Colloidal Synthesis of CsX Nanocrystals (X = Cl, Br, I)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Oleylammonium Halide
2.3. Synthesis of Cesium Oleate Precursor
2.4. Synthesis of CsX Nanocrystals Using Oleylammonium Halide
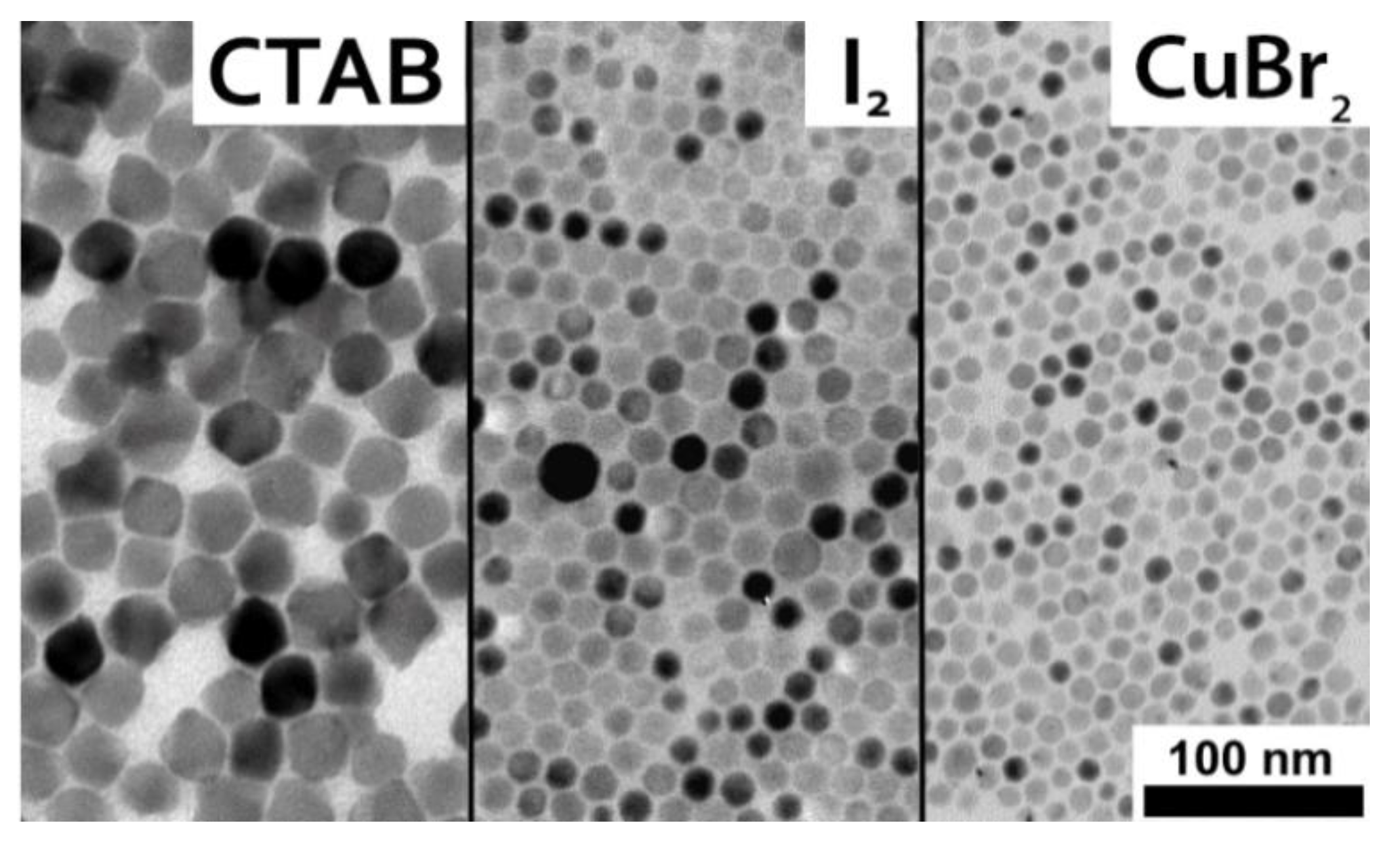
2.5. Synthesis of CsX Nanocrystals Using Novel Precursors
- In the case of cetyltrimethylammonium bromide (CTAB), CTAB (18 mg, 49 μmol) was used.
