Arabic-coated ultra-pure boron nitride nanotubes on human cells. Nanomedicine 2014, 9, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Emanet, M.; Șen, O.; Çulha, M. Evaluation of born nitride nanotubes and hexagonal boron nitride as nanocarriers for cancer drugs. Nanomedicine 2017, 12, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, H.; Yan, T.; Huang, D.; Zhi, C.; Nakanishi, H.; Gao, X.D. Folate-conjugated boron nitride nanospheres for targeted delivery of anticancer drugs. Int. J. Nanomed. 2016, 11, 4573. [Google Scholar]
- Singh, B.; Kaur, G.; Singh, P.; Singh, K.; Kumar, B.; Vij, A.; Kumar, M.; Bala, R.; Meena, R.; Singh, A.; et al. Nanostructured boron nitride with high water dispersibility for boron neutron capture therapy. Sci. Rep. 2016, 6, 35535. [Google Scholar] [CrossRef] [PubMed]
- Mateti, S.; Wong, C.S.; Liu, Z.; Yang, W.; Li, Y.; Li, L.H.; Chen, Y. Biocompatibility of boron nitride nanosheets. Nano Res. 2018, 11, 334–342. [Google Scholar] [CrossRef]
- Weyermann, J.; Lochmann, D.; Zimmer, A. A practical note on the use of cytotoxicity assays. Int. J. Pharm. 2005, 288, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; Genchi, G.G.; D’Alessandro, D.; Odorico, M.; Mattoli, V.; Giorgi, M. Pilot in vivo toxicological investigation of boron nitride nanotubes. Int. J. Nanomed. 2012, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, H.; Tang, C.; Lei, S.; Shen, W.; Wang, C.; Wang, G.; Wang, Z.; Wang, L. Toxicity evaluation of boron nitride nanospheres and water-soluble boron nitride in Caenorhabditis elegans. Int. J. Nanomed. 2017, 12, 5941. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qi, W.; Tian, L.; Li, Z.; Miao, G.; An, W.; Liu, D.; Lin, J.; Zhang, X.; Wu, W. In vivo biodistribution and toxicity of highly soluble PEG-coated boron nitride in mice. Nanoscale Res. Lett. 2015, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Salvetti, A.; Rossi, L.; Iacopetti, P.; Li, X.; Nitti, S.; Pellegrino, T.; Mattoli, V.; Golberg, D.; Ciofani, G. In vivo biocompatibility of boron nitride nanotubes: Effects on stem cell biology and tissue regeneration in planarians. Nanomedicine 2015, 10, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.R.K.; Iyer, P.R. Applications of the green synthesized gold nanoparticles-antimicrobial activity, water purification system and drug delivery system. Nanosci. Technol. 2015, 2, 1–4. [Google Scholar] [CrossRef]
- Rattanata, N.; Daduang, S.; Wongwattanakul, M.; Leelayuwat, C.; Limpaiboon, T.; Lekphrom, R.; Sandee, A.; Boonsiri, P.; Chio-Srichan, S.; Daduang, J. Gold nanoparticles enhance the anticancer activity of gallic acid against cholangiocarcinoma cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 7143–7147. [Google Scholar] [CrossRef] [PubMed]
- Geetha, R.; Ashokkumar, T.; Tamilselvan, S.; Govindaraju, K.; Sadiq, M.; Singaravelu, G. Green synthesis of gold nanoparticles and their anticancer activity. Cancer Nano 2013, 4, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Feng, Q.; Chen, Y.; Shen, Y.; Su, Q.; Zhang, Y.; Zhou, X.; Cheng, Y. Comparison of two approaches for the attachment of a drug to gold nanoparticles and their anticancer activities. Mol. Pharm. 2016, 13, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lyalin, A.; Taketsugu, T. Catalytic activity of Au and Au2 on the h-BN surface: Adsorption and activation of O2. J. Phys. Chem. C 2012, 116, 9054–9062. [Google Scholar] [CrossRef]
- Lyalin, A.; Nakayama, A.; Uosaki, K.; Taketsugu, T. Functionalization of monolayer h-BN by a metal support for the oxygen reduction reaction. J. Phys. Chem. C 2013, 117, 21359–21370. [Google Scholar] [CrossRef]
- Lyalin, A.; Nakayama, A.; Uosaki, K.; Taketsugu, T. Adsorption and catalytic activation of the molecular oxygen on the metal supported h-BN. Top. Catal. 2014, 57, 1032–1041. [Google Scholar] [CrossRef]
- Gao, M.; Adachi, M.; Lyalin, A.; Taketsugu, T. Long range functionalization of h BN monolayer by carbon doping. J. Phys. Chem. C 2016, 120, 15993–16001. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett. 2003, 540, 3–6. [Google Scholar] [CrossRef]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z. Increased oxidative stress as a selective anticancer therapy. Oxid. Med. Cell. Longev. 2015, 2015, 294303. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.S.; Bicknell, R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: Its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001, 3, 323. [Google Scholar] [CrossRef] [PubMed]
- Gorbachev, R.V.; Riaz, I.; Nair, R.R.; Jalil, R.; Britnell, L.; Belle, B.D.; Hill, E.W.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Hunting for monolayer boron nitride: Optical and Raman signatures. Small 2011, 7, 465–468. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedrzejczak-Silicka, M.; Trukawka, M.; Dudziak, M.; Piotrowska, K.; Mijowska, E. Hexagonal Boron Nitride Functionalized with Au Nanoparticles—Properties and Potential Biological Applications. Nanomaterials 2018, 8, 605. https://doi.org/10.3390/nano8080605
Jedrzejczak-Silicka M, Trukawka M, Dudziak M, Piotrowska K, Mijowska E. Hexagonal Boron Nitride Functionalized with Au Nanoparticles—Properties and Potential Biological Applications. Nanomaterials. 2018; 8(8):605. https://doi.org/10.3390/nano8080605
Chicago/Turabian StyleJedrzejczak-Silicka, Magdalena, Martyna Trukawka, Mateusz Dudziak, Katarzyna Piotrowska, and Ewa Mijowska. 2018. "Hexagonal Boron Nitride Functionalized with Au Nanoparticles—Properties and Potential Biological Applications" Nanomaterials 8, no. 8: 605. https://doi.org/10.3390/nano8080605
APA StyleJedrzejczak-Silicka, M., Trukawka, M., Dudziak, M., Piotrowska, K., & Mijowska, E. (2018). Hexagonal Boron Nitride Functionalized with Au Nanoparticles—Properties and Potential Biological Applications. Nanomaterials, 8(8), 605. https://doi.org/10.3390/nano8080605




