Variations of Major Product Derived from Conversion of 5-Hydroxymethylfurfural over a Modified MOFs-Derived Carbon Material in Response to Reaction Conditions
Abstract
:1. Introduction
2. Experimental Section
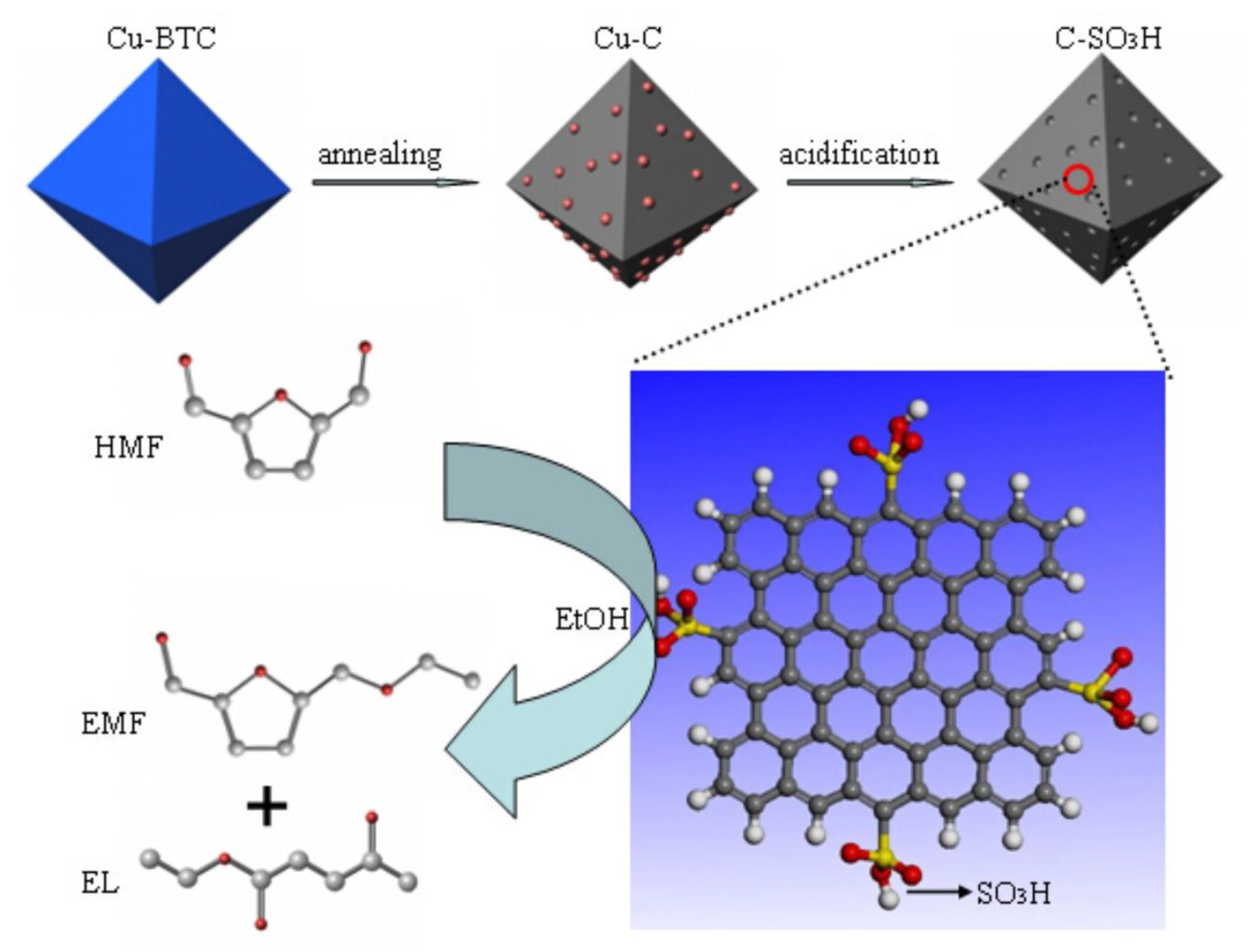
2.1. Preparation of the Catalyst
2.2. Characterization of the Catalyst
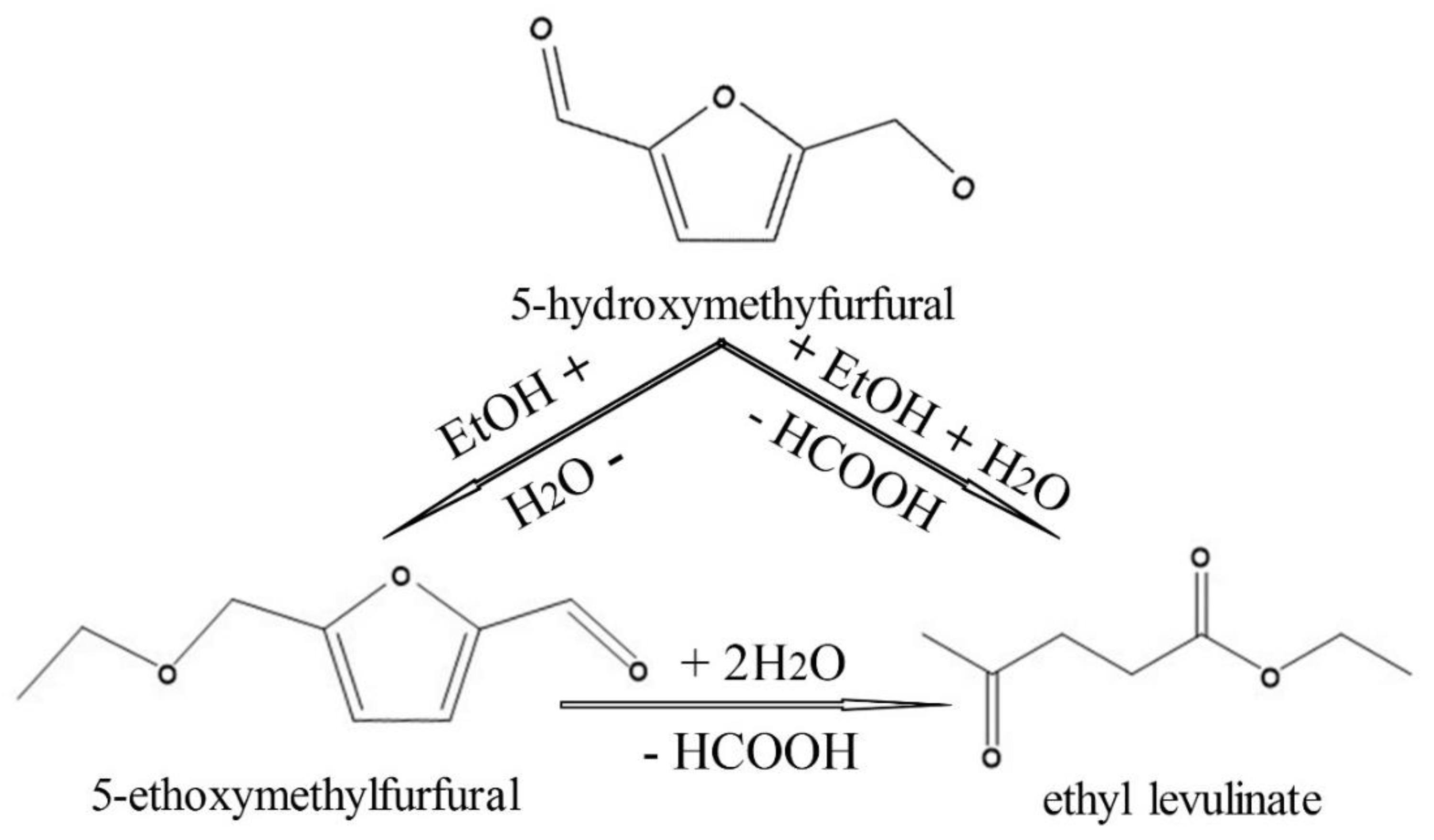
2.3. HMF Conversion into EMF and EL
3. Results and Discussion
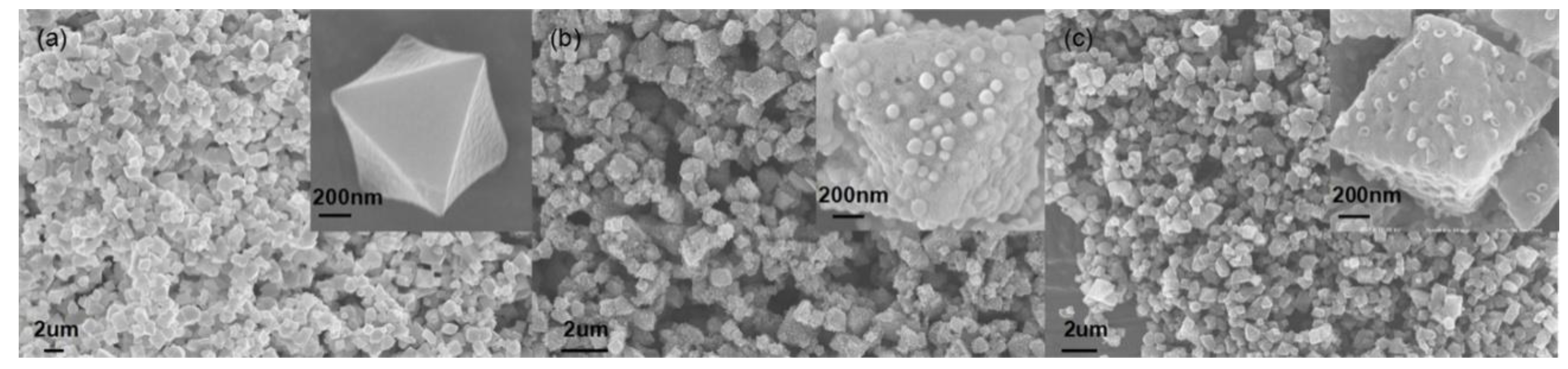
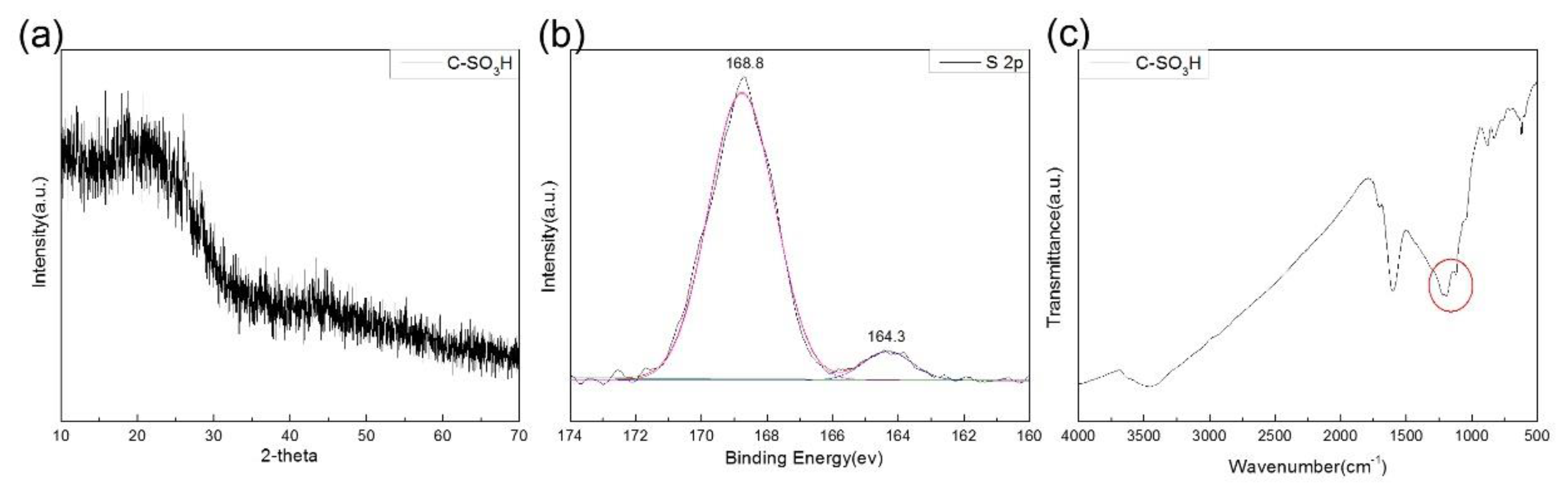
3.1. Catalyst Characterization
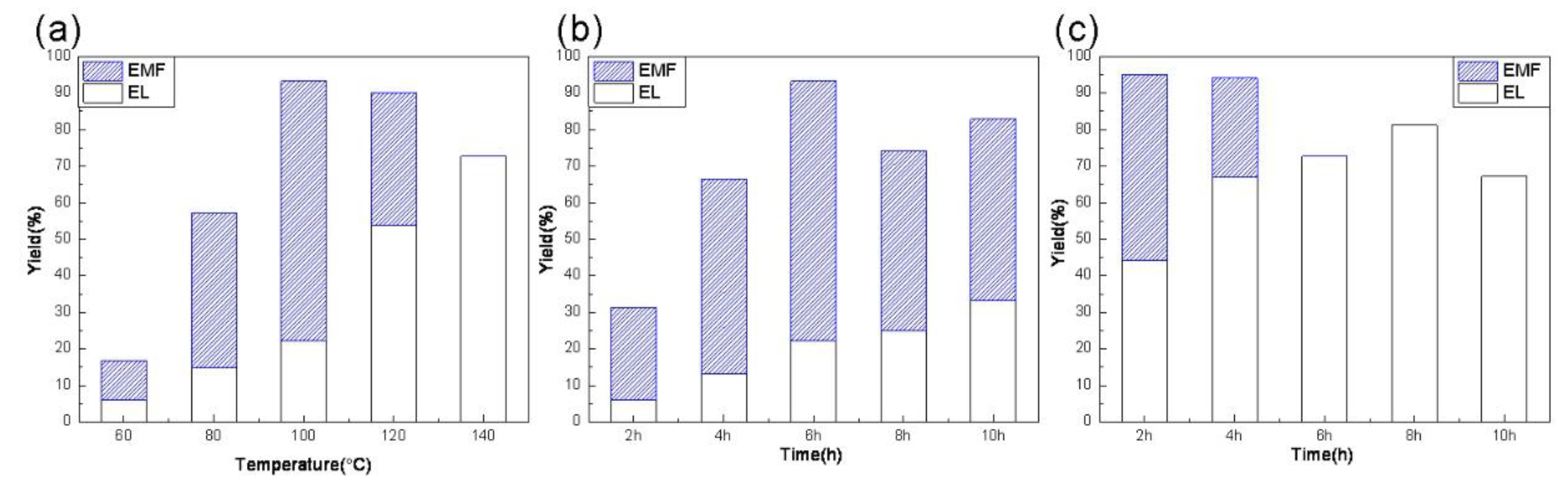
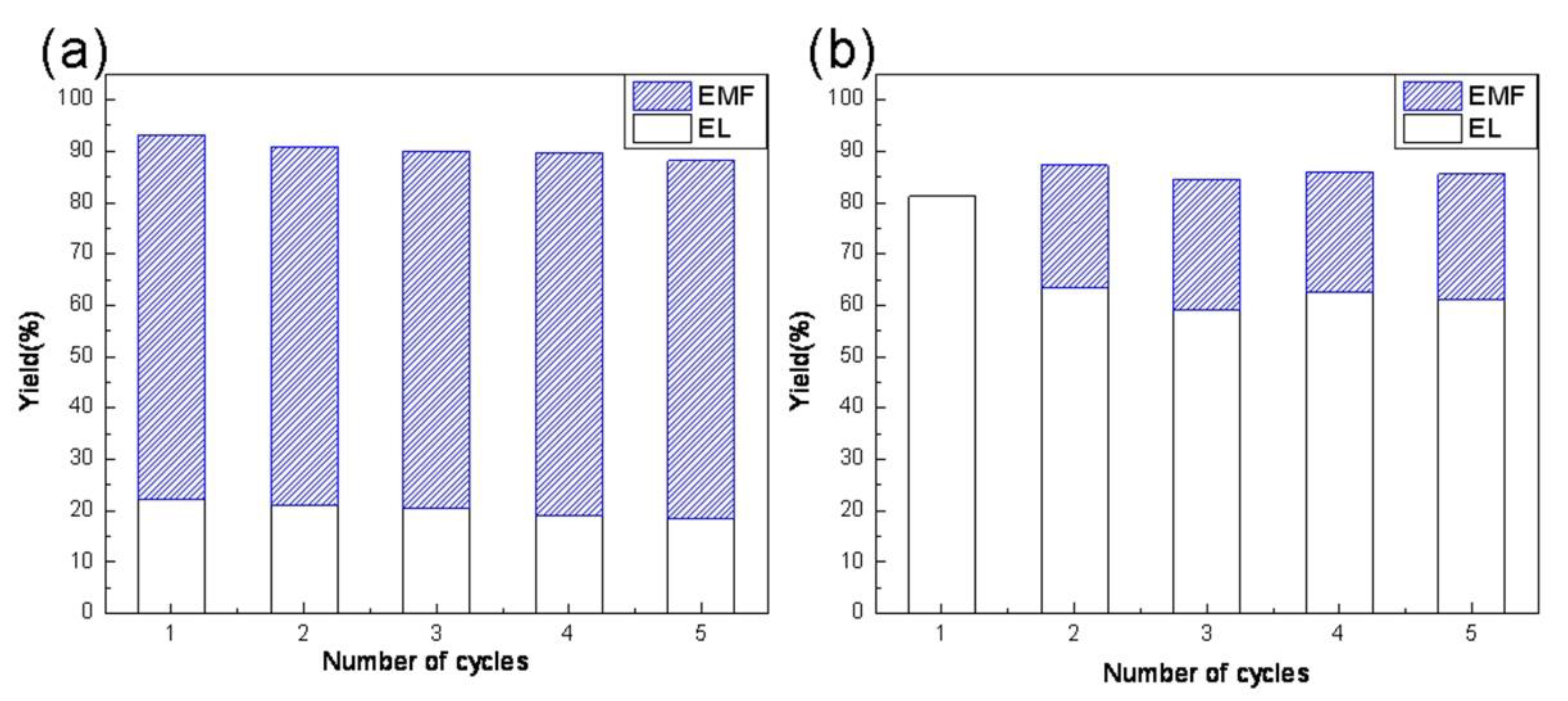
3.2. Catalyst Activity
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, T.J.; Liu, Q.Y.; Zhang, X.H.; Ma, W.C.; Zhang, Q.A. A review of thermal–chemical conversion of lignocellulosic biomass in China. Biotechnol. Adv. 2012, 30, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Luque, R.; Sepulveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuelsvia aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef] [PubMed]
- Long, H.L.; Li, X.B.; Wang, H.; Jia, J.D. Biomass resources and their bioenergy potential estimation: A review. Renew. Sustain. Energy Rev. 2013, 26, 344–352. [Google Scholar] [CrossRef]
