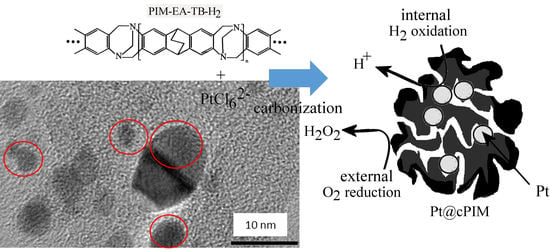
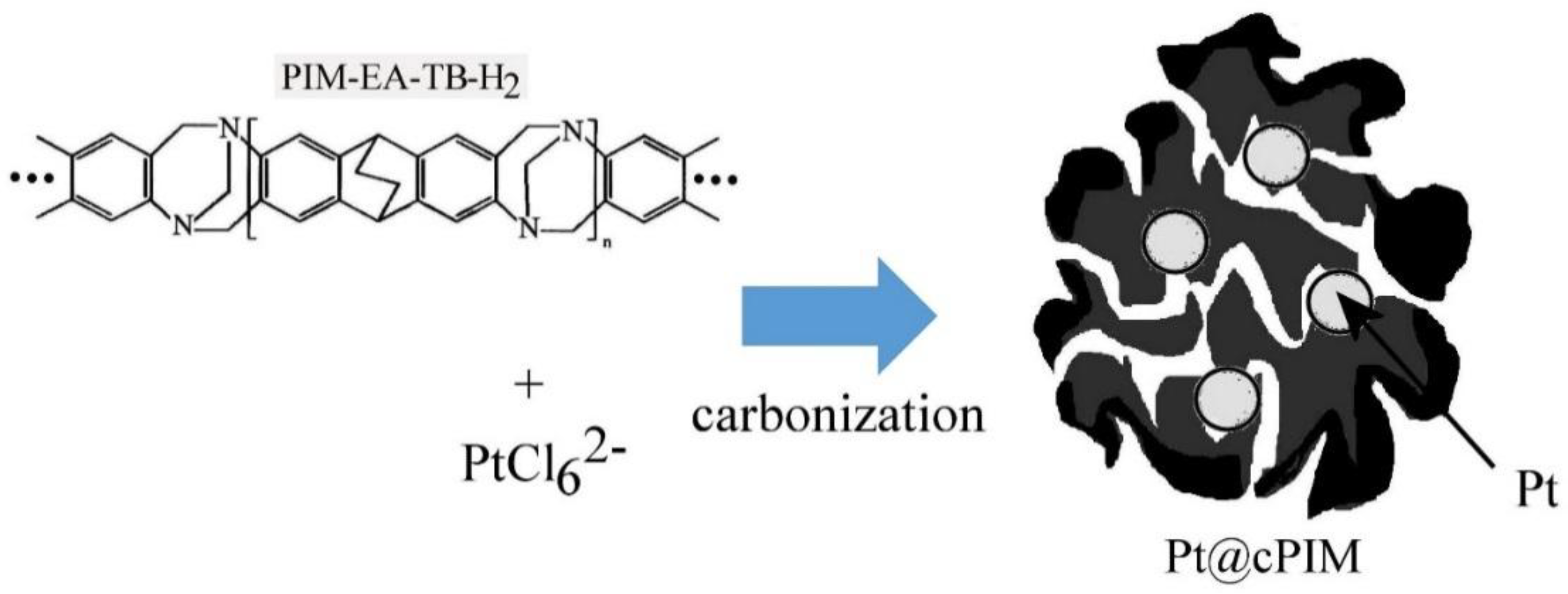
Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Catalyst and Electrode Preparation
2.4. Catalyst Testing
3. Results and Discussion
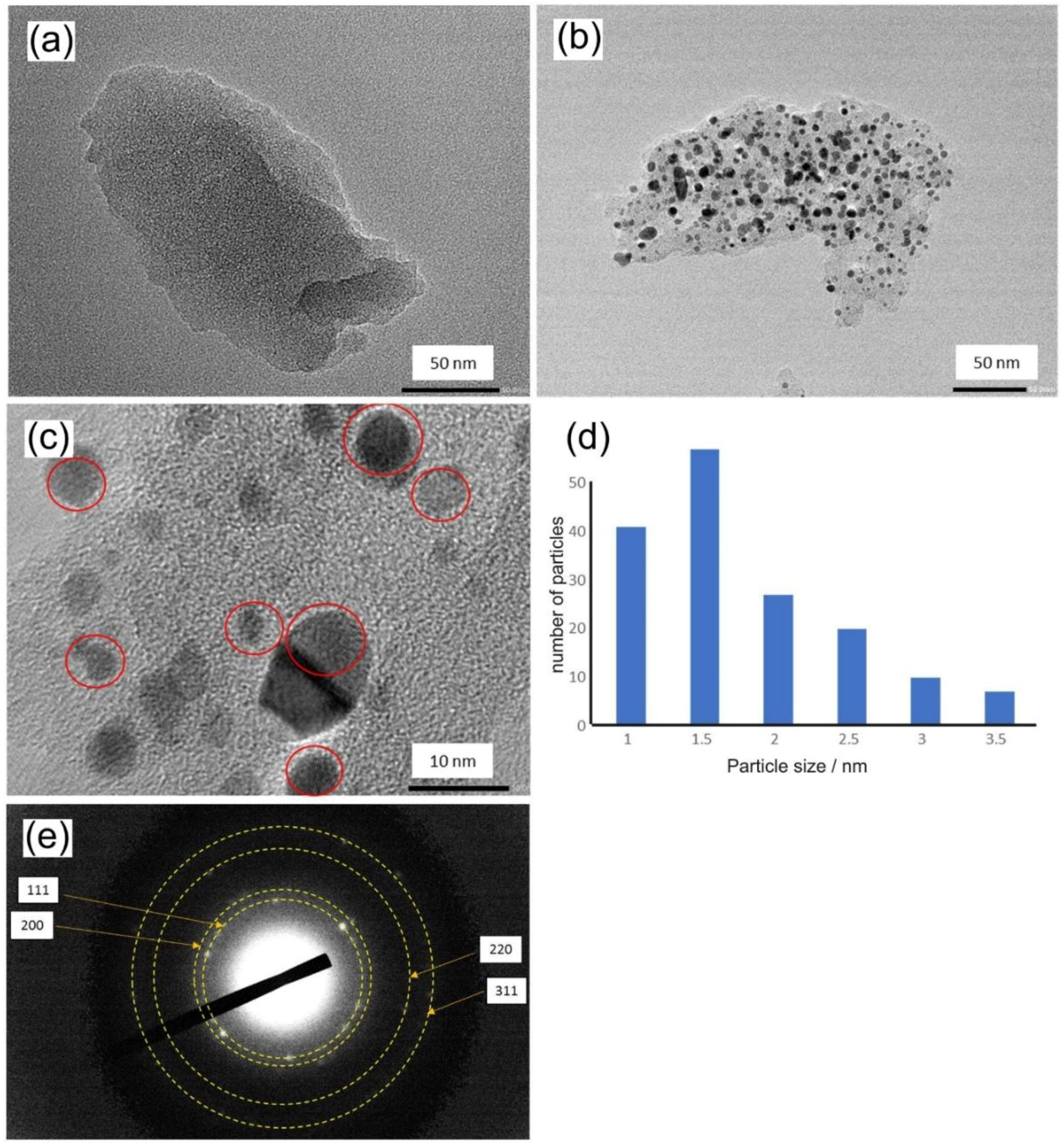
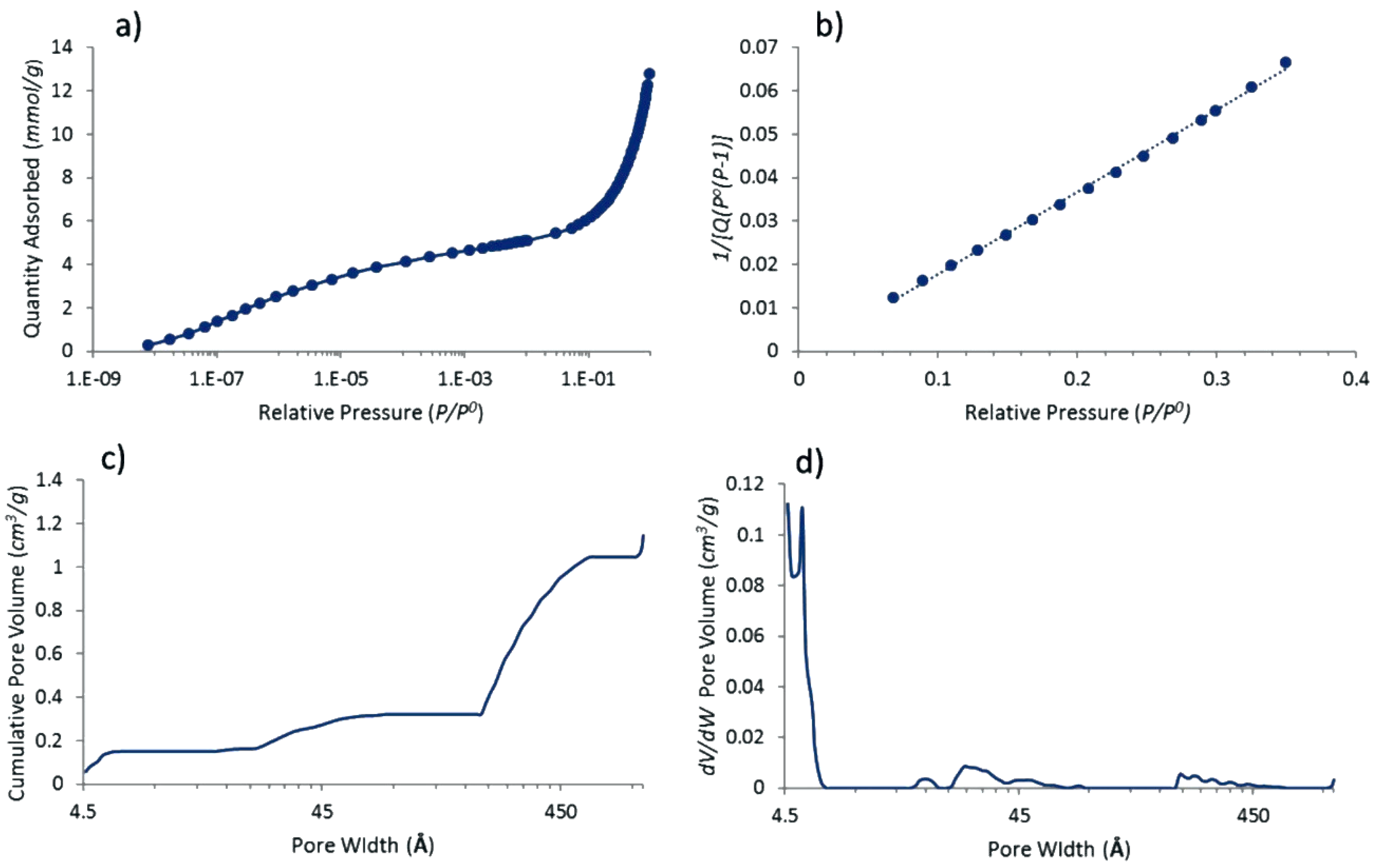
3.1. Properties of Pt@cPIM I.: Structure and Porosity
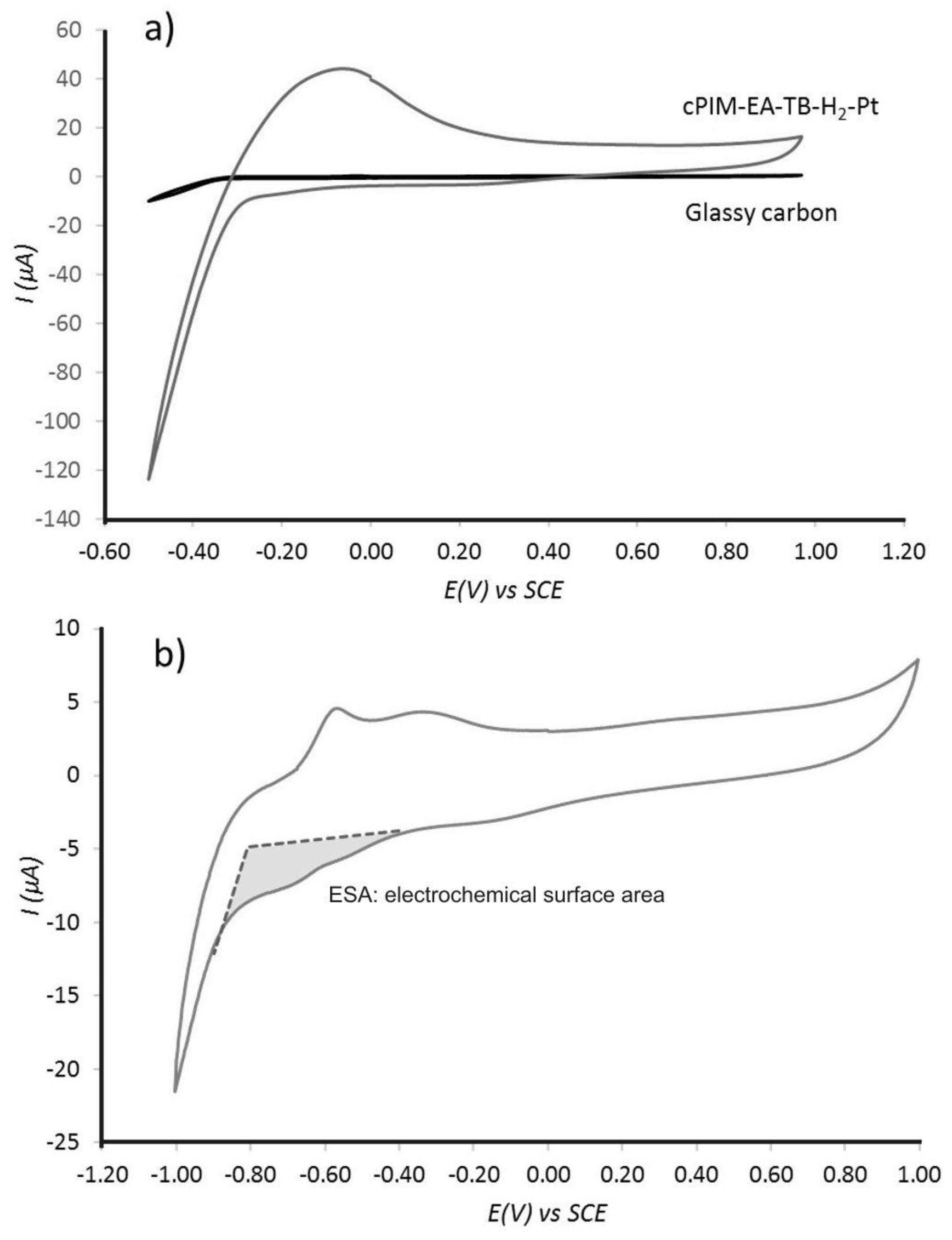
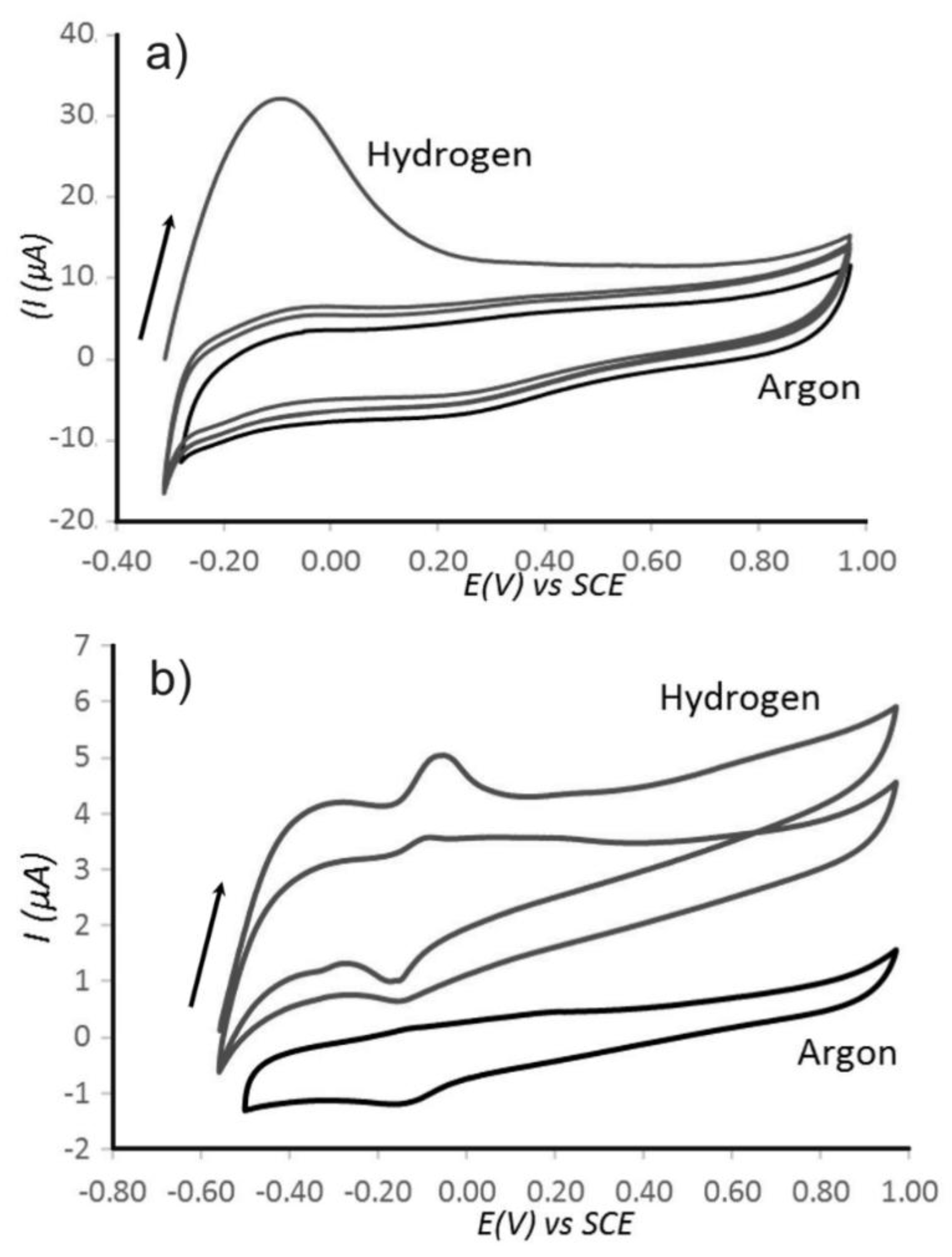
3.2. Properties of Pt@cPIM II.: Electrochemical Characterization
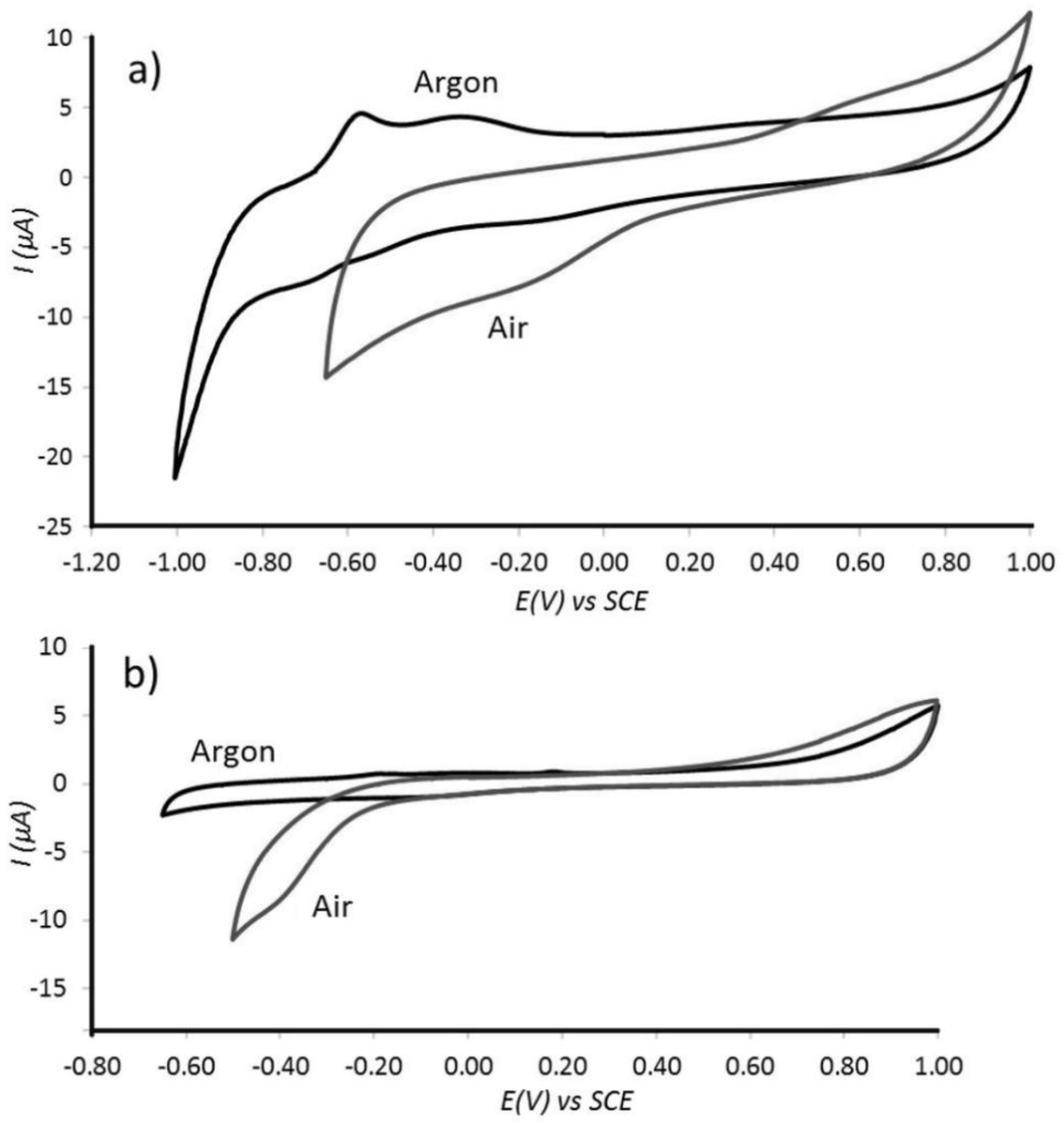
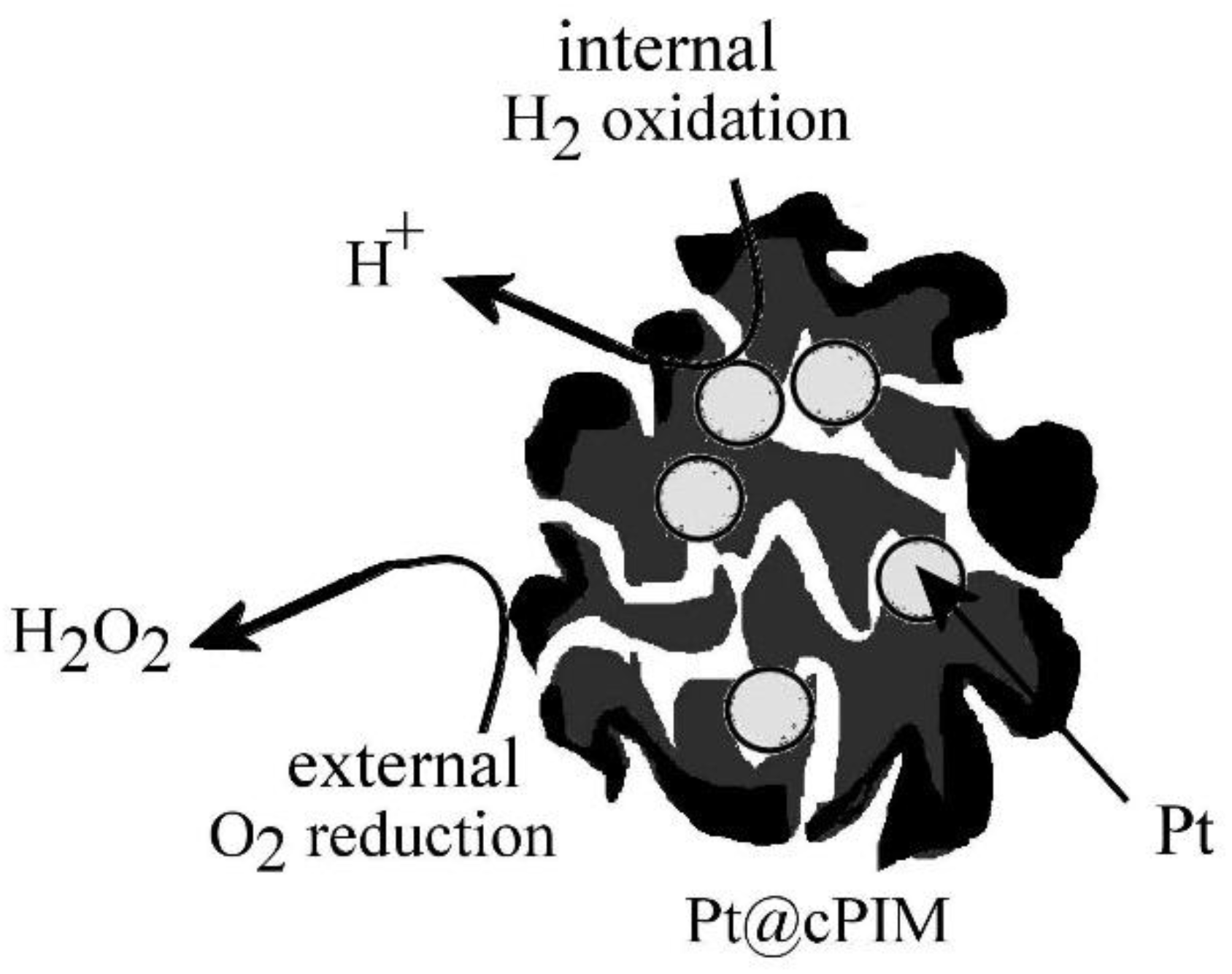
3.3. Properties of Pt@cPIM III.: Catalysis and Hydrogen Peroxide Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lavacchi, A.; Miller, H.; Vizza, F. Nanotechnology in Electrocatalysis for Energy; Springer: New York, NY, USA, 2013; pp. 1–331. [Google Scholar]
- Guo, S.J.; Zhang, S.; Sun, S.H. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Dinh, C.T.; Do, T.O. Tailoring the assembly, interfaces, and porosity of nanostructures toward enhanced catalytic activity. Chem. Commun. 2015, 51, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Madrid, E.; Lowe, J.P.; Msayib, K.J.; McKeown, N.B.; Song, Q.; Attard, G.A.; Düren, T.; Marken, F. Triphasic nature of polymers of intrinsic microporosity induces storage and catalysis effects in hydrogen and oxygen reactivity atelectrode surfaces. ChemElectroChem 2018. [Google Scholar] [CrossRef]
- Gao, J.; Zhu, M.M.; Huang, H.; Liu, Y.; Kang, Z.H. Advances, challenges and promises of carbon dots. Inorg. Chem. Front. 2017, 4, 1963–1986. [Google Scholar] [CrossRef]
- Dumitrescu, I.; Unwin, P.R.; Macpherson, J.V. Electrochemistry at carbon nanotubes: Perspective and issues. Chem. Commun. 2009, 6886–6901. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.; Xia, F.J.; Arrowsmith, R.L.; Ge, H.B.; Nelson, G.W.; Foord, J.S.; Felipe-Sotelo, M.; Evans, N.D.M.; Mitchels, J.M.; Flower, S.E.; et al. Hydrothermal conversion of one-photon-fluorescent poly(4-vinylpyridine) into two-photon-fluorescent carbon nanodots. Langmuir 2014, 30, 11746–11752. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.Y.; He, D.P.; Sanchez-Fernandez, A.; Evans, C.; Edler, K.J.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Clarke, T.J.; Taylor, S.H.; et al. Intrinsically microporous polymer retains porosity in vacuum thermolysis to electroactive heterocarbon. Langmuir 2015, 31, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.; Iniesta, J.; Leguey, V.M.; Armstrong, R.; Taylor, S.H.; Madrid, E.; Rong, Y.Y.; Castaing, R.; Malpass-Evans, R.; Carta, M. Carbonization of polymers of intrinsic microporosity to microporous heterocarbon: Capacitive pH measurements. Appl. Mater. Today 2017, 9, 136–144. [Google Scholar] [CrossRef]
- Qiu, S.L.; Ben, T. Polymers of instrinsic microporosity. In Porous Polymers: Design, Synthesis and Applications; Royal Society of Chemistry: Cambridge, UK, 2016. [Google Scholar]
- McKeown, N.B.; Budd, P.M. Polymers of intrinsic microporosity (PIMs): Organic materials for membrane separations, heterogeneous catalysis and hydrogen storage. Chem. Soc. Rev. 2006, 35, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Atilhan, M.; Anaya, B.; Al-Muhtaseb, S.; Aparicio, S.; Patel, H.; Thirion, D.; Yavuz, C.T. Investigation of ester-and amide-linker-based porous organic polymers for carbon dioxide capture and separation at wide temperatures and pressures. ACS Appl. Mater. Interfaces 2016, 8, 20772–20785. [Google Scholar] [CrossRef] [PubMed]
- Ramimoghadam, D.; Gray, E.M.; Webb, C.J. Review of polymers of intrinsic microporosity for hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 16944–16965. [Google Scholar] [CrossRef]
