Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity
Abstract
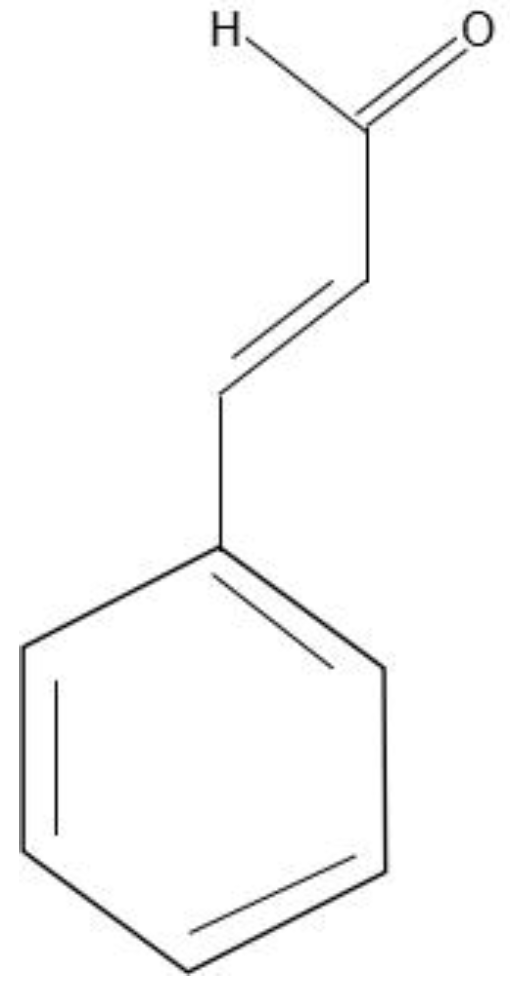
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Ion-Exchanged Permutite and Ag+/Zn2+-Permutite/CEO
2.3. Characterization and Measurement
2.4. Anti-Discoloration Property Assays and Nitrogen Physisorption
2.5. FT-IR Spectra and DSC Curve of Permutite Samples
2.6. The Loading Capacity and Release Behavior
2.7. Antimicrobial Assays
2.8. Extension of the Shelf Life of Chinese Bayberry Fruit and Migration Security Tests
2.9. Statistical Analysis
3. Results
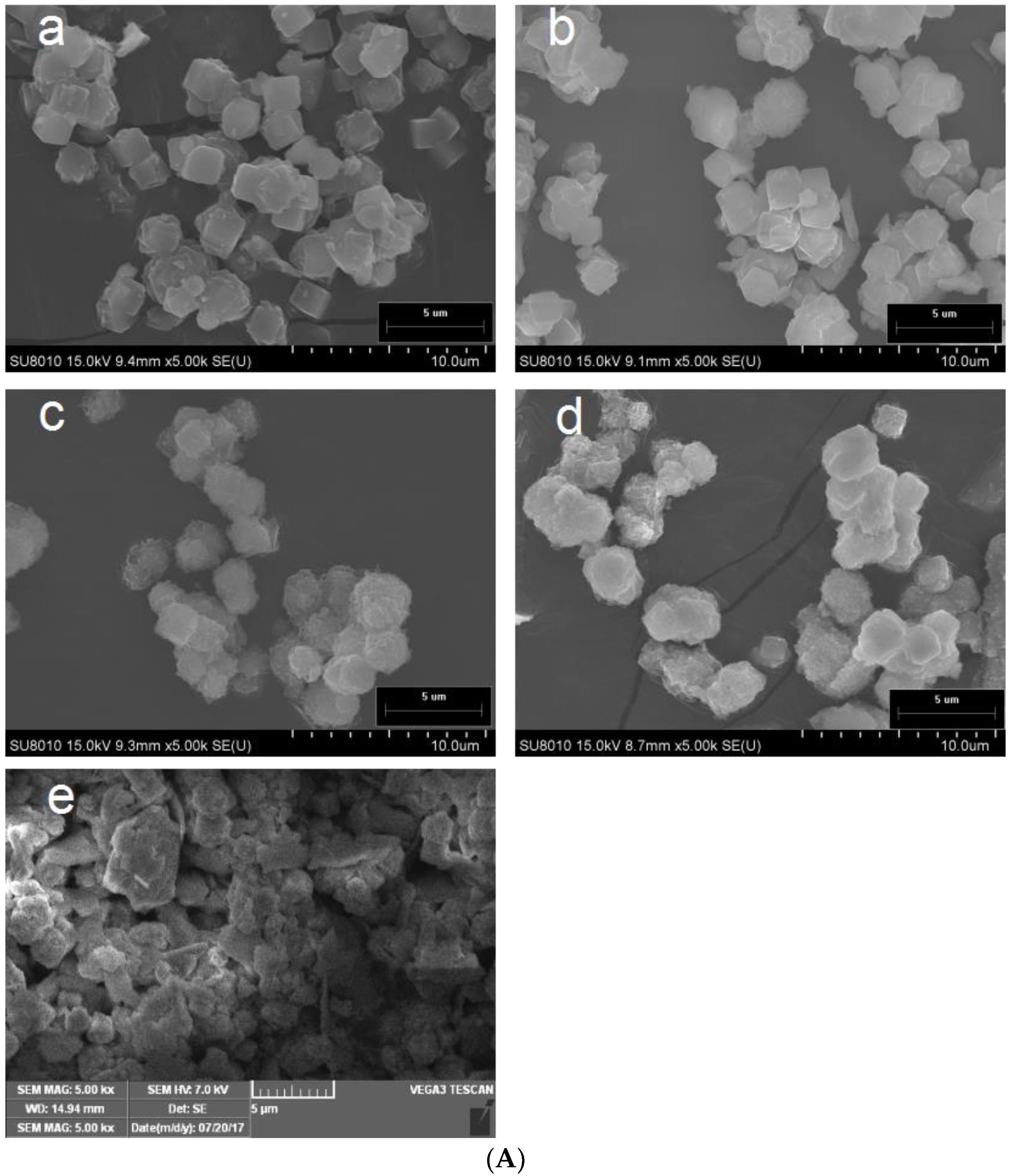
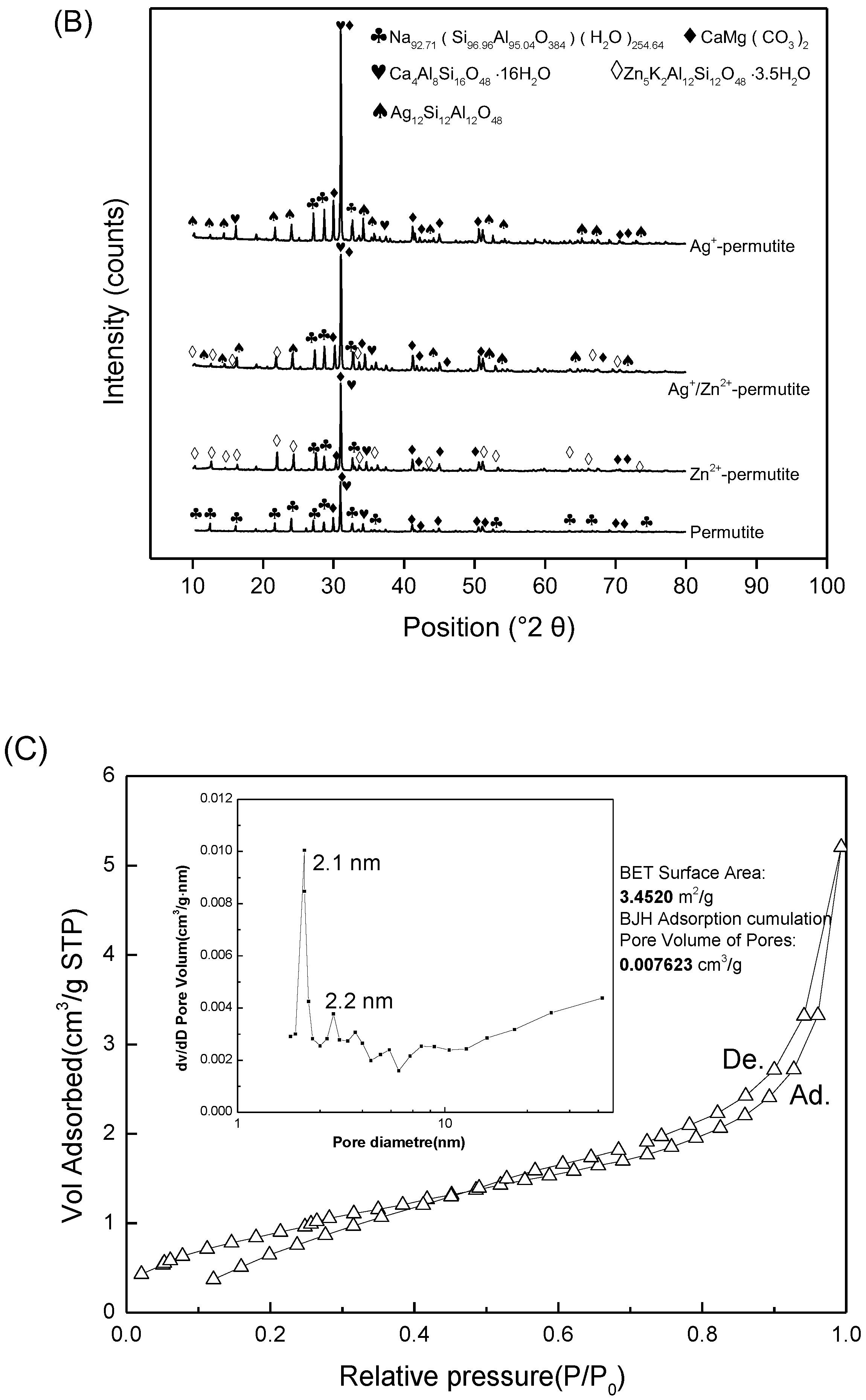
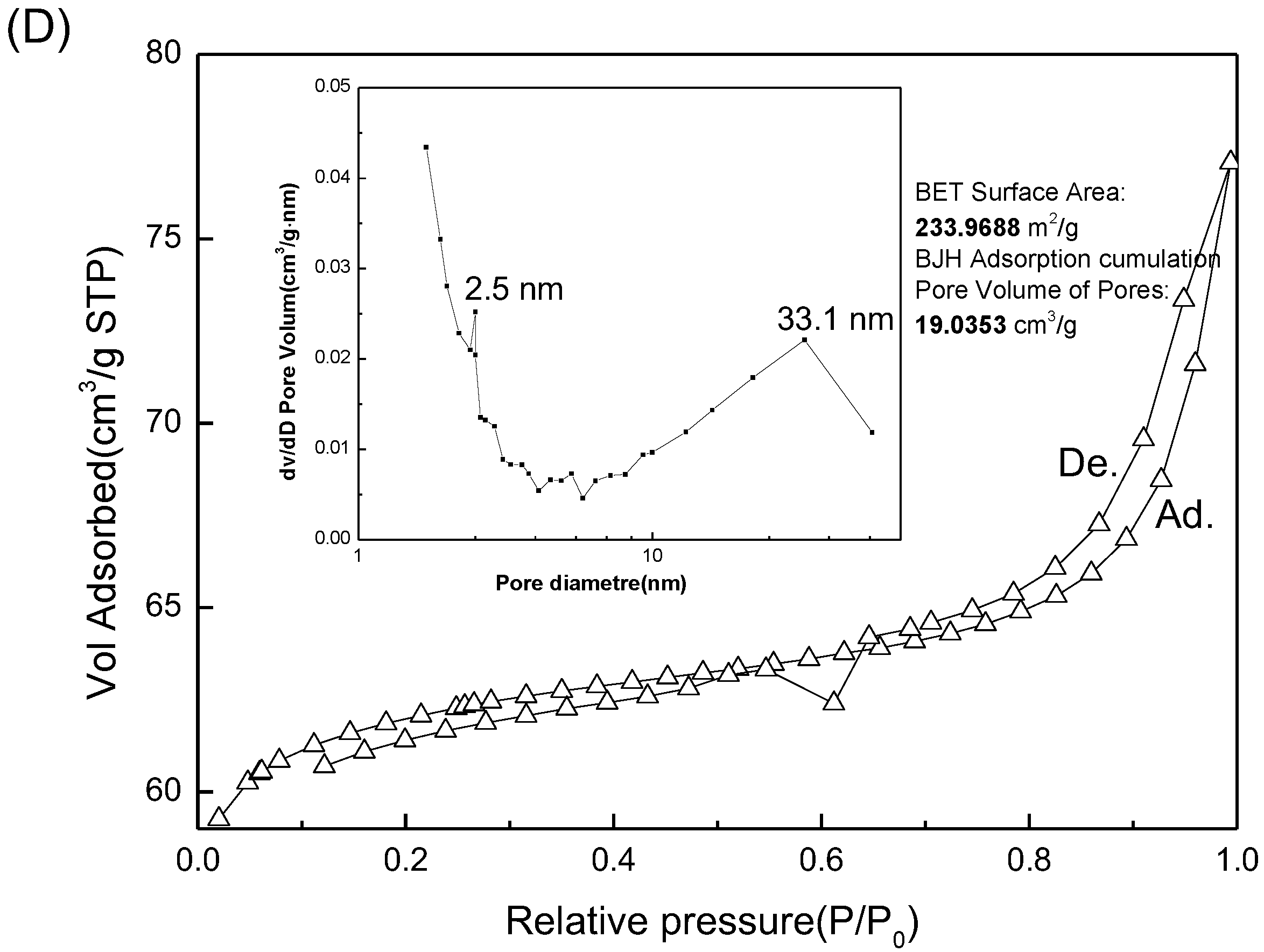
3.1. Permutite Sample Characterization
3.2. Characterization of Ag+/Zn2+-Permutite/CEO
3.3. Release Behavior of Ag+/Zn2+-Permutite/CEO
3.4. Antimicrobial Activity of Pads
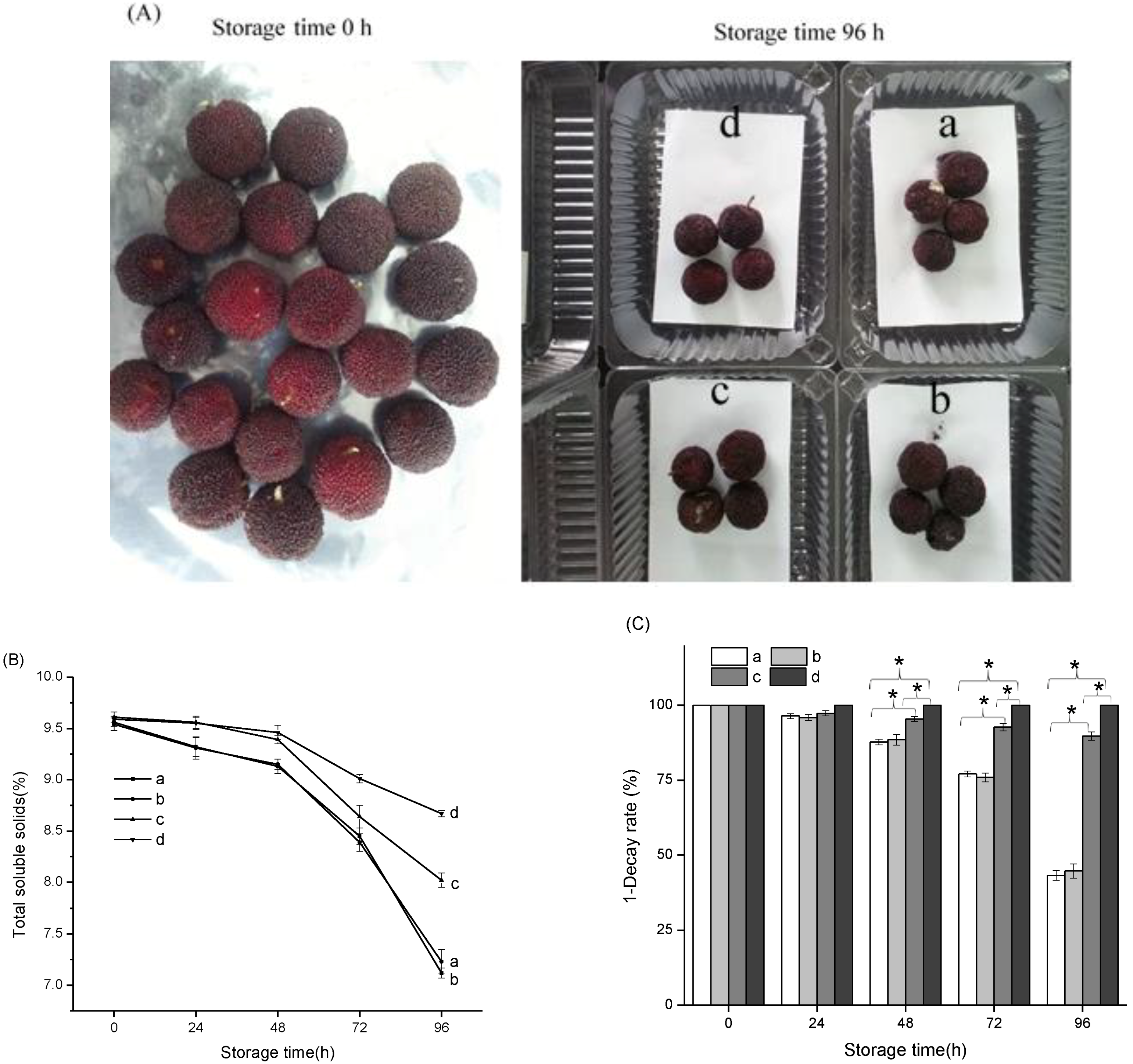
3.5. Application of the Antimicrobial Pads in Chinese Bayberry Fruit
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, L.; Zhao, C.J.; Zhang, Y.D.; Yao, J.F.; Yang, W.J.; Hu, Q.H.; Wang, C.L.; Cao, C.J. Effect of stable antimicrobial nano-silver packaging on inhibiting mildew and in storage of rice. Food Chem. 2017, 215, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Han, S.S. Dual-crosslinked poly(vinyl alcohol)/sodium alginate/silver nanocomposite beads—A promising antimicrobial material. Food Chem. 2017, 234, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Yan, Z.P.; Chen, H.J.; Xiao, J. Electrospun poly(vinyl alcohol)/permutite fibrous film loaded with cinnamaldehyde for active food packaging. J. Appl. Polym. Sci. 2018, 135, 46117. [Google Scholar] [CrossRef]