- Kocar, G.; Civas, N. An overview of biofuels from energy crops: Current status and future prospects. Renew. Sustain. Energy Rev. 2013, 28, 900–916. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lin, L.; Liu, S.J. Efficient production of furan derivatives from a sugar mixture by catalytic process. Energy Fuels 2012, 26, 4560–4567. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Li, J.; Lai, D.M.; Fu, Y.; Guo, Q.X. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply. Angew. Chem. Int. Ed. 2009, 48, 6529–6532. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Centi, G.; Macario, A.; Aloise, A.; Giordano, G. Etherification of 5-hydroxymethyl-2-furfural (HMF) with ethanol to biodiesel components using mesoporous solid acidic catalysts. Catal. Today 2011, 175, 435–441. [Google Scholar] [CrossRef]
- Mascal, M.; Nikitin, E.B. Direct, high-yield conversion of cellulose into biofuel. Angew. Chem. Int. Ed. 2008, 41, 8042–8044. [Google Scholar] [CrossRef]
- Gruter, G.J.M.; Dautzenberg, F. Method for the Synthesis of 5-Alkoxymethylfurfural Ethers and Their Use. European Patent Application No. 1834950 A1, 19 September 2007. [Google Scholar]
- Gruter, G.J.M.; Dautzenberg, F. Method for the Synthesis of 5-Hydroxymethylfurfural Ethers and Their Use. U.S. Patent Application No. 0082304 A1, 7 April 2011. [Google Scholar]
