Neurogenic Differentiation of Human Dental Pulp Stem Cells on Graphene-Polycaprolactone Hybrid Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Fiber Fabrication
2.2. Cell Culture
2.3. Cell Viability Test
2.4. Neurogenic Differentiation
3. Results and Discussions
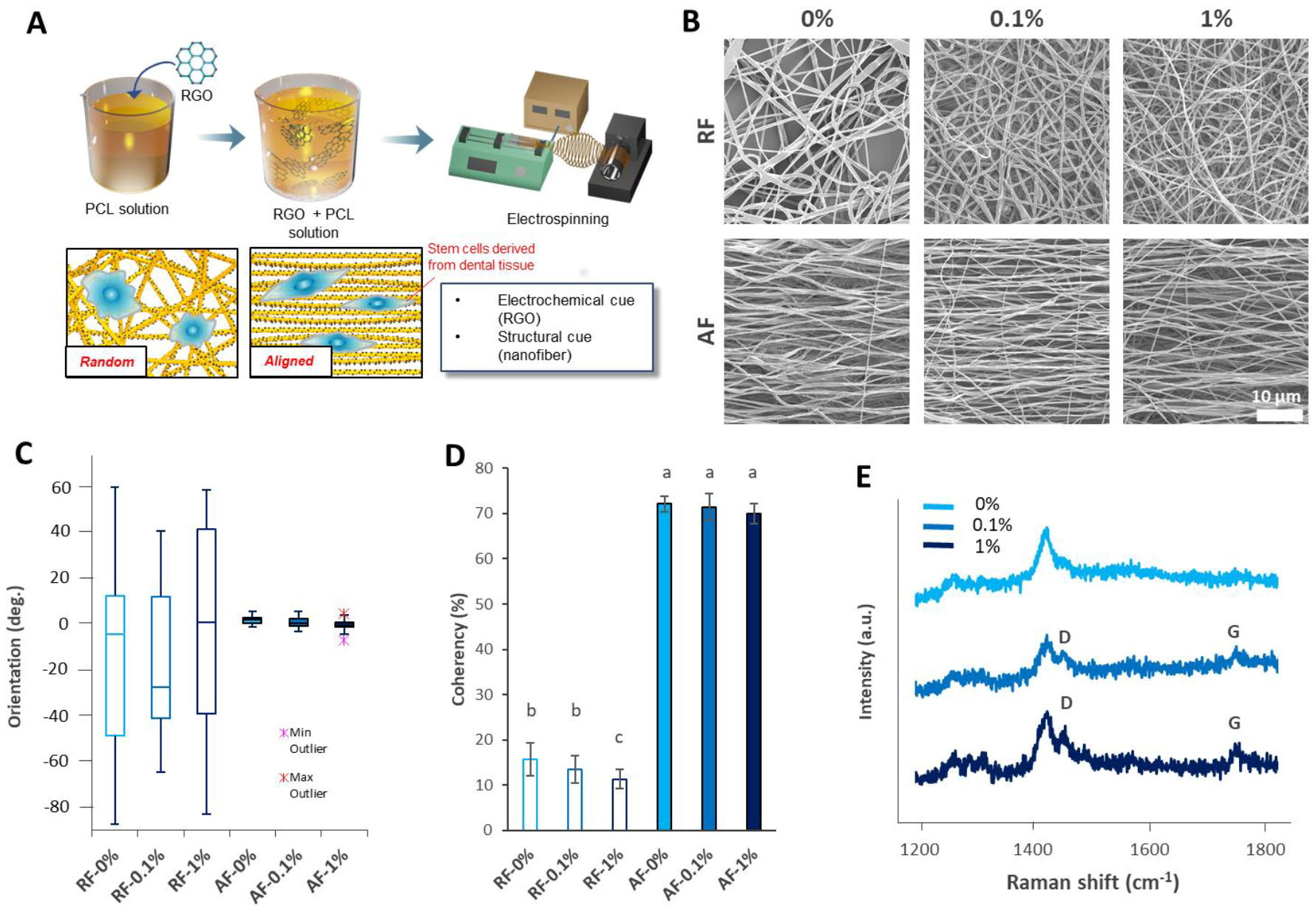
3.1. Characterization of the RGO-PCL NFs
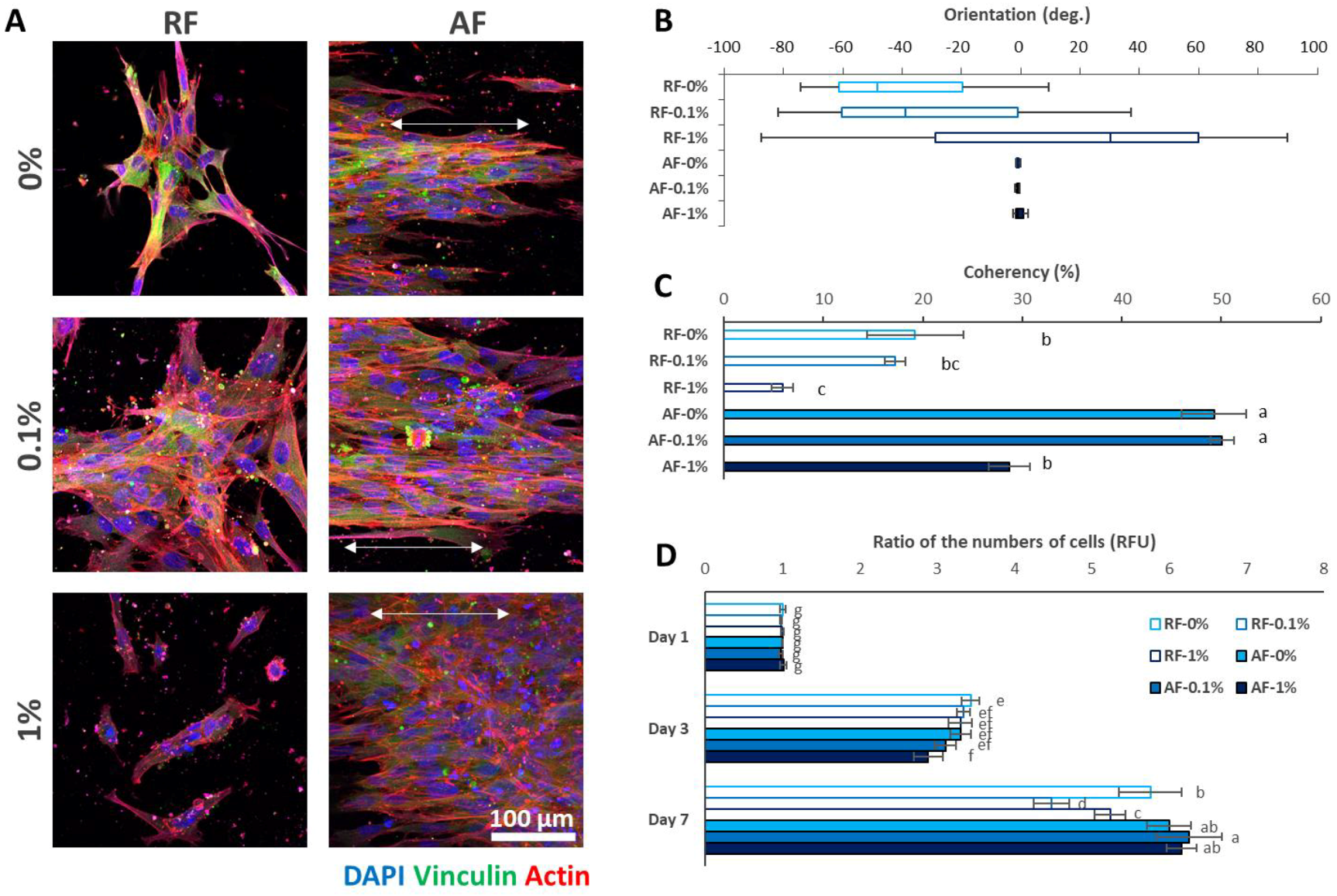
3.2. Influence of the RGO-PCL NFs on DPSC Behavior
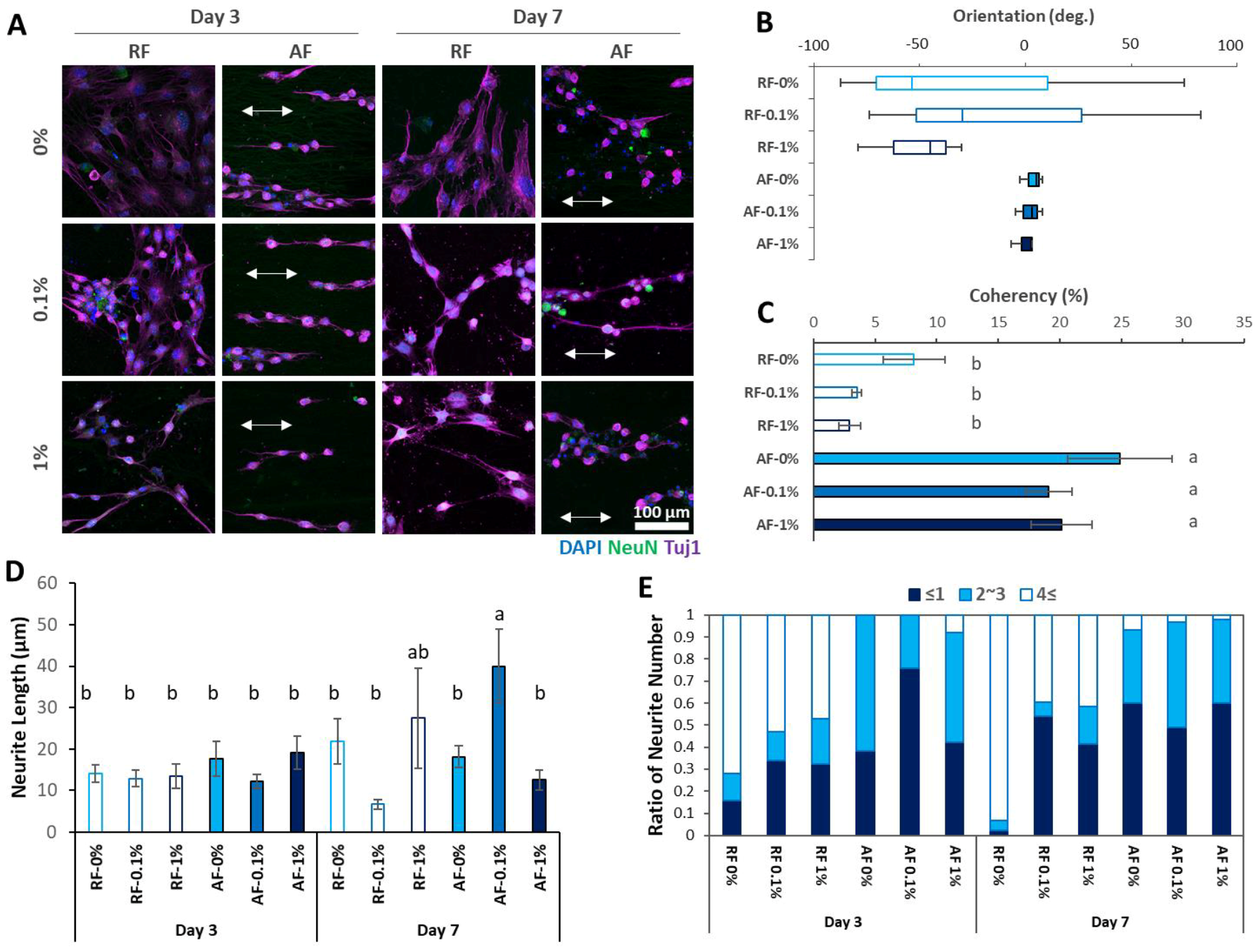
3.3. Neurogenic Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sart, S.; Agathos, S.N.; Li, Y.; Ma, T. Regulation of mesenchymal stem cell 3D microenvironment: From macro to microfluidic bioreactors. Biotechnol. J. 2016, 11, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Hughe, F.; McCulloch, C.; Melcher, A. Effects of donor age on osteogenic cells of rat bone marrow in vitro. Mech. Ageing Dev. 1990, 51, 121–132. [Google Scholar] [CrossRef]
- Dodson, S.A.; Bernard, G.W.; Kenney, E.B.; Carranza, F.A. In vitro comparison of aged and young osteogenic and hemopoietic bone marrow stem cells and their derivative colonies. J. Periodontol. 1996, 67, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-Y.; Lee, H.-J.; Kook, S.-Y.; Choung, H.-W.; Park, J.-Y.; Chung, J.-H.; Choung, Y.-H.; Kim, E.-S.; Yang, H.-C.; Choung, P.-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007, 13, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Hirose, M.; Kotobuki, N.; Shimaoka, H.; Tadokoro, M.; Maeda, M.; Hayashi, Y.; Kirita, T.; Ohgushi, H. Osteogenic differentiation of human dental papilla mesenchymal cells. Biochem. Biophys. Res. Commun. 