- Shao, P.; Niu, B.; Chen, H.J.; Sun, P.L. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2018, 107, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Inggrid, M.; Leong, S.D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Krepker, M.; Shemesh, R.; Poleg, Y.D.; Kashi, Y.; Vaxman, A.; Segal, E. Active food packaging films with synergistic antimicrobial activity. Food Control 2017, 76, 117–126. [Google Scholar] [CrossRef]
- Martínezabad, A.; Sánchez, G.; Lagaron, J.M.; Ocio, M.J. Ligands affecting silver antimicrobial efficacy on Listeria monocytogenes and Salmonella enterica. Food Chem. 2013, 139, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, J.L.; Palomares, A.E.; Rey, F.; Valencia, S.; Palou, L.; Pérez-Gago, M.B. Ag-zeolites as fungicidal material: Control of citrus green mold caused by Penicillium digitatum. Microporous Mesoporous Mater. 2017, 254, 69–76. [Google Scholar] [CrossRef]
- Ferreira, L.; Guedes, J.F.; Almeidaaguiar, C.; Fonseca, A.M.; Neves, I.C. Microbial growth inhibition caused by Zn/Ag-Y zeolite materials with different amounts of silver. Colloids Surf. B 2016, 142, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Masters, A.F.; Maschmeyer, T. Zeolites-From curiosity to cornerstone. Microporous Mesoporous Mater. 2011, 142, 423–438. [Google Scholar] [CrossRef]