- Joshi, H.; Moser, B.R.; Toler, J.; Smith, W.F.; Terry, W. Ethyl levulinate: A potential bio-based diluent for biodiesel which improves cold flow properties. Biomass Bioenergy 2011, 35, 3262–3266. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Wang, Z.H.; Chen, Q.W. Conversion of 5-hydroxymethylfurfural into 5-ethoxymethylfurfural and ethyl levulinate catalyzed by MOF-based heteropolyacid materials. Green Chem. 2016, 18, 5884–5889. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Alam, M.I.; Abu-Omar, M.M.; Saha, B. Direct conversion of cellulose and lignocellulosic biomass into chemicals and biofuel with metal chloride catalysts. J. Catal. 2012, 288, 8–15. [Google Scholar] [CrossRef]
- Kraus, G.A.; Guney, T. A direct synthesis of 5-alkoxymethylfurfural ethers from fructose via sulfonic acid-functionalizedionic liquids. Green Chem. 2012, 14, 1593–1596. [Google Scholar] [CrossRef]
- Yang, Y.; Abu-Omar, M.M.; Hu, C. Heteropolyacid catalyzed conversion of fructose, sucrose, and inulin to 5-ethoxymethylfurfural, a liquid biofuel candidate. Appl. Energy 2012, 99, 80–84. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Nguyen Van Buu, O.; Riisager, A. Conversion of mono- and disaccharides to ethyl levulinate and ethyl pyranoside with sulfonic acid-functionalized ionic liquids. ChemSusChem 2011, 4, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, J.; Huang, X.; Chen, L.; Ma, L.; Li, X. Conversion of fructose into 5-hydroxymethylfurfural and alkyl levulinates catalyzed by sulfonic acid-functionalized carbon materials. Green Chem. 2013, 15, 2895–2903. [Google Scholar] [CrossRef]
- Peng, L.; Lin, L.; Zhang, J.; Shi, J.; Liu, S. Solid acid catalyzed glucose conversion to ethyl levulinate. Appl. Catal. A Gen. 2011, 397, 259–265. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, F.C.; Xia, G.L.; Lun, Z.Y.; Chen, Q.W. Experimental and theoretical investigations of nitro-group doped porous carbon as a high performance lithium-ion battery anode. J. Mater. Chem. A 2015, 3, 18657–18666. [Google Scholar] [CrossRef]
- Struis, R.; Schildhauer, T.; Czekaj, I.; Janousch, M.; Biollaz, S.; Ludwig, C. Sulphur poisoning of Ni catalysts in the SNG production from biomass: A TPO/XPS/XAS study. Appl. Catal. A Gen. 2009, 362, 121–128. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, H.; Zhang, B.; Ren, J.; Lu, G.; Wang, Y. One-pot synthesis of carbonaceous monolith with surface sulfonic groups and its carbonization/activation. Carbon 2011, 49, 1811–1820. [Google Scholar] [CrossRef]
