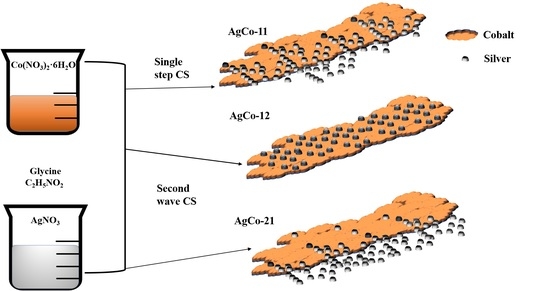
Surface Alloying in Silver-Cobalt through a Second Wave Solution Combustion Synthesis Technique
Abstract
:1. Introduction
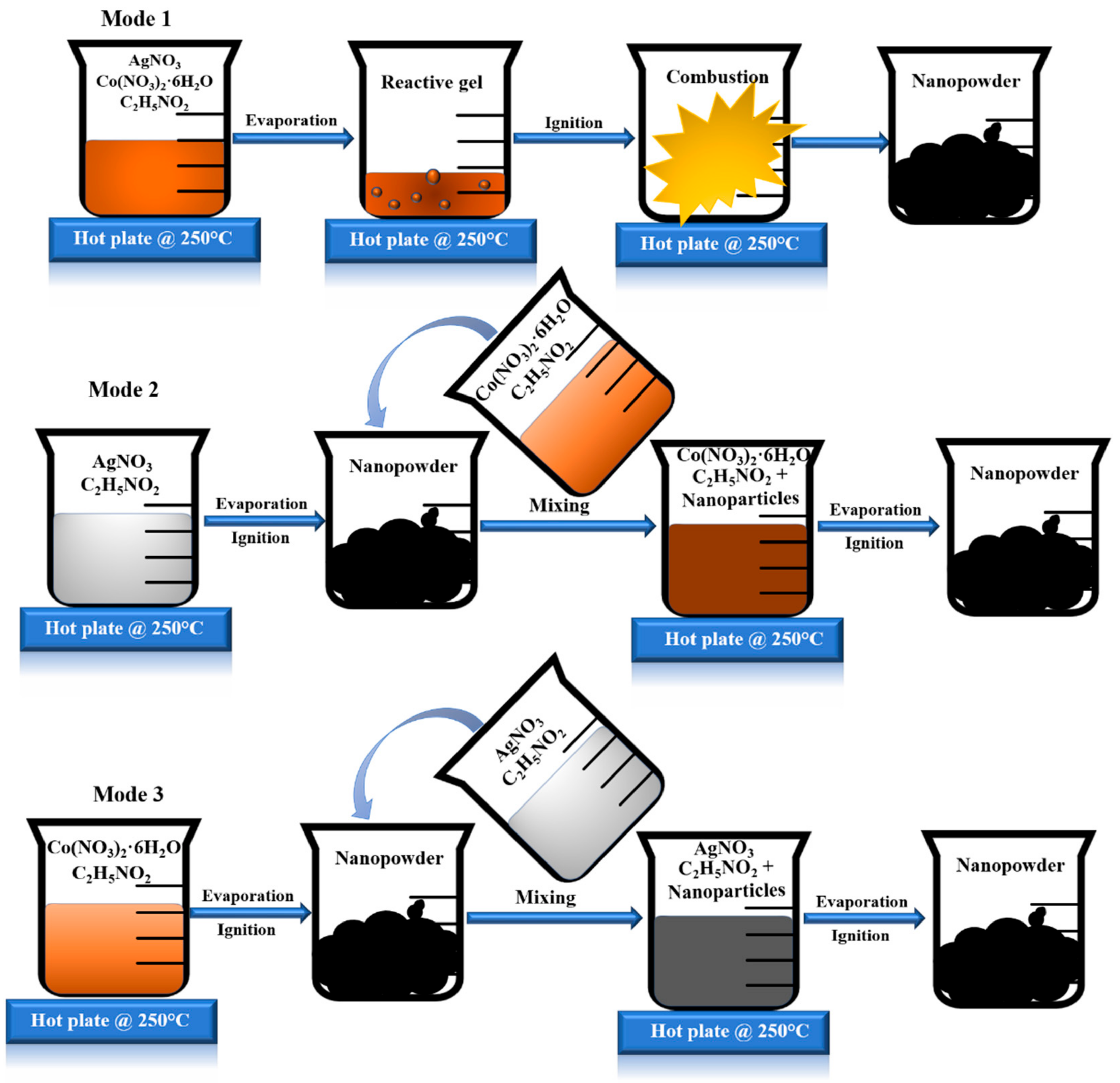
2. Experimental Section
2.1. Mode 1: AgCo-11
2.2. Mode 2: AgCo-12
2.3. Mode 3: AgCo-21
3. Material Characterization
4. Results and Discussion
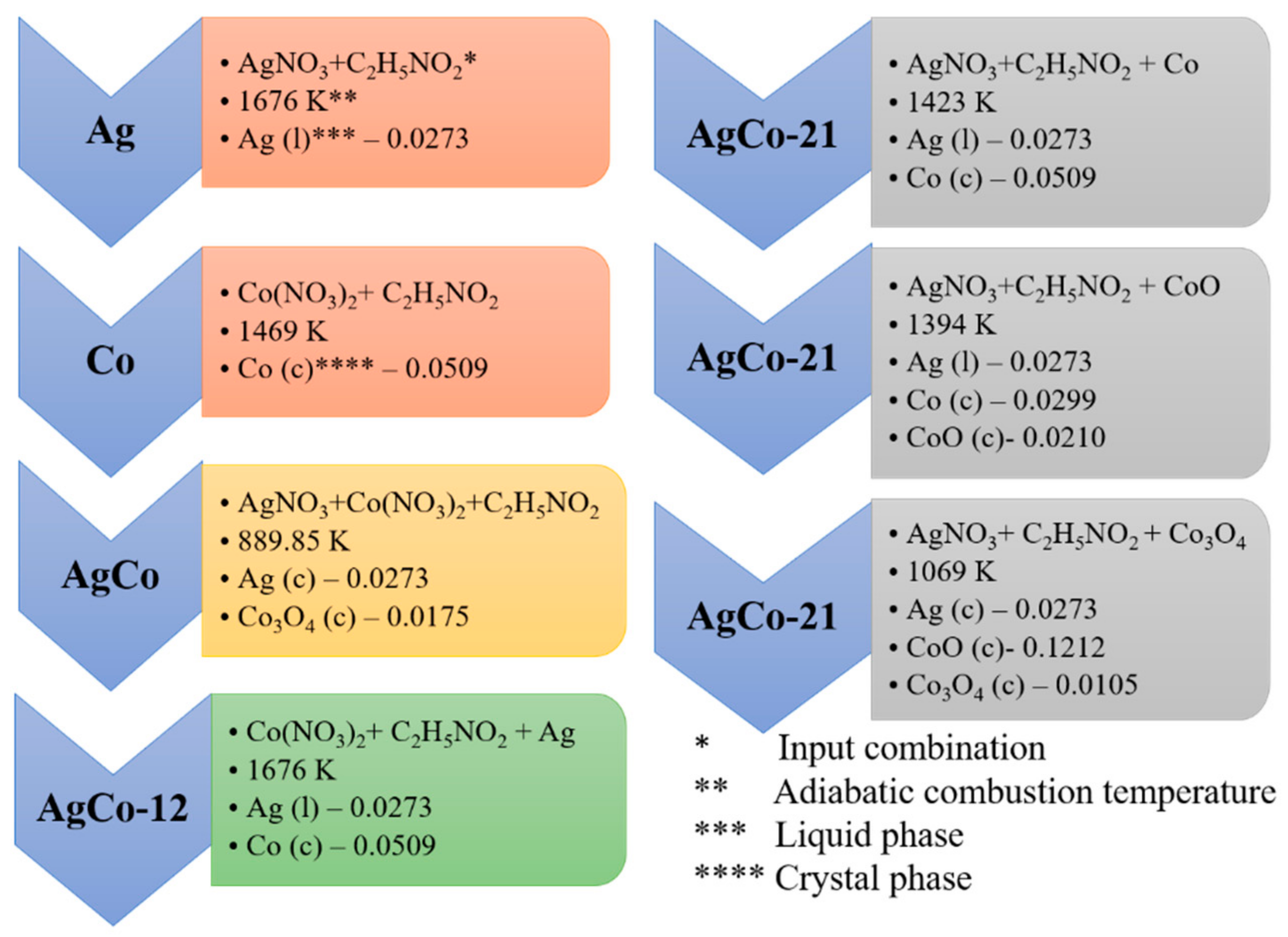
4.1. Thermodynamic Analysis
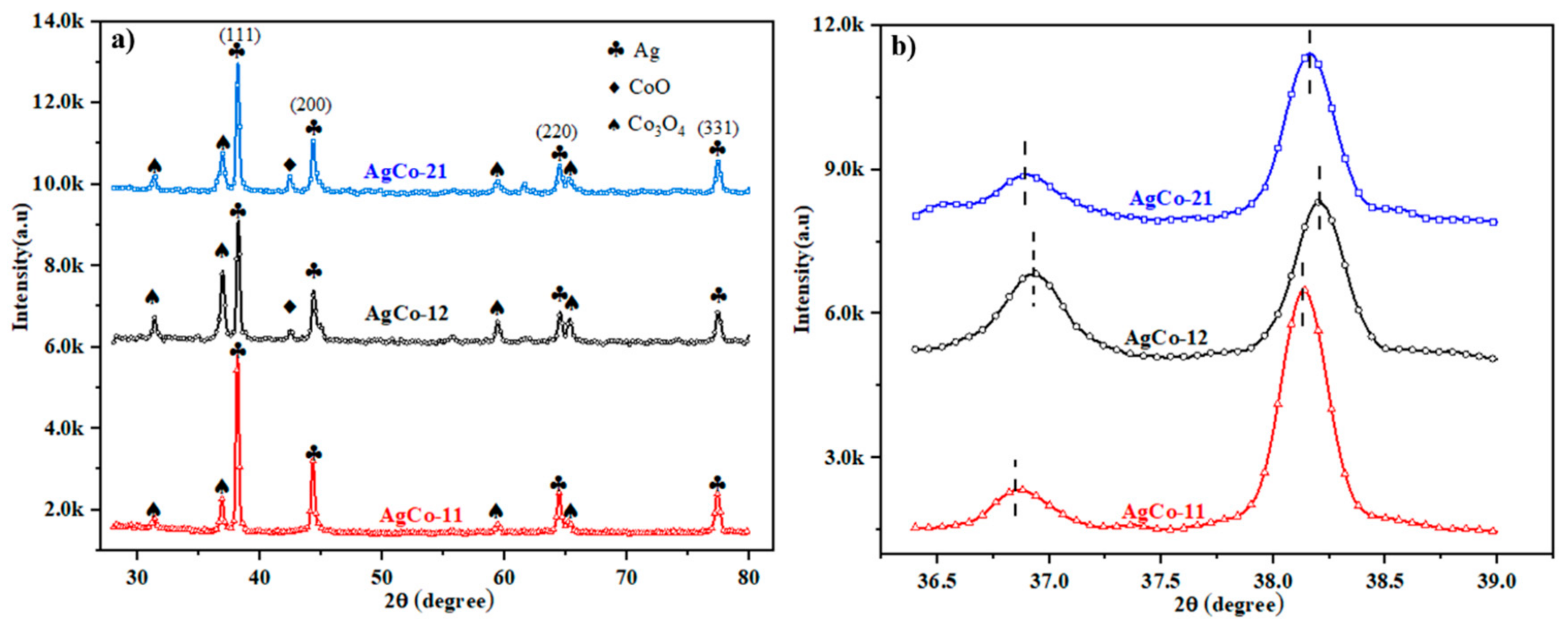
4.2. Experimental Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrer, D.; Torres-Castro, A.; Gao, X.; Sepulveda-Guzman, S.; Ortiz-Mendez, U.; Jose-Yacaman, M. Three-layer core/shell structure in Au−Pd bimetallic nanoparticles. Nano Lett. 2007, 7, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef] [PubMed]
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles—Novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Zhong, M.; Huang, C.; Wang, G. Hydrogen storage of Al-Li bimetal alloy nanostructures. J. Alloys Compd. 2017, 725, 388–392. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, L.; Li, X.; Xia, D. Structural and morphological characterization of gold–nickel electrocatalyst synthesized by taking advantage of the AuNi phase separation mechanism. J. Alloys Compd. 2010, 492, 83–87. [Google Scholar] [CrossRef]
- Rick, J.; Tsai, M.; Hwang, B.J. Biosensors incorporating bimetallic nanoparticles. Nanomaterials 2015, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Liang, X.; Chen, B. Porous Ni–Co bimetal oxides nanosheets and catalytic properties for CO oxidation. J. Alloys Compd. 2013, 574, 181–187. [Google Scholar] [CrossRef]
- Lu, D.; Yuan, L.; Chen, Z.; Zeng, R.; Cai, Y. Co-precipitation preparation of LiNi0.5Mn1.5O4 hollow hierarchical microspheres with superior electrochemical performance for 5 V Li-ion batteries. J. Alloys Compd. 2018, 730, 509–515. [Google Scholar] [CrossRef]