- Demirci, S.; Ustaoğlu, Z.; Yılmazer, G.A.; Sahin, F.; Baç, N. Antimicrobial properties of zeolite-X and zeolite-A ion-exchanged with silver, copper, and zinc against a broad range of microorganisms. Biotechnol. Appl. Biochem. 2014, 172, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Høiby-Pettersen, G.S.; Halvorsen, C.K.; Langsrud, S. Antibacterial activity of cutting boards containing silver. Food Control 2012, 28, 118–121. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Physicochemical properties of gelatin/silver nanoparticle antimicrobial composite films. Food Chem. 2014, 148, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Echegoyen, Y.; Nerín, C. Performance of an active paper based on cinnamon essential oil in mushrooms quality. Food Chem. 2015, 170, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Nobuyuki Kozukue, A.; Harden, L.A. Cinnamaldehyde Content in Foods Determined by Gas Chromatography−Mass Spectrometry. J. Agric. Food. Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; Moura, L.D.A.G.; Melo, N.R.D.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Li, M.; Xia, J.; Ding, C.; Mao, W.; Ding, H.; Xu, L.; Li, S. Development and characterization of ricinoleic acid-based sulfhydryl thiol and ethyl cellulose blended membranes. Carbohydr. Polym. 2017, 175, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Briones, V.; Aguilera, J.M. Image analysis of changes in surface color of chocolate. Food Res. Int. 2005, 8, 87–94. [Google Scholar] [CrossRef]
- Pedreschi, F.; Leon, J.; Mery, D.; Moyano, P.; Pedreschi, R.; Kaack, K.; Granby, K. Color development and acrylamide content of pre-dried potato chips. J. Food Eng. 2007, 79, 786–793. [Google Scholar] [CrossRef]