2006, 342, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Demircan, P.C.; Sariboyaci, A.E.; Unal, Z.S.; Gacar, G.; Subasi, C.; Karaoz, E. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: Comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 2011, 13, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Pinton, P.; Stellini, E.; Botticelli, D.; Sivolella, S.; Zavan, B. Donor age-related biological properties of human dental pulp stem cells change in nanostructured scaffolds. PLoS ONE 2012, 7, e49146. [Google Scholar] [CrossRef] [PubMed]
- Gandia, C.; Arminan, A.; García-Verdugo, J.M.; Lledo, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Iohara, K.; Murakami, M. Dental pulp stem cells and regeneration. Endod. Top. 2013, 28, 38–50. [Google Scholar] [CrossRef]
- Huang, A.H.C.; Snyder, B.R.; Cheng, P.H.; Chan, A.W. Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells 2008, 26, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, R.; Hayashi, Y.; Iohara, K.; Sugiyama, M.; Murakami, M.; Yamamoto, T.; Fukuta, O.; Nakashima, M. Stimulation of angiogenesis, neurogenesis and regeneration by side population cells from dental pulp. Biomaterials 2013, 34, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Takeyasu, M.; Nozaki, T.; Daito, M. Differentiation of dental pulp stem cells into a neural lineage. Pediatr. Dent. J. 2006, 16, 154–162. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009, 21, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Vacanti, J.P.; Langer, R. Progress in tissue engineering. Sci. Am. 2009, 300, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wu, L.; Qu, X. New horizons for diagnostics and therapeutic applications of graphene and graphene oxide. Adv. Mater. 2013, 25, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, K.S.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, Y.; Kim, D.-H.; Choung, P.-H.; Cho, C.-S.; Kim, S.Y.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Res. Part A 2013, 101, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Bahrami, H.; Ranjbar-Mohammadi, M. Fabrication, optimization and characterization of electrospun poly(caprolactone)/gelatin/graphene nanofibrous mats. Mater. Sci. Eng. C 2017, 78, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Wu, D.; Kuddannaya, S.; Zhang, Y.; Wang, Z. Fabrication, characterization, and biocompatibility of polymer cored reduced graphene oxide nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.T.; Seonwoo, H.; Choi, K.S.; Jin, H.; Jang, K.J.; Kim, J.; Kim, J.-W.; Kim, S.Y.; Choung, P.-H.; Chung, J.H. Pulsed-electromagnetic-field-assisted reduced graphene oxide substrates for multidifferentiation of human mesenchymal stem cells. Adv. Healthc. Mater. 2016, 5, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-T.; Park, S.-H.; Kim, J.; Seonwoo, H.; Choung, P.-H.; Chung, J.H. Cell image processing method for automatic cell pattern recognition and morphological analysis of mesenchymal stem cells-an algorithm for cell classification and adaptive brightness correction. J. Biosyst. Eng. 2013, 38, 55–63. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bae, W.