- In the case of iodine, iodine (24 mg, 0.19 mmol) was dissolved in 0.5 mL (80–90%) of oleylamine by stirring and this mixture was used.
- In the case of CuBr2, CuBr2 (40 mg, 0.188 mmol) was dissolved in 0.5 mL (80–90%) of oleylamine by stirring at room temperature and a lower reaction temperature of 90 °C was used.
2.6. Purification of Crude CsX Nanocrystals
2.7. Characterization
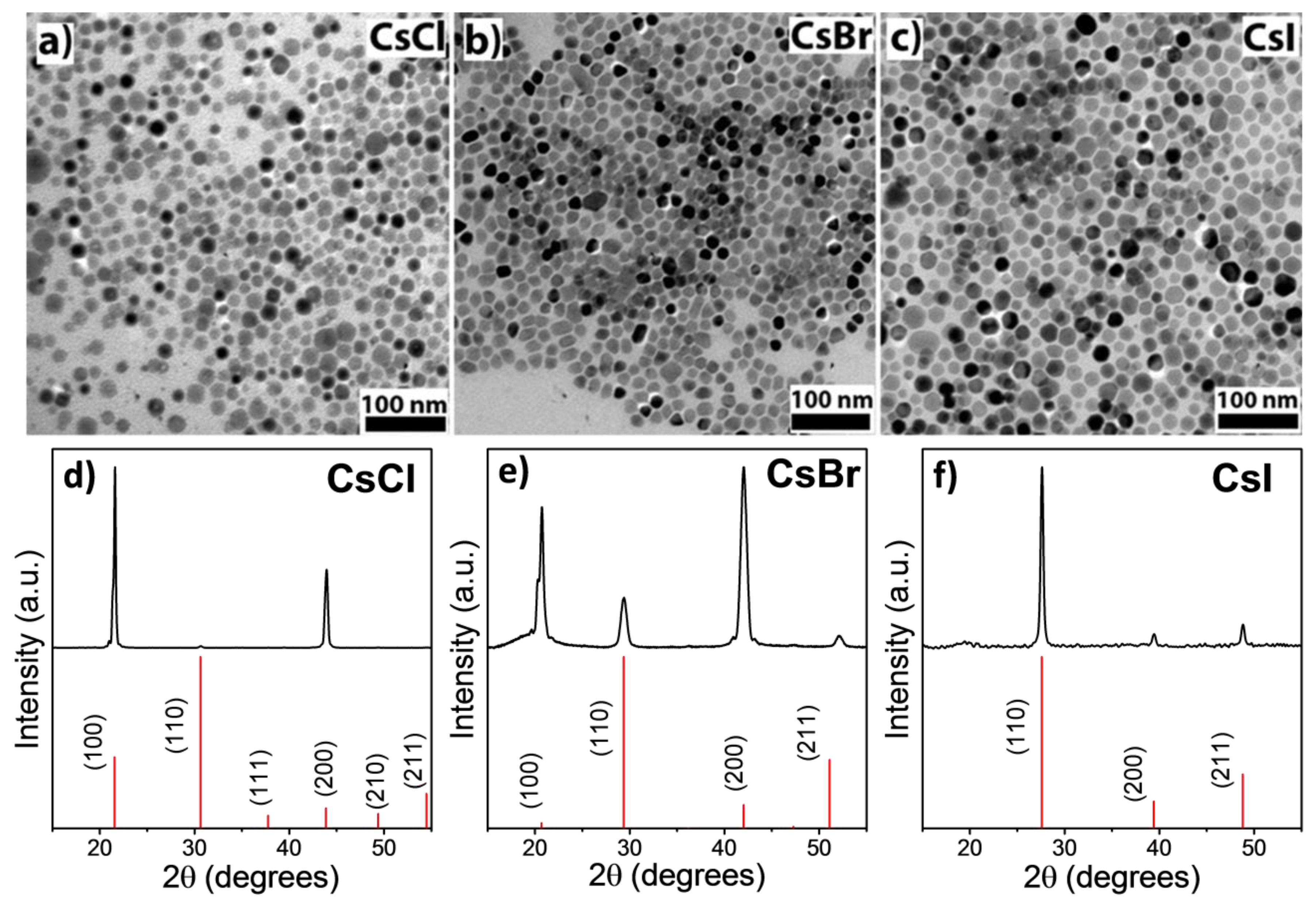
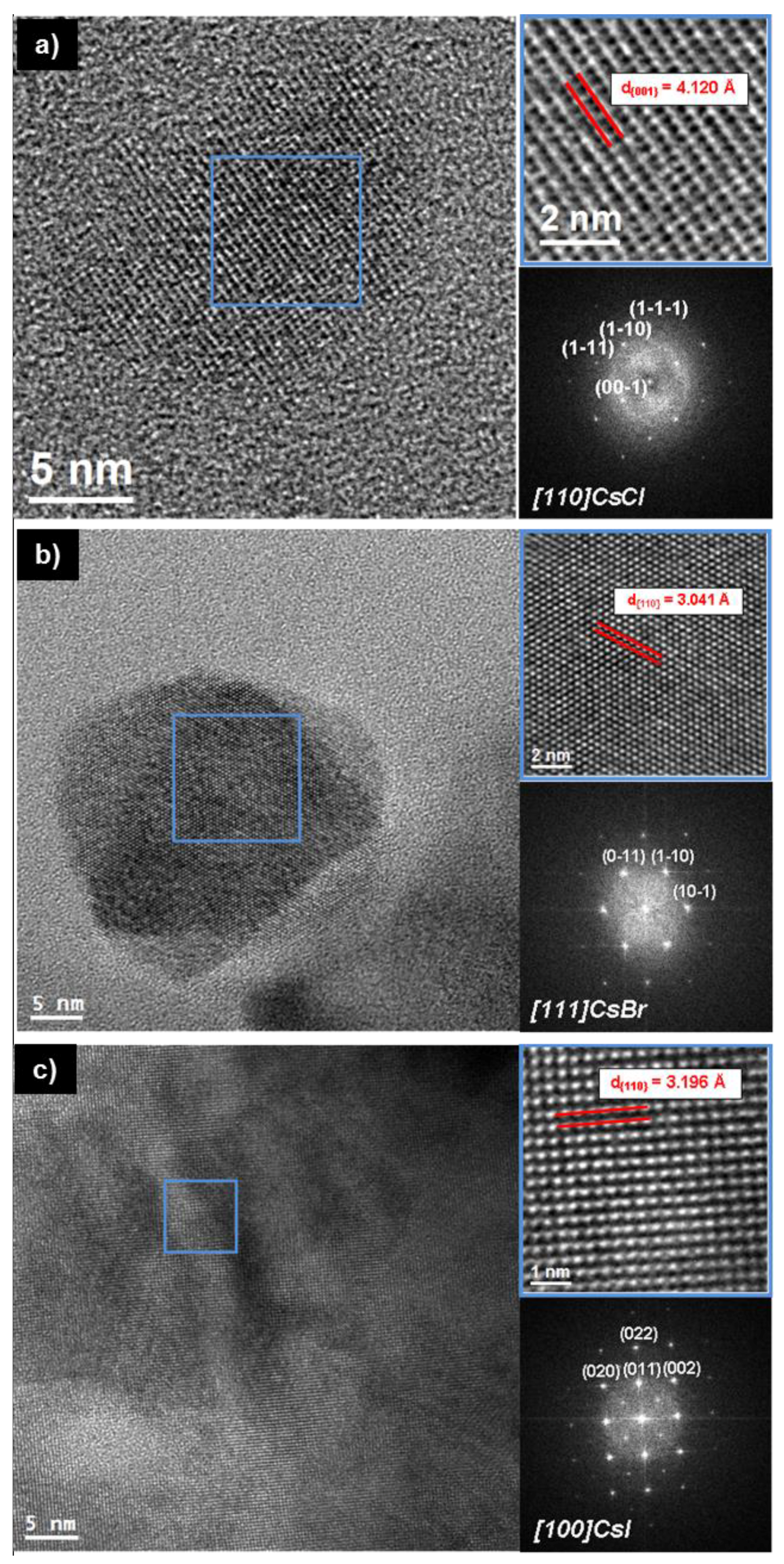
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sarkas, H.W.; Kidder, L.H.; Bowen, K.H. Photoelectron spectroscopy of color centers in negatively charged cesium iodide nanocrystals. J. Chem. Phys. 1995, 102, 57–66. [Google Scholar] [CrossRef]
- Halliday, M.T.E.; Hess, W.P.; Shluger, A.L. Structure and properties of electronic and hole centers in CsBr from theoretical calculations. J. Phys. Condens. Matter 2015, 27, 245501. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Arun, P.; Ravi Kant, C.; Mehra, N.C.; Makinistian, L.; Albanesi, E.A. Effect of residual stress on the optical properties of CsCl thin films. J. Phys. Chem. Solids 2010, 71, 163–169. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, C.; Li, Q.; Zhou, Y.; Li, Q.; Ma, Y. High-pressure phase transition of cesium chloride and cesium bromide. Phys. Chem. Chem. Phys. 2014, 16, 17924–17929. [Google Scholar] [CrossRef] [PubMed]
- Korovkin, E.V. Detection of defects binding free polarons in colored alkali halide crystals. Phys. Solid State 2017, 59, 490–494. [Google Scholar] [CrossRef]
- Flórez, M.; Recio, J.M.; Francisco, E.; Blanco, M.A.; Pendás, A.M. First-principles study of the rocksalt–cesium chloride relative phase stability in alkali halides. Phys. Rev. B 2002, 66, 144112. [Google Scholar] [CrossRef]