- Bergenholtz, K.P.; Nielsen, P.V. New Improved Method for Evaluation of Growth by Food Related Fungi on Biologically Derived Materials. J. Food Sci. 2002, 67, 2745–2749. [Google Scholar] [CrossRef]
- Edge, M.; Allen, N.S.; Turner, D.; Robinson, J.; Seal, K. The enhanced performance of biocidal additives in paints and coatings. Prog. Org. Coat. 2001, 43, 10–17. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Z.; Chen, X. Effect of high oxygen atmospheres on fruit decay and quality in Chinese bayberries, strawberries and blueberries. Food Control 2008, 19, 470–474. [Google Scholar] [CrossRef]
- Pereyra, A.M.; Gonzalez, M.R.; Rosato, V.G.; Basaldella, E.I. A-type zeolite containing Ag+/Zn2+ as inorganic antifungal for waterborne coating formulations. Prog. Org. Coat. 2014, 77, 213–218. [Google Scholar] [CrossRef]
- Dai, J.M.; Hou, W.; Wei, L.Q.; Jia, H.S.; Liu, X.G.; Xu, B.S. Study on the Color Change Resistant Property of Silver and Zinc-loading Zeolite 4A Antibacterial Agent. J. Inorg. Mater. 2008, 23, 1011–1015. [Google Scholar] [CrossRef]
- Ho, T.M.; Howes, T.; Bhandari, B.R. Encapsulation of gases in powder solid matrices and their applications: A review. Powder Technol. 2014, 259, 87–108. [Google Scholar] [CrossRef]
- Li, D.; Lv, D.Y.; Zhu, Q.X.; Li, H.; Chen, H.; Wu, M.M.; Chai, Y.F.; Lu, F. Chromatographic separation and detection of contaminants from whole milk powder using a chitosan-modified silver nanoparticles surface-enhanced Raman scattering device. Food Chem. 2017, 224, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yu, C.; Li, Q. Regenerable antimicrobial activity in polyamide thin film nanocomposite membranes. J. Membr. Sci. 2015, 476, 119–127. [Google Scholar] [CrossRef]