-G.; Park, S.; Kim, Y.J.; Jo, I.; Park, S.; Jeon, N.L.; Kwak, W.; Cho, S.; Park, J.; et al. Engineering structures and functions of mesenchymal stem cells by suspended large-area graphene nanopatterns. 2D Mater. 2016, 3, 035013. [Google Scholar] [CrossRef]
- Seonwoo, H.; Bae, W.-G.; Park, S.; Kim, H.-N.; Choi, K.S.; Lim, K.T.; Hyun, H.; Kim, J.-W.; Kim, J.; Chung, J.H. Hierarchically micro-and nanopatterned topographical cues for modulation of cellular structure and function. IEEE Trans. Nanobiosci. 2016, 15, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.-E.; Petrie, T.-A.; Creighton, F.-P.; Garcia, A.-J. Human mesenchymal stem cell differentiation on self-assembled monolayers presenting different surface chemistries. Acta Biomater. 2010, 6, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K.-S.; Kim, D.; Kim, W.; Lee, D.; Kim, H.-N.; Hyun, H.; Lim, K.-T.; Kim, J.-W.; Kim, Y.-R.; et al. Controlled extracellular topographical and chemical cues for acceleration of neuronal development. J. Ind. Eng. Chem. 2018, 61, 65–70. [Google Scholar] [CrossRef]
- Kim, J.; Choung, P.-H.; Kim, I.Y.; Lim, K.T.; Son, H.M.; Choung, Y.-H.; Cho, C.-S.; Chung, J.H. Electrospun nanofibers composed of poly (ε-caprolactone) and polyethylenimine for tissue engineering applications. Mater. Sci. Eng. C 2009, 29, 1725–1731. [Google Scholar] [CrossRef]
- Seo, Y.-R.; Kim, J.-W.; Seonwoo, H.; Kim, J.; Chung, J.H.; Lim, K.-T. Cellulose-based nanocrystals: Sources and applications via agricultural byproducts. J. Biosyst. Eng. 2018, 43, 59–71. [Google Scholar]