- Long, N.V.; Hien, T.D.; Asaka, T.; Ohtaki, M.; Nogami, M. Synthesis and characterization of Pt-Pd nanoparticles with core-shell morphology: Nucleation and overgrowth of the Pd shells on the as-prepared and defined Pt seeds. J. Alloys Compd. 2011, 509, 7702–7709. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Z.; Cui, R.; Wu, H.; Cheng, D. Structures, thermal stability, and chemical activity of crown-jewel-structured Pd-Pt nanoalloys. J. Phys. Chem. C 2014, 119, 10888–10895. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Y.; Zhao, Y.; Chu, H. Effect of the composition of lanthanide complexes on their luminescence enhancement by Ag@SiO2 core-shell nanoparticles. Nanomaterials 2018, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, E.; Soled, S.L.; Fiato, R.A.; Via, G.H. Bimetallic synergy in cobalt ruthenium Fischer-Tropsch synthesis catalysts. J. Catal. 1993, 143, 345–368. [Google Scholar] [CrossRef]
- Zafferoni, C.; Cioncoloni, G.; Foresti, M.L.; Dei, L.; Carretti, E.; Vizza, F.; Lavacchi, A.; Innocenti, M. Synergy of Cobalt and Silver Microparticles Electrodeposited on Glassy Carbon for the Electrocatalysis of the Oxygen Reduction Reaction: An Electrochemical Investigation. Molecules 2015, 20, 14386–14401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nairan, A.; Khan, U.; Iqbal, M.; Khan, M.; Javed, K.; Riaz, S.; Naseem, S.; Han, X. Structural and magnetic response in bimetallic core/shell magnetic nanoparticles. Nanomaterials 2016, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Salker, A.; Desai, M.F. Low-temperature nitric oxide reduction over silver-substituted cobalt oxide spinels. Catal. Sci. Technol. 