- Hanim, S.A.M.; Malek, N.A.N.N.; Ibrahim, Z. Analyses of surface area, porosity, silver release and antibacterial activity of amine-functionalized, silver-exchanged zeolite NaY. Vacuum 2017, 143, 344–347. [Google Scholar] [CrossRef]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active composite starch films containing green synthetized silver nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Sepehri, S.; García, B.B.; Zhang, Q.; Cao, G. Enhanced electrochemical and structural properties of carbon cryogels by surface chemistry alteration with boron and nitrogen. Carbon 2009, 47, 1436–1443. [Google Scholar] [CrossRef]
- Sun, L.N.; Lu, L.X.; Qiu, X.L.; Tang, Y.L. Development of low-density polyethylene antioxidant active films containing α-tocopherol loaded with MCM-41 (Mobil Composition of Matter No. 41) mesoporous silica. Food Control 2017, 71, 193–199. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, H.; Liu, J.; Gao, X.; Martens, W.N.; Frost, R.L.; Shen, Y.; Yuan, Z. A mesoporous structure for efficient photocatalysts: Anatase nanocrystals attached to leached clay layers. Microporous Mesoporous Mater. 2008, 112, 32–44. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, H.G.; Kim, J.H. Preparation and Properties of Chitosan/Poly(vinyl alcohol) Nanofibers Containing Silver Zeolite. J. Manag. Inq. 2013, 50, 119–121. [Google Scholar] [CrossRef]