- Polak-Krasna, K.; Dawson, R.; Holyfield, L.T.; Bowen, C.R.; Burrows, A.D.; Mays, T.J. Mechanical characterization of polymer of intrinsic microporosity PIM-1 for hydrogen storage applications. J. Mater. Sci. 2017, 52, 3862–3875. [Google Scholar] [CrossRef]
- Ghanem, B.S.; McKeown, N.B.; Budd, P.M.; Al-Harbi, N.M.; Fritsch, D.; Heinrich, K.; Starannikova, L.; Tokarev, A.; Yampolskii, Y. Synthesis, characterization, and gas permeation properties of a novel group of polymers with intrinsic microporosity: PIM-polyimides. Macromolecules 2009, 42, 7881–7888. [Google Scholar] [CrossRef]
- Rong, Y.Y.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Attard, G.A.; Marken, F. High density heterogenization of molecular electrocatalysts in a rigid intrinsically microporous polymer. Electrochem. Commun. 2014, 46, 26–29. [Google Scholar] [CrossRef]
- Song, Q.L.; Cao, S.; Pritchard, R.H.; Ghalei, B.; Al-Muhtaseb, S.A.; Terentjev, E.M.; Cheetham, A.K.; Sivaniah, E. Controlled thermal oxidative crosslinking of polymers of intrinsic microporosity towards tunable molecular sieve membranes. Nat. Commun. 2014, 5, 12–14. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.B.; Budd, P.M. Exploitation of intrinsic microporosity in polymer-based materials. Macromolecules 2010, 43, 5163–5176. [Google Scholar] [CrossRef]
- Thomas, A.; Kuhn, P.; Weber, J.; Titirici, M.M.; Antonietti, M. Porous polymers: Enabling solutions for energy applications. Macromol. Rapid Commun. 2009, 30, 221–236. [Google Scholar] [CrossRef] [PubMed]
- He, D.P.; Rong, Y.Y.; Kou, Z.K.; Mu, S.C.; Peng, T.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Marken, F. Intrinsically microporous polymer slows down fuel cell catalyst corrosion. Electrochem. Commun. 2015, 59, 72–76. [Google Scholar] [CrossRef]
- Rong, Y.Y.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Attard, G.A.; Marken, F. Intrinsically porous polymer protects catalytic gold particles for enzymeless glucose oxidation. Electroanalysis 2014, 26, 904–909. [Google Scholar] [CrossRef]