2016, 6, 430–433. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, C.; Ming, M.; Yang, Y.; Xu, B.; Wang, Y.; Zhang, Y.; Wu, J.; Fan, G. In Situ Formation of AgCo Stabilized on Graphitic Carbon Nitride and Concomitant Hydrolysis of Ammonia Borane to Hydrogen. Nanomaterials 2018, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Munoz-Berbel, X.; Vigués, N.; Macanás, J.; Munoz, M.; Mas, J.; Muraviev, D.N. Characterization of fibrous polymer silver/cobalt nanocomposite with enhanced bactericide activity. Langmuir 2011, 28, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.M.; Baltruschat, H.; Wittmaier, D.; Friedrich, K.A. A highly efficient bifunctional catalyst for alkaline air-electrodes based on a Ag and Co3O4 hybrid: RRDE and online DEMS insights. Electrochim. Acta 2015, 151, 332–339. [Google Scholar] [CrossRef]
- Holewinski, A.; Idrobo, J.; Linic, S. High-performance Ag-Co alloy catalysts for electrochemical oxygen reduction. Nat. Chem. 2014, 6, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.; de Castro, J.; Ticianelli, E.A. Silver-cobalt bimetallic particles for oxygen reduction in alkaline media. J. Power Sources 2006, 161, 806–812. [Google Scholar] [CrossRef]
- Gulari, E.; Güldür, Ç.; Srivannavit, S.; Osuwan, S. Co oxidation by silver cobalt composite oxide. Appl. Catal. A Gen. 1999, 182, 147–163. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Saleh, M.A.H.; van den Broeke, L.J.P. Cellulose assisted combustion synthesis of porous Cu–Ni nanopowders. RSC Adv. 2015, 5, 28703–28712. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Bhosale, R.R.; Saleh, M.A.H.; Ghosh, U.K.; Al-Marri, M.; Almomani, F.A.; Khader, M.M.; Tarlochan, F. Cobalt oxide nanopowder synthesis using cellulose assisted combustion technique. Ceram. Int. 2016, 42, 12771–12777. [Google Scholar] [CrossRef]