- Kowalski, M.P.; Fritz, G.G.; Cruddace, R.G.; Unzicker, A.E.; Swanson, N. Quantum efficiency of cesium iodide photocathodes at soft X-ray and extreme ultraviolet wavelengths. Appl. Opt. 1986, 25, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Boutboul, T.; Breskin, A.; Chechik, R.; Braem, A.; Lion, G. Ultraviolet photoabsorption measurements in alkali iodide and caesium bromide evaporated films. J. Appl. Phys. 1998, 83, 7896–7899. [Google Scholar] [CrossRef]
- Wei, Z.; Perumal, A.; Su, R.; Sushant, S.; Xing, J.; Zhan, Q.; Tan, S.T.; Demir, H.V.; Xiong, Q. Solution-processed highly bright and durable cesium lead halide perovskite light-emitting diodes. Nanoscale 2016, 8, 18021–18026. [Google Scholar] [CrossRef] [PubMed]
- Lü, Z.; Deng, Z.; Chen, Z.; Du, H.; Zou, Y.; Xu, D.; Wang, Y. Improved performance of organic light-emitting diodes with cesium chloride inside tris (8-hydroxyquinoline) aluminum. Curr. Appl. Phys. 2011, 11, 573–577. [Google Scholar] [CrossRef]
- Lü, Z.; Deng, Z.; Hou, Y.; Zhang, X.; Xu, H. Enhanced properties of organic electroluminescent devices with cesium chloride ultra-thin layer. Displays 2013, 34, 69–74. [Google Scholar] [CrossRef]
- Shahi, M.; Gautam, S.; Shah, P.V.; Rawat, J.S.; Chaudhury, P.K.; Tandon, R.P. Decoration of cesium iodide nano particles on patterned carbon nanotube emitter arrays to improve their field emission. J. Nanopart. Res. 2013, 15, 1497. [Google Scholar] [CrossRef]
- Farzaneh, A.; Abdi, M.R.; Saraee, K.R.E.; Mostajaboddavati, M.; Quaranta, A. Cesium-iodide-based nanocrystal for the detection of ionizing radiation. Opt. Mater. 2016, 55, 22–26. [Google Scholar] [CrossRef]
- Tao, T.; Yang, Z.; Su, Y.; Wei, H.; Sharma, P.; Wei, L.; Kong, E.S.-W.; Zhang, Y. Photolithography enhancement by incorporating photoluminescent nanoscale cesium iodide molecular dots into the photoresists. J. Nanopart. Res. 2013, 15, 1991. [Google Scholar] [CrossRef]