- Madrid, E.; Rong, Y.Y.; Carta, M.; McKeown, N.B.; Malpass-Evans, R.; Attard, G.A.; Clarke, T.J.; Taylor, S.H.; Long, Y.T.; Marken, F. Metastable ionic diodes derived from an amine-based polymer of intrinsic microporosity. Angew. Chem. Int. Ed. 2014, 53, 10751–10754. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.X.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Madrid, E.; Marken, F. One-step preparation of microporous Pd@cPIM composite catalyst film for triphasic electrocatalysis. Electrochem. Commun. 2018, 86, 17–20. [Google Scholar] [CrossRef]
- Rong, Y.Y.; Song, Q.L.; Mathwig, K.; Madrid, E.; He, D.P.; Niemann, R.G.; Cameron, P.J.; Dale, S.E.C.; Bending, S.; Carta, M.; et al. Rectifier pH-induced reversal of ionic diode polarity in 300 nm thin membranes based on a polymer of intrinsic microporosity. Electrochem. Commun. 2016, 69, 41–45. [Google Scholar] [CrossRef]
- Al-Kutubi, H.; Rassaei, L.; Olthuis, W.; Nelson, G.W.; Foord, J.S.; Holdway, P.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Tsang, S.C.; et al. Polymers of intrinsic microporosity as high temperature templates for the formation of nanofibrous oxides. RSC Adv. 2015, 5, 73323–73326. [Google Scholar] [CrossRef]
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; McKeown, N.B. An efficient polymer molecular sieve for membrane gas separations. Science 2013, 339, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Salinas, O.; Ma, X.H.; Litwiller, E.; Pinnau, I. High-performance carbon molecular sieve membranes for ethylene/ethane separation derived from an intrinsically microporous polyimide. J. Membr. Sci. 2016, 500, 115–123. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.G.; Lee, K.; Baek, Y.; Yoo, Y.; Kim, Y.S.; Kim, B.G.; Lee, J.C. A carbonaceous membrane based on a polymer of intrinsic microporosity (PIM-1) for water treatment. Sci. Rep. 2016, 6, 36078. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.Y.; He, D.P.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Gromboni, M.F.; Mascaro, L.H.; Nelson, G.W.; Foord, J.S.; Holdway, P.; et al. High-utilization nanoplatinum catalyst (Pt@cPIM) obtained via vacuum carbonization in a molecularly rigid polymer of intrinsic microporosity. Electrocatalysis 2017, 8, 132–143. [Google Scholar] [CrossRef]
- Xia, F.J.; Pan, M.; Mu, S.C.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Attard, G.A.; Brew, A.; Morgan, D.J.; Marken, F. Polymers of intrinsic microporosity in electrocatalysis: Novel pore rigidity effects and lamella palladium growth. Electrochim. Acta 2014, 128, 3–9. [Google Scholar] [CrossRef]
- You, P.Y.; Kamarudin, S.K. Recent progress of carbonaceous materials in fuel cell applications: An overview. Chem. Eng. J. 2017, 309, 489–502. [Google Scholar] [CrossRef]
- Afraz, A.; Rafati, A.A.; Hajian, A.; Khoshnood, M. Electrodeposition of Pt nanoparticles on new porous graphitic carbon nanostructures prepared from biomass for fuel cell and methanol sensing applications. Electrocatalysis 2015, 6, 220–228. [Google Scholar] [CrossRef]
- Kakaei, K. Electrochemical characteristics and performance of platinum nanoparticles supported by Vulcan/polyaniline for oxygen reduction in PEMFC. Fuel Cells 2012, 12, 939–945. [Google Scholar] [CrossRef]
- Bernardo, P.; Scorzafave, V.; Clarizia, G.; Tocci, E.; Jansen, J.C.; Borgogno, A.; Malpass-Evans, R.; McKeown, N.B.; Carta, M.; Tasselli, F. Thin film composite membranes based on a polymer of intrinsic microporosity derived from Tröger’s base: A combined experimental and computational investigation of the role of residual casting solvent. J. Membr. Sci 2018, in press. [Google Scholar]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin, Germany, 2004. [Google Scholar]