- Kumar, A.; Cross, A.; Manukyan, K.; Bhosale, R.; van den Broeke, L.; Miller, J.; Mukasyan, A.; Wolf, E. Combustion synthesis of copper–nickel catalysts for hydrogen production from ethanol. Chem. Eng. J. 2015, 278, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Mukasyan, A.; Wolf, E. Combustion synthesis of Ni, Fe and Cu multi-component catalysts for hydrogen production from ethanol reforming. Appl. Catal. A Gen. 2011, 401, 20–28. [Google Scholar] [CrossRef]
- Kumar, A.; Wolf, E.; Mukasyan, A. Solution combustion synthesis of metal nanopowders: Copper and copper/nickel alloys. AIChE J. 2011, 57, 3473–3479. [Google Scholar] [CrossRef]
- Kumar, A.; Wolf, E.; Mukasyan, A. Solution combustion synthesis of metal nanopowders: Nickel—Reaction pathways. AIChE J. 2011, 57, 2207–2214. [Google Scholar] [CrossRef]
- Rogachev, A.; Mukasyan, A. Combustion of heterogeneous nanostructural systems (Review). Combust. Explos. Shock Waves 2010, 46, 243–266. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Martirosyan, K.S. Combustion of Heterogeneous Systems: Fundamentals and Applications for Materials Synthesis 2007; Transworld Research Network: Kerala, India, 2007. [Google Scholar]
- Aruna, S.T.; Mukasyan, A.S. Combustion synthesis and nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- Małecka, B.; Łącz, A.; Drożdż, E.; Małecki, A. Thermal decomposition of d-metal nitrates supported on alumina. J. Therm. Anal. Calorim. 2015, 119, 1053–1061. [Google Scholar] [CrossRef]