- Pehlivan, H.; Balkoese, D.; Uelkue, S.; Tihminlioglu, F. Characterization of pure and silver exchanged natural zeolite filled polypropylene composite films. Compos. Sci. Technol. 2005, 65, 2049–2058. [Google Scholar] [CrossRef]
- Fernández, A.; Soriano, E.; Hernández-Muñoz, P.; Gavara, R. Migration of antimicrobial silver from composites of polylactide with silver zeolites. J. Food Sci. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of Bactericidal Action of Silver Zeolite and Its Comparison with That of Silver Nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Zhu, Y.; Jin, W. Physical and chemical stabilities of β-carotene emulsions stabilized by Ulva fasciata polysaccharide. Food Hydrocoll. 2017, 64, 28–35. [Google Scholar] [CrossRef]
- Zhang, W.S.; Chen, K.S.; Zhang, B.; Sun, C.D.; Cai, C.; Zhou, C.H.; Xu, W.P.; Zhang, W.Q.; Ferguson, I.B. Postharvest responses of Chinese bayberry fruit. Postharvest Biol. Technol. 2005, 37, 241–251. [Google Scholar] [CrossRef]
- Ma, R.; Yu, S.; Tian, Y.; Wang, K.; Sun, C.; Li, X.; Zhang, J.; Chen, K.; Fang, J. Effect of Non-Thermal Plasma-Activated Water on Fruit Decay and Quality in Postharvest Chinese Bayberries. Food Bioprocess Technol. 2016, 9, 1825–1834. [Google Scholar] [CrossRef]
- Wang, K.; Jin, P.; Tang, S.; Shang, H.; Rui, H.; Di, H.; Cai, Y.; Zheng, Y. Improved control of postharvest decay in Chinese bayberries by a combination treatment of ethanol vapor with hot air. Food Control 2011, 22, 82–87. [Google Scholar] [CrossRef]