- Weber, J.; Wain, A.J.; Attard, G.A.; Marken, F. Electrothermal Annealing of Catalytic Platinum Microwire Electrodes: Towards Membrane-Free pH 7 Glucose Micro-Fuel Cells. Electroanalysis 2017, 29, 38–44. [Google Scholar] [CrossRef]
- Biegler, T.; Rand, D.A.J.; Woods, R. Limiting oxygen coverage on platinized platinum; relevance to determination of real platinum area by hydrogen adsorption. J. Electroanal. Chem. 1971, 29, 269–272. [Google Scholar] [CrossRef]
- Mtukula, A.C.; Bo, X.J.; Guo, L.P. Highly active non-precious metal electrocatalyst for the hydrogen evolution reaction based on nitrogen-doped graphene supported MoO2/WN/Mo2N. J. Alloys Compd. 2017, 692, 614–621. [Google Scholar] [CrossRef]
- Zhou, W.J.; Jia, J.; Lu, J.; Yang, L.J.; Hou, D.M.; Li, G.Q.; Chen, S.W. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Yu, Z.T.; Ye, J.B.; Chen, W.X.; Xu, S.R. Fabrication of MoS2/reduced graphene oxide hybrid as an earth-abundant hydrogen evolution electrocatalyst. Mater. Lett. 2017, 188, 48–51. [Google Scholar] [CrossRef]
- Sheng, W.C.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs. alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Wang, D.Z.; Shen, Y.L.; Zhang, X.Y.; Wu, Z.Z. Enhanced hydrogen evolution from the MoP/C hybrid by the modification of Ketjen black. J. Mater. Sci. 2017, 52, 3337–3343. [Google Scholar] [CrossRef]
- Hotchen, C.E.; Attard, G.A.; Bull, S.D.; Marken, F. One-step electroless growth of nano-fibrous platinum catalyst from “paint-on” PtCl62-solution in poly-(ethylene-glycol). Electrochim. Acta 2014, 137, 484–488. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Bedford, N.M.; Woehl, T.J.; Rentz, N.S.; Showalter, A.R.; Pylypenko, S.; Bunker, B.A.; Lee, S.; Reinhart, B.; Ren, Y.; Ertem, S.P.; et al. Multi-component Fe-Ni hydroxide nanocatalyst for oxygen evolution and methanol oxidation reactions under alkaline conditions. ACS Catal. 2017, 7, 365–379. [Google Scholar] [CrossRef]