- Waterhouse, G.I.; Bowmaker, G.A.; Metson, J.B. The thermal decomposition of silver (I, III) oxide: A combined XRD, FT-IR and Raman spectroscopic study. Phys. Chem. Chem. Phys. 2001, 3, 3838–3845. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Goya, M.; Arevalo, M.; Rodríguez, J.; Pastor, E. Carbon supported Ag and Ag-Co catalysts tolerant to methanol and ethanol for the oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2016, 41, 19789–19798. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Sun, K.; Li, W. Pd–Ni electrocatalysts for efficient ethanol oxidation reaction in alkaline electrolyte. Int. J. Hydrogen Energy 2011, 36, 12686–12697. [Google Scholar] [CrossRef]
- Joo, Y.; Ahmed, M.S.; Han, H.S.; Jeon, S. Preparation of electrochemically reduced graphene oxide-based silver-cobalt alloy nanocatalysts for efficient oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 21751–21761. [Google Scholar] [CrossRef]
- Jamale, A.P.; Shanmugam, S.; Bhosale, C.; Jadhav, L. Physiochemical properties of combustion synthesized La0.6Sr0.4Co0.8Fe0.2O3—δ perovskite: A role of fuel to oxidant ratio. Mater. Sci. Semicond. Process. 2015, 40, 855–860. [Google Scholar] [CrossRef]
- Jadhav, L.; Patil, S.; Jamale, A.; Chavan, A. Solution combustion synthesis: Role of oxidant to fuel ratio on powder properties. Mater. Sci. Forum 2013, 757, 85–98. [Google Scholar] [CrossRef]
- Farhadi, S.; Safabakhsh, J.; Zaringhadam, P. Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (Co3O4) nanoparticles. J. Nanostruct. Chem. 2013, 3, 69. [Google Scholar] [CrossRef]
- Makhlouf, M.T.; Abu-Zied, B.; Mansoure, T. Direct fabrication of cobalt oxide nano-particles employing glycine as a combustion fuel. Phys. Chem. 2012, 2, 86–93. [Google Scholar] [CrossRef]
- Zhou, W.; Shao, Z.; Ran, R.; Gu, H.; Jin, W.; Xu, N. LSCF Nanopowder from cellulose-glycine-nitrate process and its application in intermediate-temperature solid-oxide fuel cells. J. Am. Ceram. Soc. 2008, 91, 1155–1162. [Google Scholar] [CrossRef]