- Arsic, J.; Reynhout, I.C.; van Enckevort, W.J.P.; Vlieg, E. Growth and characterization of cesium halides with cubic morphologies. J. Cryst. Growth 2003, 253, 472–480. [Google Scholar] [CrossRef]
- Jiang, C.B.; Wu, B.; Zhang, Z.Q.; Lu, L.; Li, S.X.; Mao, S.X. Lithium fluoride nanowires via vapor-liquid-solid growth. Appl. Phys. Lett. 2006, 88, 93103. [Google Scholar] [CrossRef]
- Abdelkader, E.; Buckner, S.W. Synthesis of NaX (X = F, Cl, Br, I) Nanoparticles. Soft Nanosci. Lett. 2013, 3, 22–27. [Google Scholar] [CrossRef]
- Zheng, F.; Wang, D.; Fang, H.; Wang, H.; Wang, M.; Huang, K.; Chen, H.; Feng, S. Controlled Crystallization of Sodium Chloride Nanocrystals in Microdroplets Produced by Electrospray from an Ultra-Dilute Solution. Eur. J. Inorg. Chem. 2016, 2016, 1860–1865. [Google Scholar] [CrossRef]
- Lim, S.-C.; Lin, H.-P.; Tsai, W.-L.; Lin, H.-W.; Hsu, Y.-T.; Tuan, H.-Y. Binary halide, ternary perovskite-like, and perovskite-derivative nanostructures: Hot injection synthesis and optical and photocatalytic properties. Nanoscale 2017, 9, 3747–3751. [Google Scholar] [CrossRef] [PubMed]
- De Roo, J.; Ibáñez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.C.; Van Driessche, I.; Kovalenko, M.V.; Hens, Z. Highly Dynamic Ligand Binding and Light Absorption Coefficient of Cesium Lead Bromide Perovskite Nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the Ligand-Mediated Synthesis of Colloidal CsPbBr3 Perovskite Nanocrystals: The Role of Organic Acid, Base, and Cesium Precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. Handbook of Chemistry and Physics, 97th ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498754293. [Google Scholar]
- Wise, M.E.; Biskos, G.; Martin, S.T.; Russell, L.M.; Buseck, P.R. Phase Transitions of Single Salt Particles Studied Using a Transmission Electron Microscope with an Environmental Cell. Aerosol Sci. Technol. 2005, 39, 849–856. [Google Scholar] [CrossRef]