- Yang, C.Z.; van der Laak, N.K.; Chan, K.Y.; Zhang, X. Microwave-assisted microemulsion synthesis of carbon supported Pt-WO3 nanoparticles as an electrocatalyst for methanol oxidation. Electrochim. Acta 2012, 75, 262–272. [Google Scholar] [CrossRef]
- He, D.P.; Rong, Y.Y.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Marken, F. Fuel cell anode catalyst performance can be stabilized with a molecularly rigid film of polymers of intrinsic microporosity (PIM). RSC Adv. 2016, 6, 9315–9319. [Google Scholar] [CrossRef]
- Gupta, S.; Qiao, L.; Zhao, S.; Xu, H.; Lin, Y.; Devaguptapu, S.V.; Wang, X.L.; Swihart, M.T.; Wu, G. Highly active and stable graphene tubes decorated with FeCoNi alloy nanoparticles via a template-free graphitization for bifunctional oxygen reduction and evolution. Adv. Energy Mater. 2016, 6, 12–14. [Google Scholar] [CrossRef]
- Wu, H.J.; Guo, C.Z.; Li, J.Q.; Ma, Z.L.; Feng, Q.Y.; Chen, C.G. A graphene-based electrocatalyst co-doped with nitrogen and cobalt for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 20494–20501. [Google Scholar] [CrossRef]
- Song, W.Q.; Ren, Z.; Chen, S.Y.; Meng, Y.T.; Biswas, S.; Nandi, P.; Elsen, H.A.; Gao, P.X.; Suib, S.L. Ni- and Mn-promoted mesoporous Co3O4: A stable bifunctional catalyst with surface-structure-dependent activity for oxygen reduction reaction and oxygen evolution reaction. ACS Appl. Mater. Interfaces 2016, 8, 20802–20813. [Google Scholar] [CrossRef] [PubMed]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Antoine, O.; Durand, R. RRDE study of oxygen reduction on Pt nanoparticles inside Nafion: H2O2 production in PEMFC cathode conditions. J. Appl. Electrochem. 2000, 30, 839–844. [Google Scholar] [CrossRef]