- Xu, R.; Zeng, H.C. Self-generation of tiered surfactant superstructures for one-pot synthesis of Co3O4 nanocubes and their close-and non-close-packed organizations. Langmuir 2004, 20, 9780–9790. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.K.; Rao, G.R. Effect of microwave on the nanowire morphology, optical, magnetic, and pseudocapacitance behavior of Co3O4. J. Phys. Chem. C 2011, 115, 25543–25556. [Google Scholar] [CrossRef]
- Mailu, S.N.; Waryo, T.T.; Ndangili, P.M.; Ngece, F.R.; Baleg, A.A.; Baker, P.G.; Iwuoha, E.I. Determination of anthracene on Ag–Au alloy nanoparticles/overoxidized-polypyrrole composite modified glassy carbon electrodes. Sensors 2010, 10, 9449–9465. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Cheng, H.; Hsu, H.; Lin, L.; Hsieh, W. Band gap variation of size-controlled ZnO quantum dots synthesized by sol-gel method. Chem. Phys. Lett. 2005, 409, 208–211. [Google Scholar] [CrossRef]
- Lim, S.P.; Pandikumar, A.; Huang, N.M.; Lim, H.N. Enhanced photovoltaic performance of silver@ titania plasmonic photoanode in dye-sensitized solar cells. RSC Adv. 2014, 4, 38111–38118. [Google Scholar] [CrossRef]
- Srivastava, M.; Ojha, A.K.; Chaubey, S.; Materny, A. Synthesis and optical characterization of nanocrystalline NiFe2O4 structures. J. Alloys Compd. 2009, 481, 515–519. [Google Scholar] [CrossRef]
- Mahmoud, K.H. Synthesis and spectroscopic investigation of cobalt oxide nanoparticles. Polym. Compos. 2016, 37, 1881–1885. [Google Scholar] [CrossRef]
- Dhas, C.R.; Venkatesh, R.; Jothivenkatachalam, K.; Nithya, A.; Benjamin, B.S.; Raj, A.M.E.; Jeyadheepan, K.; Sanjeeviraja, C. Visible light driven photocatalytic degradation of Rhodamine B and Direct Red using cobalt oxide nanoparticles. Ceram. Int. 2015, 41, 9301–9313. [Google Scholar] [CrossRef]