- Lanzafame, P.; Barbera, K.; Perathoner, S.; Centi, G.; Aloise, A.; Migliori, M.; Macarib, A.; Nagy, J.B.; Giordano, G. The role of acid sites induced by defects in the etherification of HMF on Silicalite-1 catalysts. J. Catal. 2015, 330, 558–568. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Z.; Huang, K. Cellulose sulfuric acid as a bio-supported and recyclable solid acid catalyst for the synthesis of 5-hydroxymethylfurfural and 5-ethoxymethylfurfural from fructose. Cellulose 2013, 20, 2081–2089. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Liu, J.; Yin, D.; Su, S.; Ding, H. Hydrolysis of cellulose into reducing sugars in ionic liquids. Fuel 2016, 164, 46–50. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, Z.; Zheng, J.; Lin, J. Efficient synthesis of promising liquid fuels 5-ethoxymethylfurfural from carbohydrates. Fuel 2015, 150, 236–242. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Bell, A.T. Etherification and reductive etherification of 5-(hydroxymethyl)furfural: 5-(alkoxymethyl)furfurals and 2,5-bis(alkoxymethyl)furans as potential bio-diesel candidates. Green Chem. 2012, 14, 1626–1634. [Google Scholar] [CrossRef]
- Yin, S.; Sun, J.; Liu, B.; Zhang, Z. Magnetic material grafted cross-linked imidazolium based polyionic liquids: An efficient acid catalyst for the synthesis of promising liquid fuel 5-ethoxymethylfurfural from carbohydrates. J. Mater. Chem. A 2015, 3, 4992–4999. [Google Scholar] [CrossRef]