- Semanová, J.; Skláršová, B.; Šimon, P.; Šimko, P. Elimination of polycyclic aromatic hydrocarbons from smoked sausages by migration into polyethylene packaging. Food Chem. 2016, 201, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cushen, M.; Kerry, J.; Morris, M.; Cruzromero, M.; Cummins, E. Migration and exposure assessment of silver from a PVC nanocomposite. Food Chem. 2013, 139, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Störmer, A.; Bott, J.; Kemmer, D.; Franz, R. Critical review of the migration potential of nanoparticles in food contact plastics. Trends Food Sci. Technol. 2017, 63, 39–50. [Google Scholar] [CrossRef]
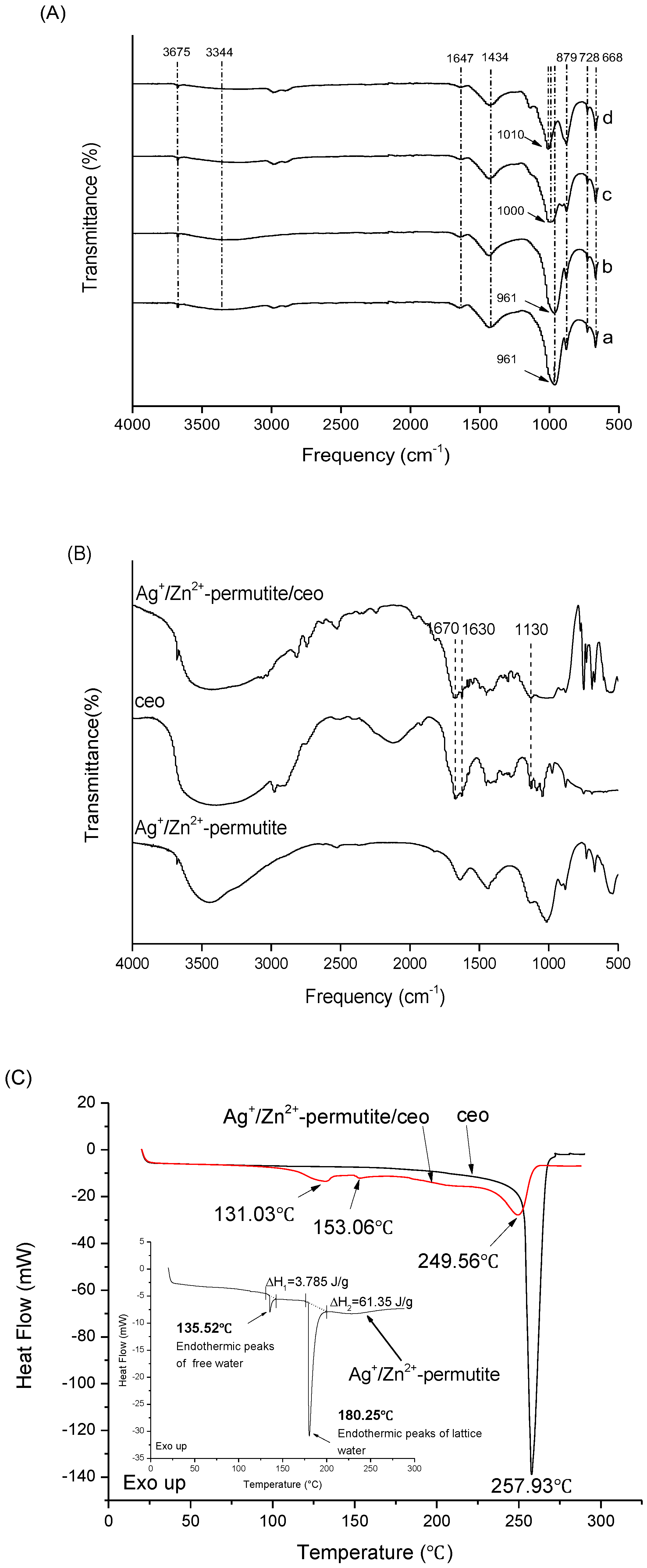
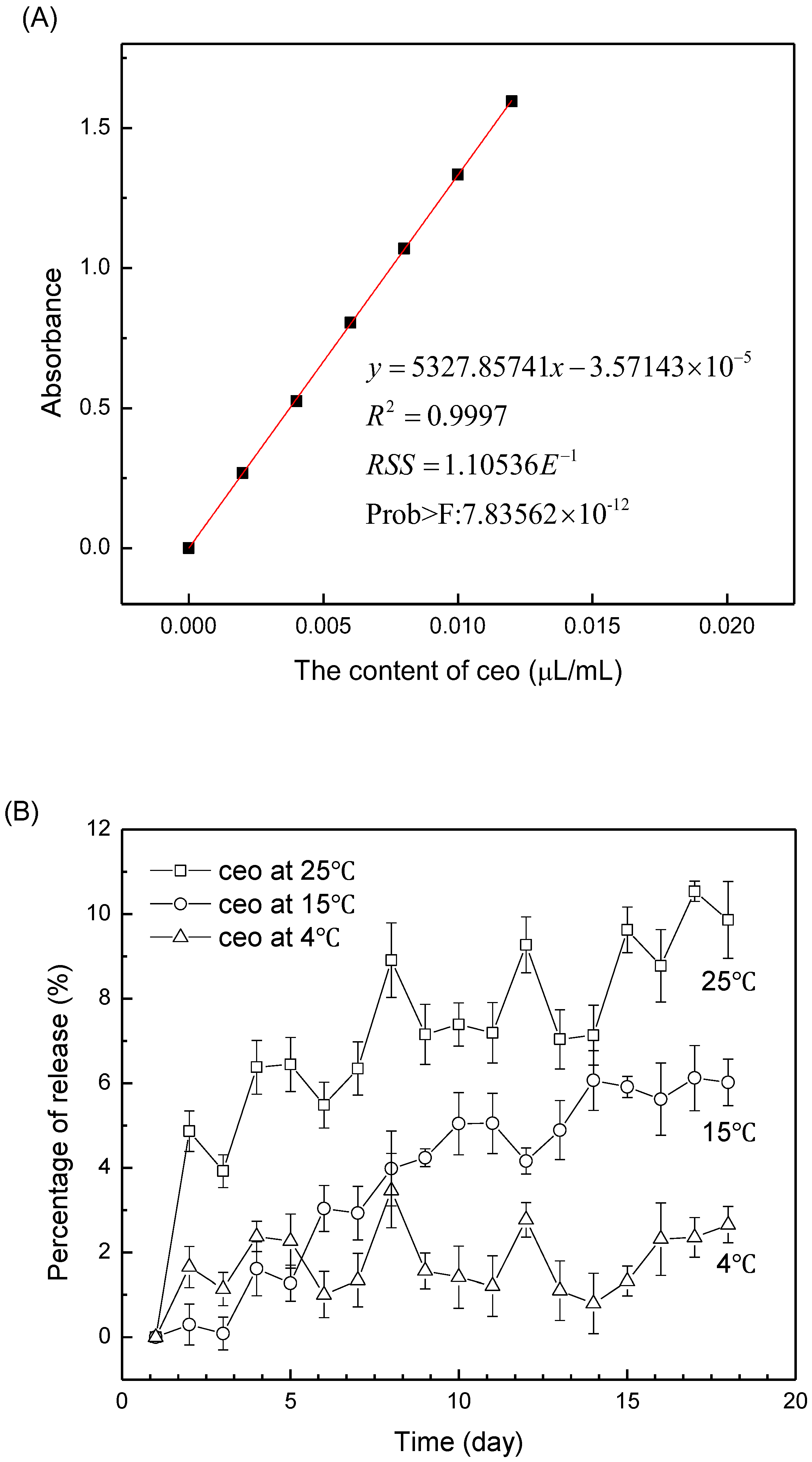
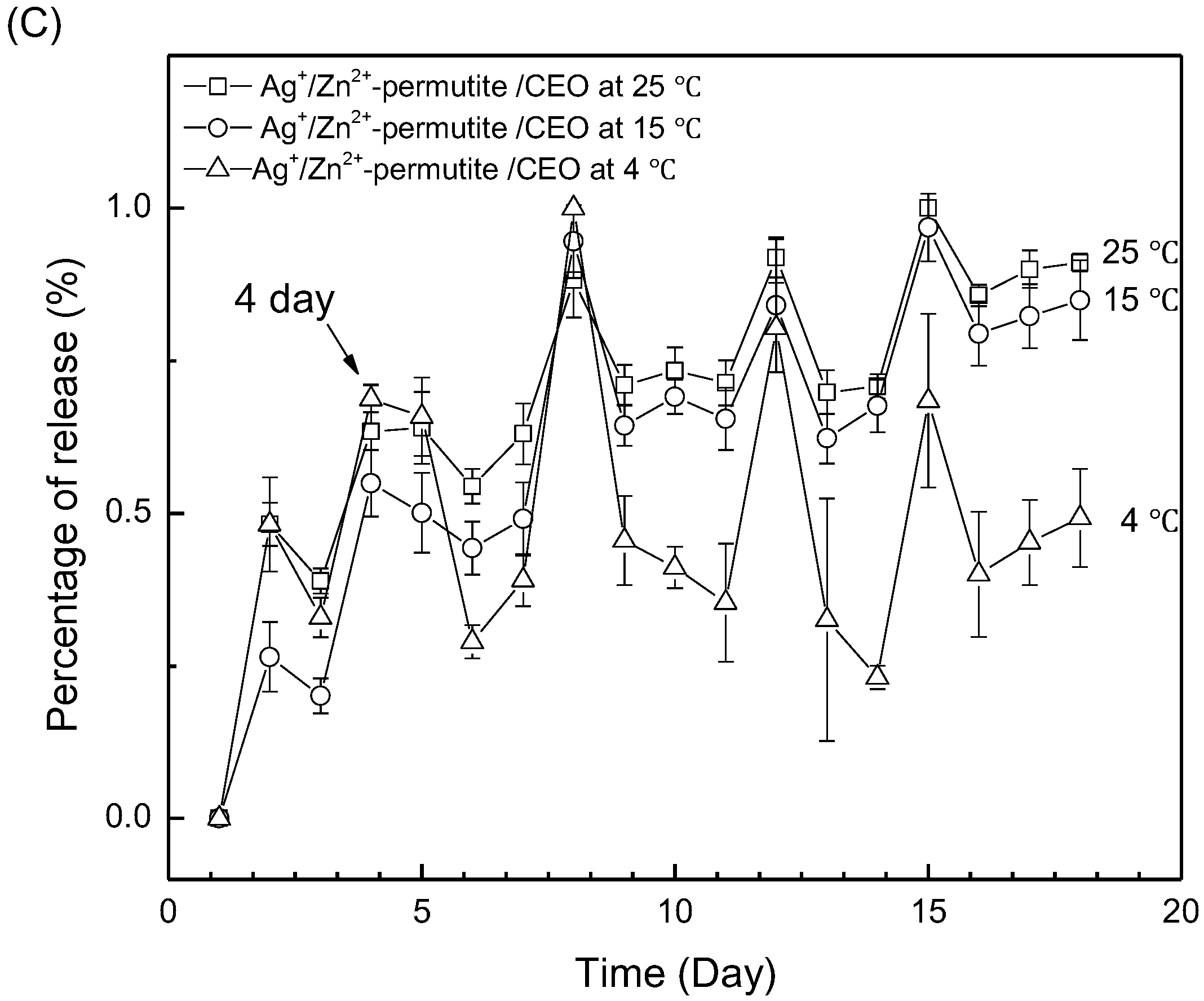

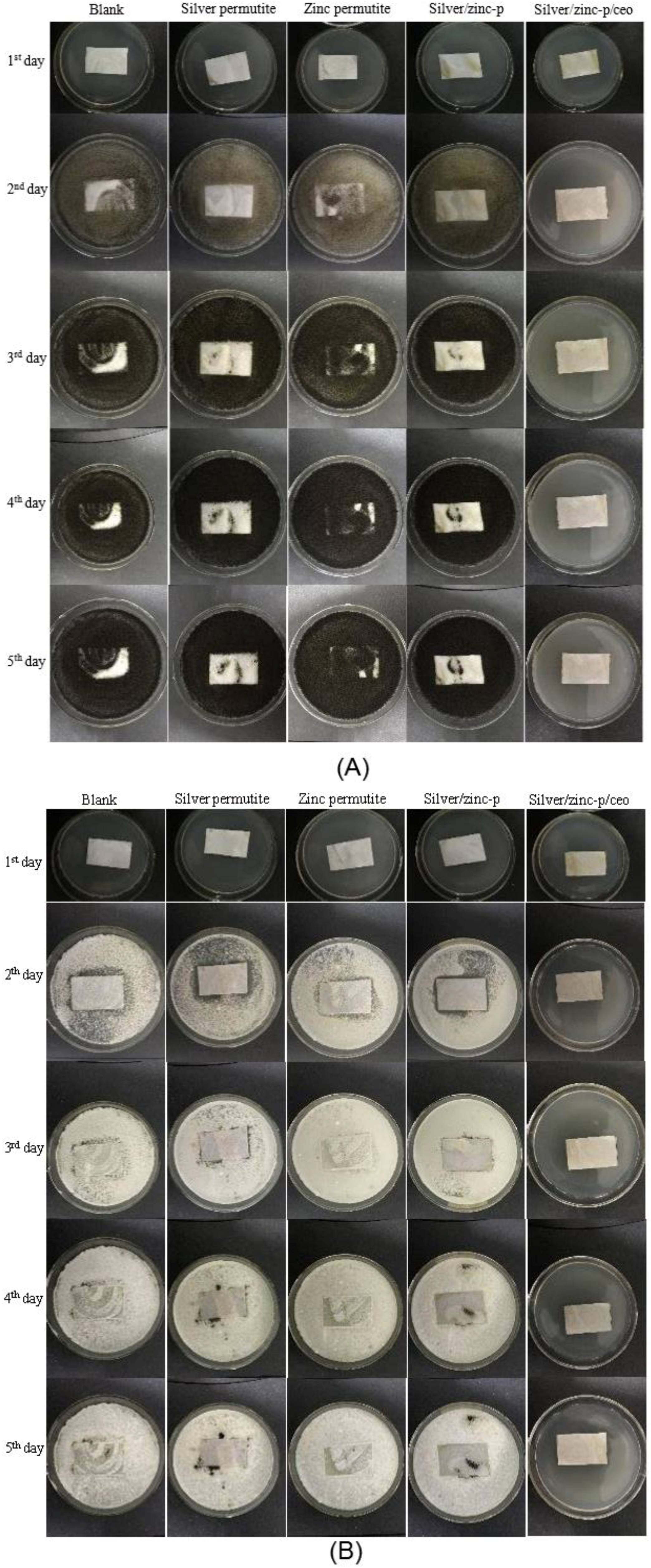
| Samples | Content of Metal Ion (wt%) | |||||
|---|---|---|---|---|---|---|
| Na+ | Mg2+ | K+ | Ca2+ | Ag+ | Zn2+ | |
| a | 11.73 ± 0.5 | 1.35 ± 0.09 | 0.40 ± 0.03 | 0.77 ± 0.05 | ND | ND |
| b | 6.16 ± 0.3 | 0.52 ± 0.06 | ND | 0.49 ± 0.02 | 18.47 ± 1.32 | ND |
| c | ND | 0.87 ± 0.04 | ND | 0.69 ± 0.04 | ND | 9.55 ± 0.74 |
| d | 1.10 ± 0.3 | 0.65 ± 0.03 | ND | 0.47 ± 0.06 | 9.68 ± 0.94 | 7.80 ± 0.61 |
| Specimens | L* | a* | b* | W (L, a, b) | ∆E | ||||
|---|---|---|---|---|---|---|---|---|---|
| Original | UV | Original | UV | Original | UV | Original | UV | ||
| a | 91.26 ± 2.42 | 88.20 ± 3.62 | 0.00 | −0.09 ± 0.02 | −0.38 ± 0.06 | −0.48 ± 0.09 | 91.3 ± 3.98 | 88.2 ± 2.13 | 3.1 ± 0.41 |
| b | 72.04 ± 1.42 | 82.08 ± 2.41 | 1.00 ± 0.12 | −0.42 ± 0.04 | 5.08 ± 0.24 | 4.45 ± 0.29 | 71.6 ± 2.12 | 81.5 ± 2.94 | 10.2 ± 0.67 |