- Pang, M.; Hu, J.; Zeng, H.C. Synthesis, morphological control, and antibacterial properties of hollow/solid Ag2S/Ag heterodimers. J. Am. Chem. Soc. 2010, 132, 10771–10785. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jewell, S.; She, Y.; Leung, M.K. In situ deposition of Ag–Ag2 S hybrid nanoparticles onto TiO2 nanotube arrays towards fabrication of photoelectrodes with high visible light photoelectrochemical properties. Phys. Chem. Chem. Phys. 2014, 16, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef] [PubMed]
- Ferraria, A.M.; Carapeto, A.P.; Rego, D.; Botelho, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Liu, J.; Zou, S.; Xiao, L.; Fan, J. Well-dispersed bimetallic nanoparticles confined in mesoporous metal oxides and their optimized catalytic activity for nitrobenzene hydrogenation. Catal. Sci. Technol. 2014, 4, 441–446. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Chao, D.; Baikie, T.; Bai, L.; Chen, S.; Zhao, Y.; Sum, T.C.; Lin, J.; Shen, Z. Hierarchical porous LiNi1/3Co1/3Mn1/3O2 nano-/micro spherical cathode material: Minimized cation mixing and improved Li mobility for enhanced electrochemical performance. Sci. Rep. 2016, 6, 25771. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, Y.; Deng, J.; Yang, J.; Zhao, X.; Han, Z.; Zhang, K.; Dai, H. Insights into the active sites of ordered mesoporous cobalt oxide catalysts for the total oxidation of O-xylene. J. Catal. 2017, 352, 282–292. [Google Scholar] [CrossRef]
- Hashimoto, S.; Sugie, T.; Zhang, Z.; Yamashita, K.; Noda, M. Effects of final annealing in oxygen on characteristics of BaTiO3 thin films for resistance random access memory. Jpn. J. Appl. Phys. 2015, 54, 10NA12. [Google Scholar] [CrossRef]