- Sinha, A.; Martin, E.M.; Lim, K.-T.; Carrier, D.J.; Han, H.; Zharov, V.P.; Kim, J.-W. Cellulose nanocrystals as advanced “Green” materials for biological and biomedical engineering. J. Biosyst. Eng. 2015, 40, 373–393. [Google Scholar] [CrossRef]
- Kim, T.M.; Lee, T.; El-Said, W.A.; Choi, J.W. Graphene-based materials for stem cell applications. Materials 2015, 8, 8674–8690. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seonwoo, H.; Jang, K.-J.; Lee, D.; Park, S.; Lee, M.; Park, S.; Lim, K.-T.; Kim, J.; Chung, J.H. Neurogenic Differentiation of Human Dental Pulp Stem Cells on Graphene-Polycaprolactone Hybrid Nanofibers. Nanomaterials 2018, 8, 554. https://doi.org/10.3390/nano8070554
Seonwoo H, Jang K-J, Lee D, Park S, Lee M, Park S, Lim K-T, Kim J, Chung JH. Neurogenic Differentiation of Human Dental Pulp Stem Cells on Graphene-Polycaprolactone Hybrid Nanofibers. Nanomaterials. 2018; 8(7):554. https://doi.org/10.3390/nano8070554
Chicago/Turabian StyleSeonwoo, Hoon, Kyung-Je Jang, Dohyeon Lee, Sunho Park, Myungchul Lee, Sangbae Park, Ki-Taek Lim, Jangho Kim, and Jong Hoon Chung. 2018. "Neurogenic Differentiation of Human Dental Pulp Stem Cells on Graphene-Polycaprolactone Hybrid Nanofibers" Nanomaterials 8, no. 7: 554. https://doi.org/10.3390/nano8070554
APA StyleSeonwoo, H., Jang, K.-J., Lee, D., Park, S., Lee, M., Park, S., Lim, K.-T., Kim, J., & Chung, J. H. (2018). Neurogenic Differentiation of Human Dental Pulp Stem Cells on Graphene-Polycaprolactone Hybrid Nanofibers. Nanomaterials, 8(7), 554. https://doi.org/10.3390/nano8070554