- Liu, B.; Gou, Z.; Liu, A.; Zhang, Z. Synthesis of furan compounds from HMF and fructose catalyzed by aluminum-exchanged K-10 clay. J. Ind. Eng. Chem. 2015, 21, 338–339. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, Z.; Fang, Z.; Liu, B.; Huang, K. Synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose in ethanol catalyzed by MCM-41 supported phosphotungstic acid. J. Ind. Eng. Chem. 2014, 20, 1977–1984. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Liu, B.; Li, J. Silica coated magnetic Fe3O4 nanoparticles supported phosphotungstic acid: A novel environmentally friendly catalyst for the synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose. Catal. Sci. Technol. 2013, 3, 2104–2112. [Google Scholar] [CrossRef]
| Entry | Catalyst | Reaction Condition | Yield of EMF % | Yield of EL % |
|---|---|---|---|---|
| 1 | None | 100 °C/6 h | 0 | 0 |
| 2 | None | 140 °C/6 h | 26 | 4 |
| 3 | C-SO3H | 100 °C/6 h | 71 | 22 |
| 4 | C-SO3H | 140 °C/6 h | <1 | 73 |
| 5 | Cu-BTC | 100 °C/6 h | 0 | 0 |
| 6 | Cu-C | 100 °C/6 h | 0 | 0 |
| Entry | Catalyst | Conversion % | Yield of EMF % | Yield of EL % |
|---|---|---|---|---|
| 1 [11] | Z-SBA-15 | 100 | 76 | 23 |
| 2 [27] | NH4-S5.5 | 69 | 21 | 8 |
| 3 [22] | CNT-PSSA | 99 | - | 84 |
| 4 [23] | SO42−/ZrO2 | 100 | - | 30 |