- Swanson, H.E.; Fuyat, R.K. Standard X-Ray Diffraction Powder Patterns. Vol. II, Data for 30 Inorganic Substances. In National Bureau of Standards Circular 539, 2nd ed.; National Bureau of Standards: Washington, DC, USA, 1953. [Google Scholar]
- Swanson, H.E.; Ugrinic, G.M.; Fuyat, R.K. Standard X-Ray Diffraction Powder Patterns. Vol. III, Data for 34 Inorganic Substances. In National Bureau of Standards Circular 539, 3rd ed.; National Bureau of Standards: Washington, DC, USA, 1954. [Google Scholar]
- Hobbs, L.W. Transmission Electron Microscopy of Defects in Alkali Halides. J. Phys. Colloq. 1973, 34, C9-227–C9-241. [Google Scholar] [CrossRef]
- Whetten, R.L. Alkali halide nanocrystals. Acc. Chem. Res. 1993, 26, 49–56. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, P.J.; Meyns, M.; Zuo, Y.; Grau-Carbonell, A.; Lagoudakis, P.G.; Charlton, M.D.B.; Martí-Sánchez, S.; Arbiol, J.; Cabot, A.; Kanaras, A.G. Colloidal Synthesis of CsX Nanocrystals (X = Cl, Br, I). Nanomaterials 2018, 8, 506. https://doi.org/10.3390/nano8070506
Shaw PJ, Meyns M, Zuo Y, Grau-Carbonell A, Lagoudakis PG, Charlton MDB, Martí-Sánchez S, Arbiol J, Cabot A, Kanaras AG. Colloidal Synthesis of CsX Nanocrystals (X = Cl, Br, I). Nanomaterials. 2018; 8(7):506. https://doi.org/10.3390/nano8070506
Chicago/Turabian StyleShaw, Peter J., Michaela Meyns, Yong Zuo, Albert Grau-Carbonell, Pavlos G. Lagoudakis, Martin D. B. Charlton, Sara Martí-Sánchez, Jordi Arbiol, Andreu Cabot, and Antonios G. Kanaras. 2018. "Colloidal Synthesis of CsX Nanocrystals (X = Cl, Br, I)" Nanomaterials 8, no. 7: 506. https://doi.org/10.3390/nano8070506
APA StyleShaw, P. J., Meyns, M., Zuo, Y., Grau-Carbonell, A., Lagoudakis, P. G., Charlton, M. D. B., Martí-Sánchez, S., Arbiol, J., Cabot, A., & Kanaras, A. G. (2018). Colloidal Synthesis of CsX Nanocrystals (X = Cl, Br, I). Nanomaterials, 8(7), 506. https://doi.org/10.3390/nano8070506