- Tian, J.; Gao, H.; Kong, H.; Yang, P.; Zhang, W.; Chu, J. Influence of transition metal doping on the structural, optical, and magnetic properties of TiO2 films deposited on Si substrates by a sol-gel process. Nanoscale Res. Lett. 2013, 8, 533. [Google Scholar] [CrossRef] [PubMed]
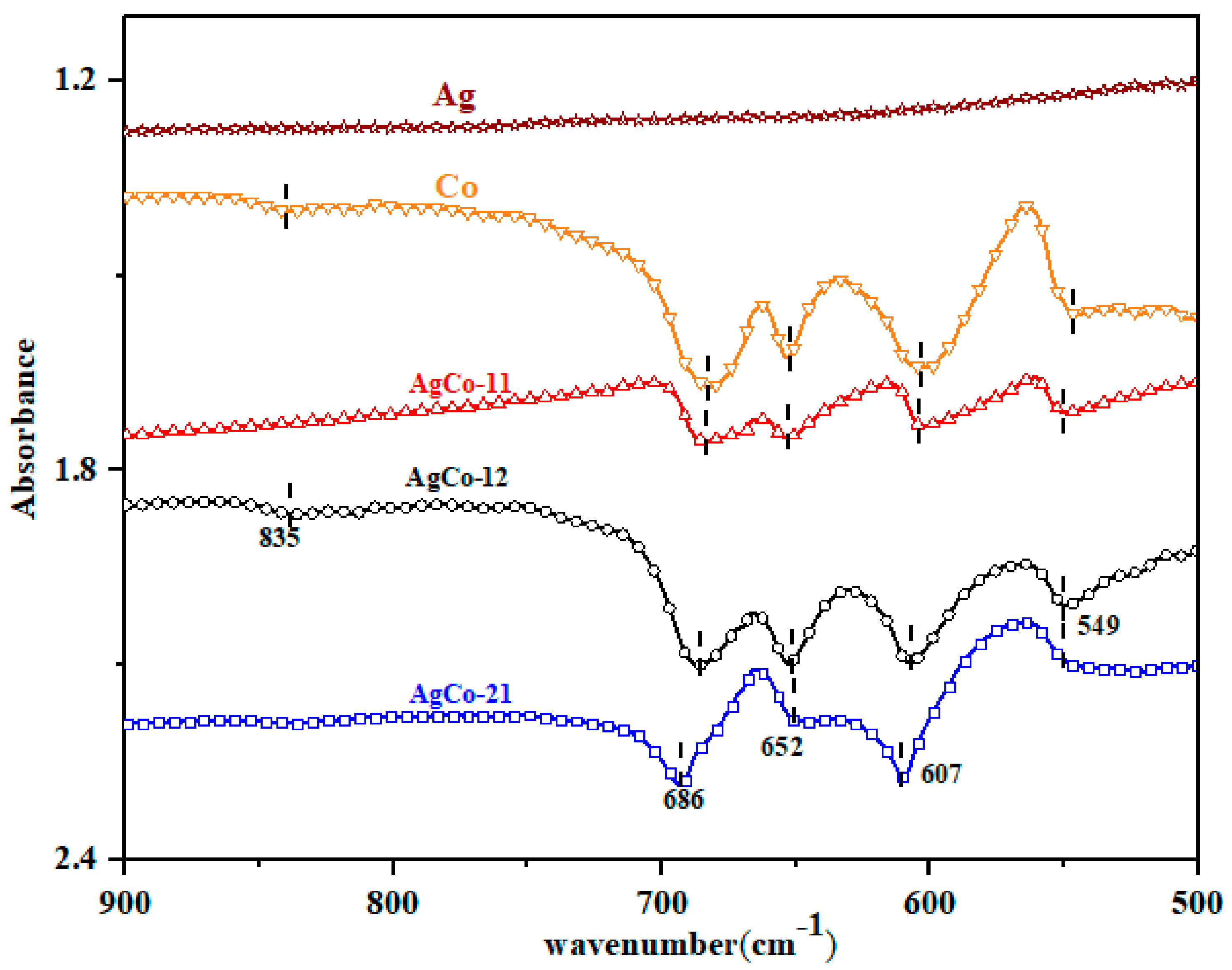

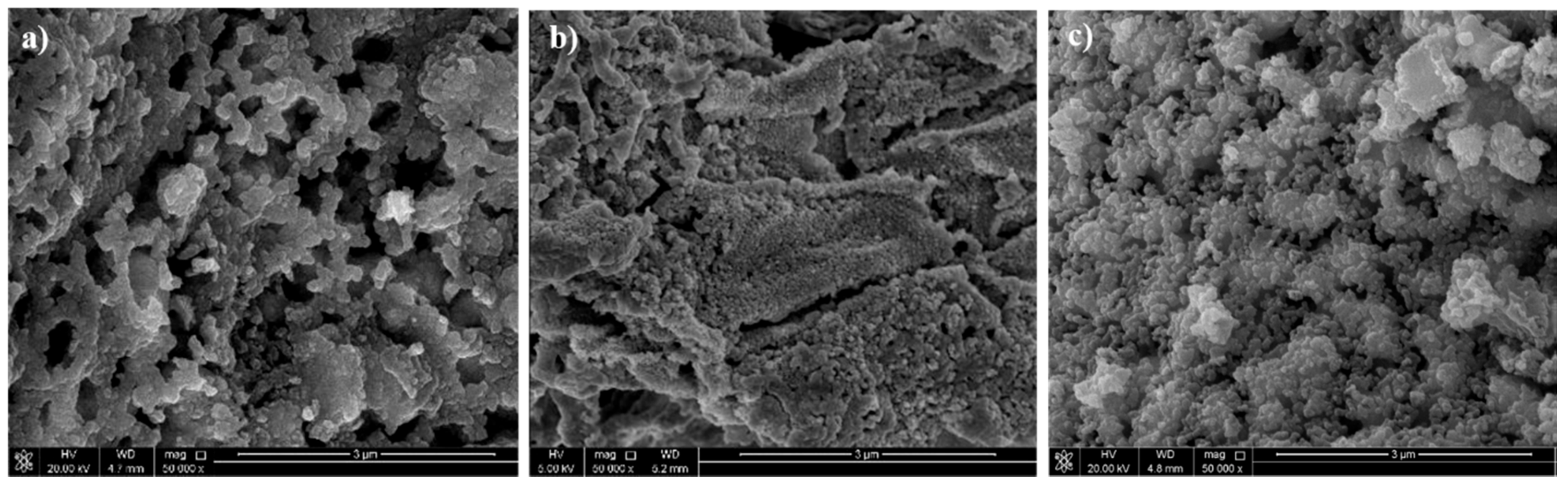
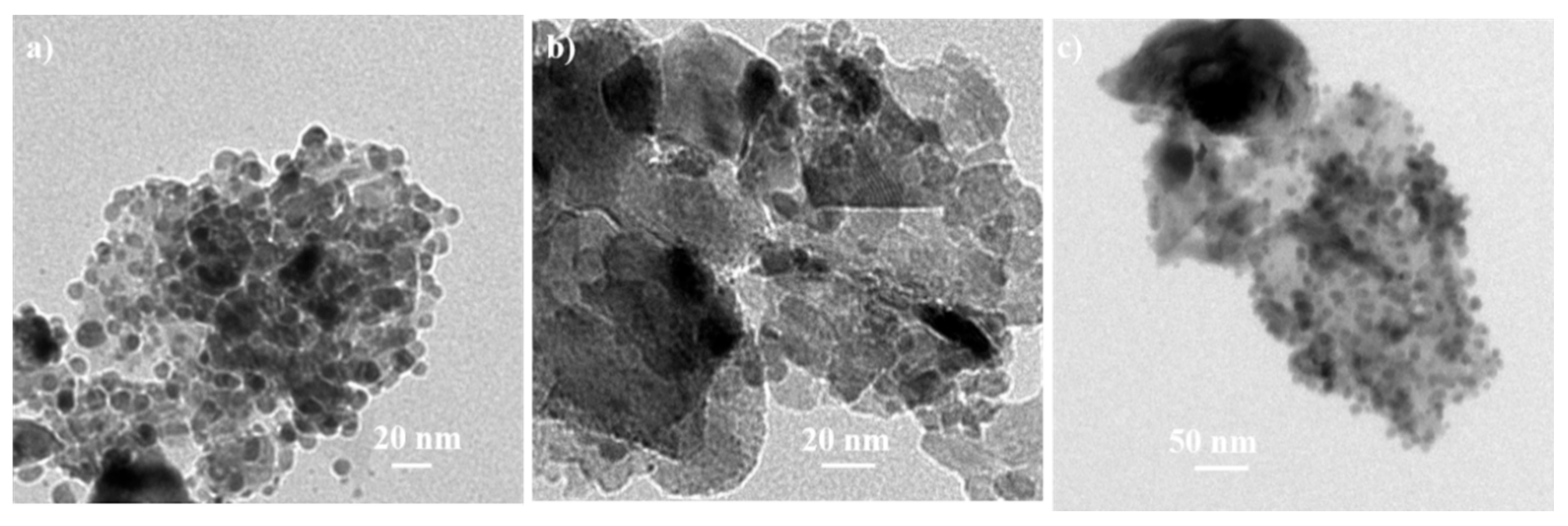
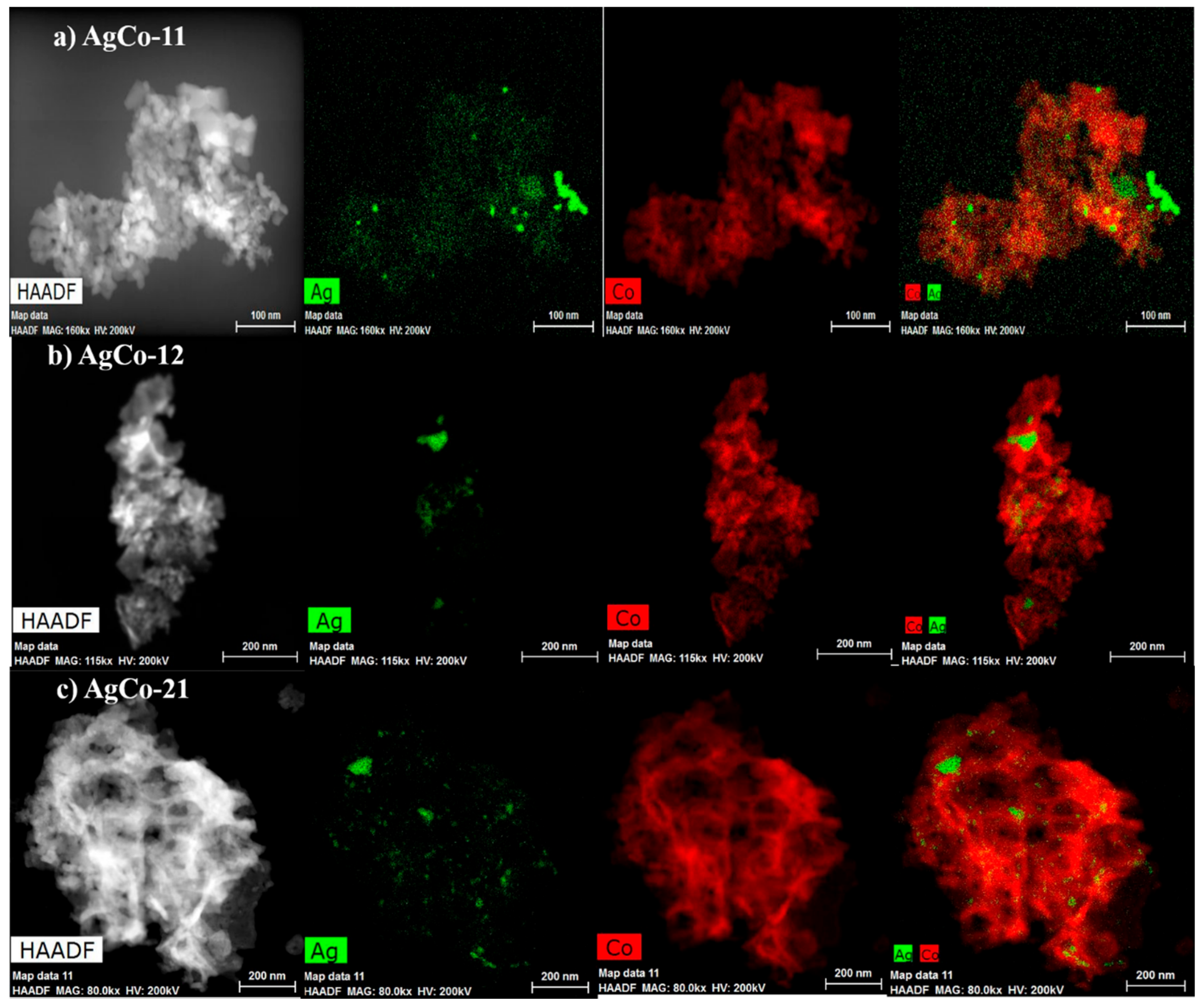
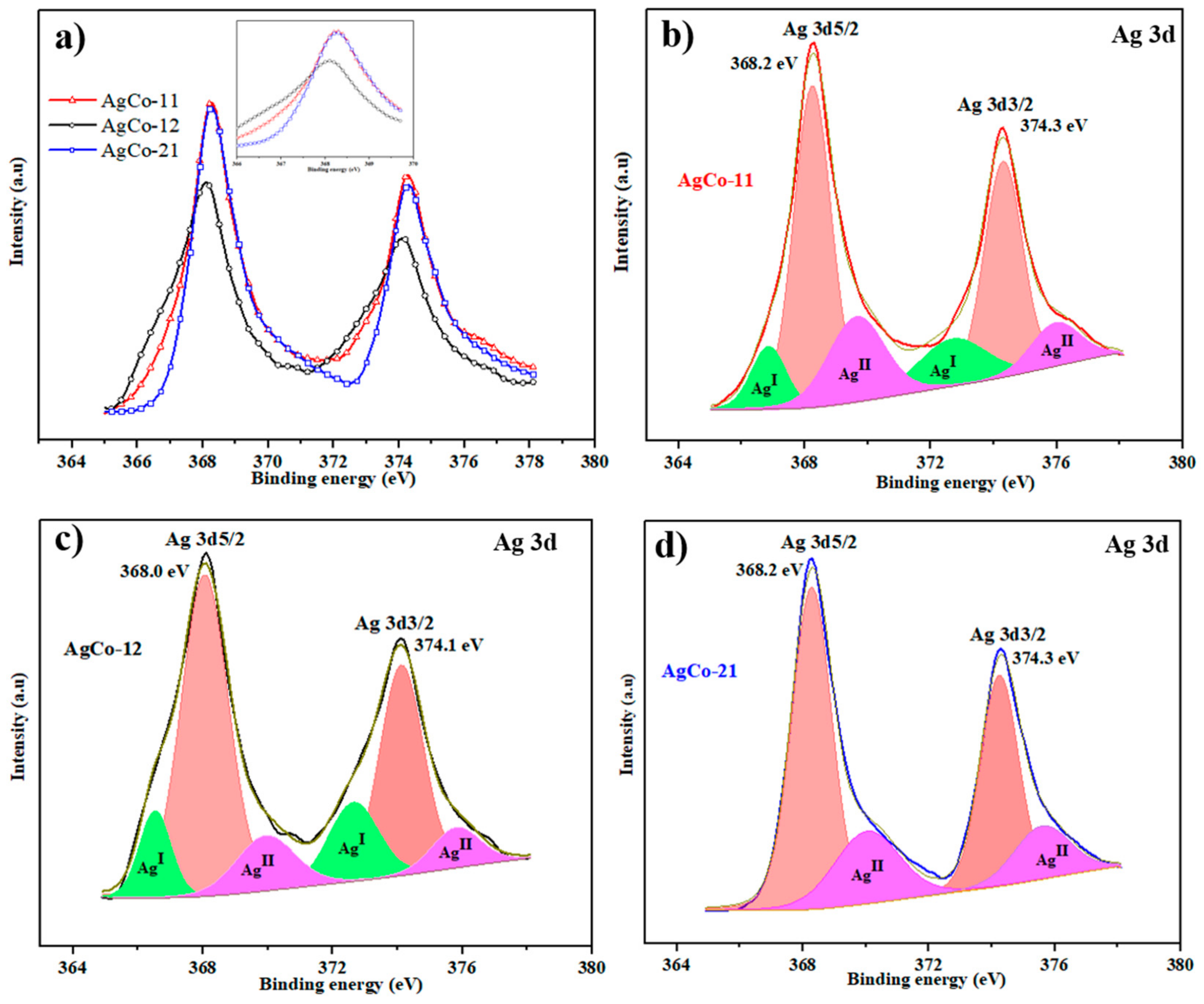
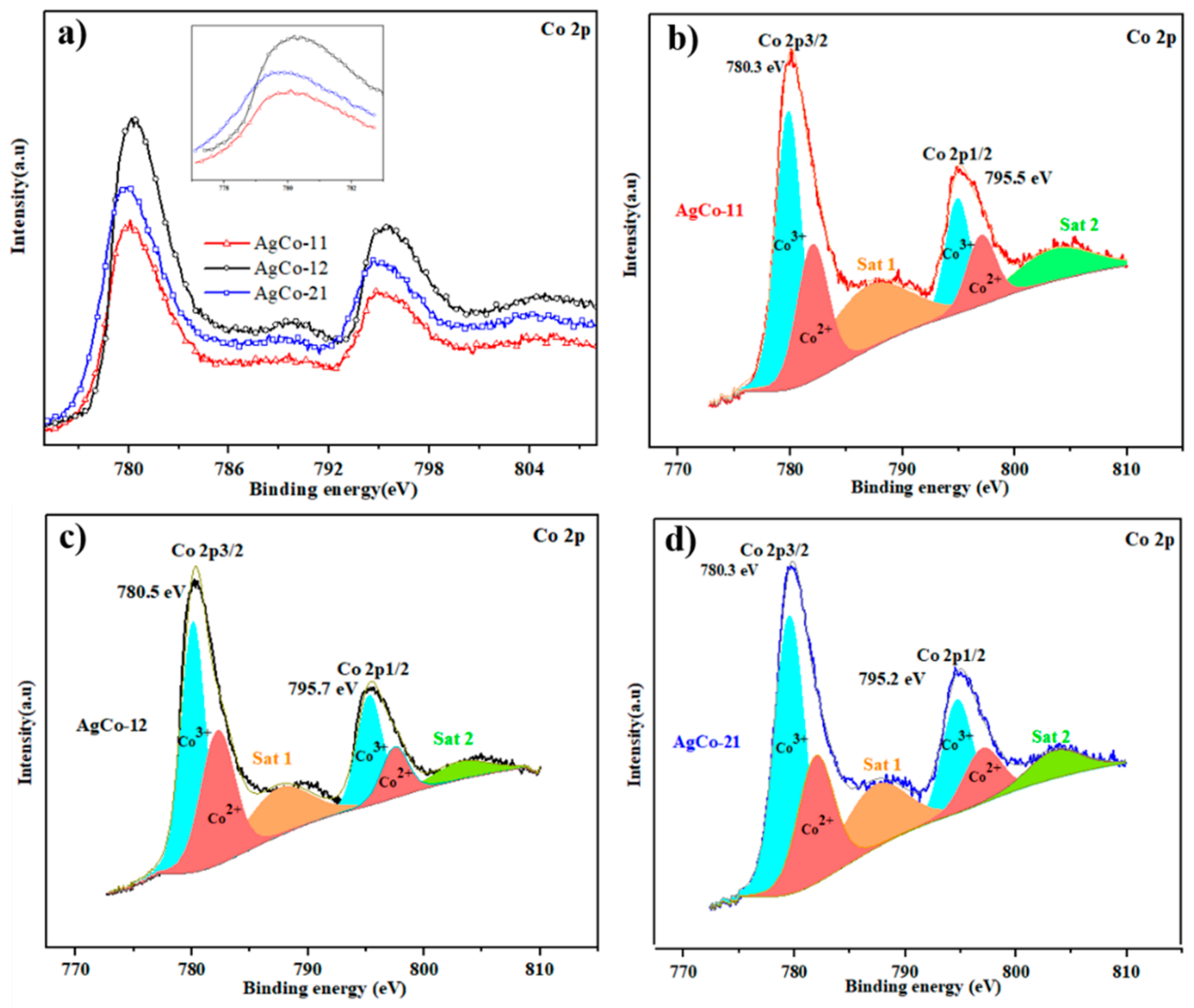
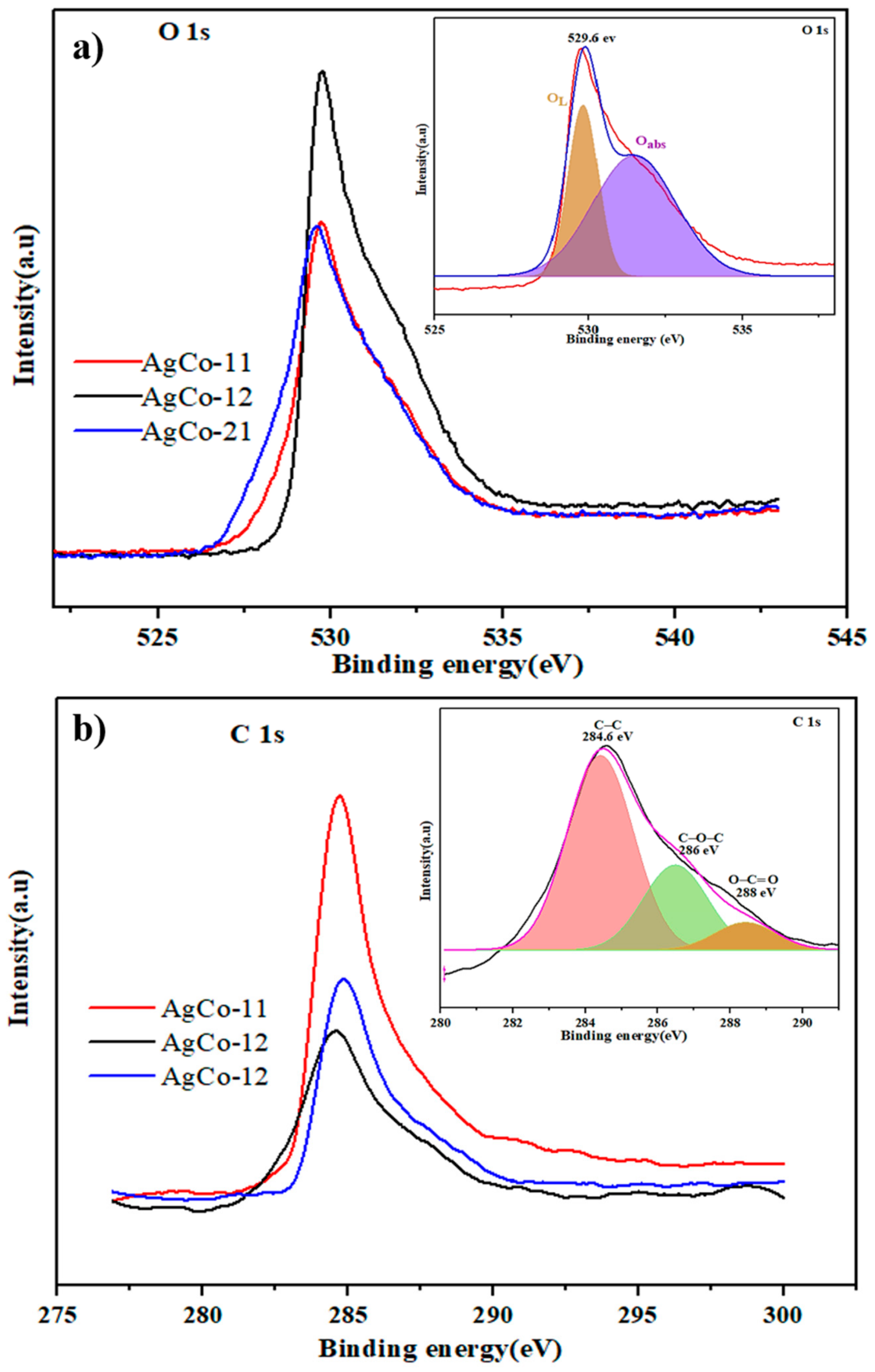
| Alloys | Ag 3d5/2 | Ag 3d3/2 | ||||
|---|---|---|---|---|---|---|
| B.E. (eV) | Area | %Area | B.E. (eV) | Area | %Area | |
| AgCo-11 | 368.2 | 4743.26 | 34.51 | 374.2 | 3387 | 24.64 |
| AgCo-12 | 368.02 | 4552.08 | 39.90 | 374.0 | 2974 | 26.07 |
| AgCo-21 | 368.27 | 5488.33 | 44.73 | 374.2 | 3448 | 28.13 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashok, A.; Kumar, A.; Tarlochan, F. Surface Alloying in Silver-Cobalt through a Second Wave Solution Combustion Synthesis Technique. Nanomaterials 2018, 8, 604. https://doi.org/10.3390/nano8080604
Ashok A, Kumar A, Tarlochan F. Surface Alloying in Silver-Cobalt through a Second Wave Solution Combustion Synthesis Technique. Nanomaterials. 2018; 8(8):604. https://doi.org/10.3390/nano8080604
Chicago/Turabian StyleAshok, Anchu, Anand Kumar, and Faris Tarlochan. 2018. "Surface Alloying in Silver-Cobalt through a Second Wave Solution Combustion Synthesis Technique" Nanomaterials 8, no. 8: 604. https://doi.org/10.3390/nano8080604
APA StyleAshok, A., Kumar, A., & Tarlochan, F. (2018). Surface Alloying in Silver-Cobalt through a Second Wave Solution Combustion Synthesis Technique. Nanomaterials, 8(8), 604. https://doi.org/10.3390/nano8080604