- Akram, A.; Freakley, S.J.; Reece, C.; Piccinini, M.; Shaw, G.; Edwards, J.K.; Desmedt, F.; Miquel, P.; Seuna, E.; Willock, D.J.; et al. Gas phase stabilizer-free production of hydrogen peroxide using supported gold-palladium catalysts. Chem. Sci. 2016, 7, 5833–5837. [Google Scholar] [CrossRef]
- Edwards, J.K.; Pritchard, J.; Lu, L.; Piccinini, M.; Shaw, G.; Carley, A.F.; Morgan, D.J.; Kiely, C.J.; Hutchings, G.J. The direct synthesis of hydrogen peroxide using platinum-promoted gold–palladium catalysts. Angew. Chem. Int. Ed. 2014, 53, 2381–2384. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamik, R.K.; Hernández-Ibáñez, N.; Iniesta, J.; Edwards, J.K.; Howe, A.G.R.; Armstrong, R.D.; Taylor, S.H.; Roldan, A.; Rong, Y.; Malpass-Evans, R.; et al. Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production. Nanomaterials 2018, 8, 542. https://doi.org/10.3390/nano8070542
Adamik RK, Hernández-Ibáñez N, Iniesta J, Edwards JK, Howe AGR, Armstrong RD, Taylor SH, Roldan A, Rong Y, Malpass-Evans R, et al. Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production. Nanomaterials. 2018; 8(7):542. https://doi.org/10.3390/nano8070542
Chicago/Turabian StyleAdamik, Robert K., Naiara Hernández-Ibáñez, Jesus Iniesta, Jennifer K. Edwards, Alexander G. R. Howe, Robert D. Armstrong, Stuart H. Taylor, Alberto Roldan, Yuanyang Rong, Richard Malpass-Evans, and et al. 2018. "Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production" Nanomaterials 8, no. 7: 542. https://doi.org/10.3390/nano8070542
APA StyleAdamik, R. K., Hernández-Ibáñez, N., Iniesta, J., Edwards, J. K., Howe, A. G. R., Armstrong, R. D., Taylor, S. H., Roldan, A., Rong, Y., Malpass-Evans, R., Carta, M., McKeown, N. B., He, D., & Marken, F. (2018). Platinum Nanoparticle Inclusion into a Carbonized Polymer of Intrinsic Microporosity: Electrochemical Characteristics of a Catalyst for Electroless Hydrogen Peroxide Production. Nanomaterials, 8(7), 542. https://doi.org/10.3390/nano8070542