| c | 76.65 ± 2.38 | 74.95 ± 1.46 | −0.17 ± 0.06 | −0.40 ± 0.04 | 0.23 ± 0.06 | 0.73 ± 0.14 | 76.6 ± 2.65 | 74.9 ± 2.45 | 1.8 ± 0.27 |
| d | 71.99 ± 2.23 | 77.72 ± 1.77 | 0.45 ± 0.09 | −0.10 ± 0.01 | 2.53 ± 0.14 | 2.22 ± 0.19 | 71.9 ± 2.71 | 77.6 ± 2.38 | 5.8 ± 0.38 |
| Time (d) | Control | Silver Permutite | Zinc Permutite | Silver/Zinc Permutite | Silver/Zinc p-CEO | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | P | A | P | A | P | A | P | A | P | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 0 |
| 3 | 3 | 3 | 2 | 2 | 2 | 3 | 1 | 2 | 0 | 0 |
| 4 | 4 | 4 | 2 | 3 | 3 | 3 | 2 | 3 | 0 | 0 |
| 5 | 4 | 4 | 3 | 4 | 3 | 4 | 2 | 3 | 0 | 0 |
| Simulants | Ions Migrate from Ag+/Zn2+-Permutite/CEO Antibacterial Pads (mg/L) | ||
|---|---|---|---|
| Ag+ | Zn2+ | Al3+ | |
| Water | 0.04330 ± 0.002 | 0.9420 ± 0.005 | ND |
| Limit value | 0.5 | 25 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, B.; Yan, Z.; Shao, P.; Kang, J.; Chen, H. Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity. Nanomaterials 2018, 8, 598. https://doi.org/10.3390/nano8080598
Niu B, Yan Z, Shao P, Kang J, Chen H. Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity. Nanomaterials. 2018; 8(8):598. https://doi.org/10.3390/nano8080598
Chicago/Turabian StyleNiu, Ben, Zhipeng Yan, Ping Shao, Ji Kang, and Hangjun Chen. 2018. "Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity" Nanomaterials 8, no. 8: 598. https://doi.org/10.3390/nano8080598
APA StyleNiu, B., Yan, Z., Shao, P., Kang, J., & Chen, H. (2018). Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity. Nanomaterials, 8(8), 598. https://doi.org/10.3390/nano8080598