| 5 [33] | K-10 clay-Al | 78.9 | 73.2 | - |
| 6 [34] | 40 wt % MCM-41-HPW | 96 | 85 | 7 |
| 7 [35] | Fe3O4@SiO2-HPW | 98 | 84 | 6 |
| 8 (in this work) a | C-SO3H | 100 | 71 | 22 |
| 9 (in this work) b | C-SO3H | 100 | - | 81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Chen, Q. Variations of Major Product Derived from Conversion of 5-Hydroxymethylfurfural over a Modified MOFs-Derived Carbon Material in Response to Reaction Conditions. Nanomaterials 2018, 8, 492. https://doi.org/10.3390/nano8070492
Wang Z, Chen Q. Variations of Major Product Derived from Conversion of 5-Hydroxymethylfurfural over a Modified MOFs-Derived Carbon Material in Response to Reaction Conditions. Nanomaterials. 2018; 8(7):492. https://doi.org/10.3390/nano8070492
Chicago/Turabian StyleWang, Zhenhua, and Qianwang Chen. 2018. "Variations of Major Product Derived from Conversion of 5-Hydroxymethylfurfural over a Modified MOFs-Derived Carbon Material in Response to Reaction Conditions" Nanomaterials 8, no. 7: 492. https://doi.org/10.3390/nano8070492
APA StyleWang, Z., & Chen, Q. (2018). Variations of Major Product Derived from Conversion of 5-Hydroxymethylfurfural over a Modified MOFs-Derived Carbon Material in Response to Reaction Conditions. Nanomaterials, 8(7), 492. https://doi.org/10.3390/nano8070492





