Insights into Metal Oxide and Zero-Valent Metal Nanocrystal Formation on Multiwalled Carbon Nanotube Surfaces during Sol-Gel Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Nanohybrid Synthesis
2.3. Physical Morphology and Elemental Composition
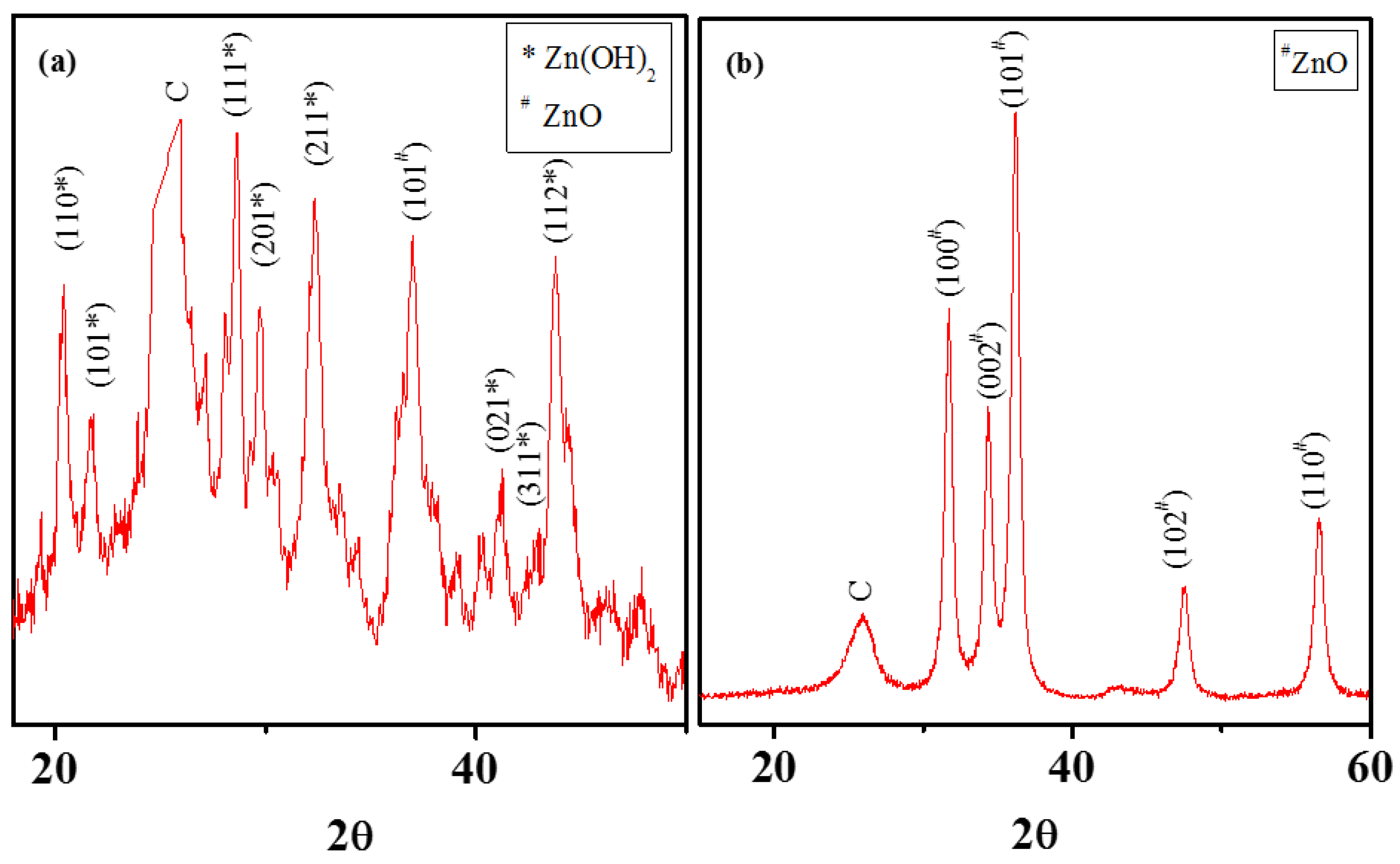
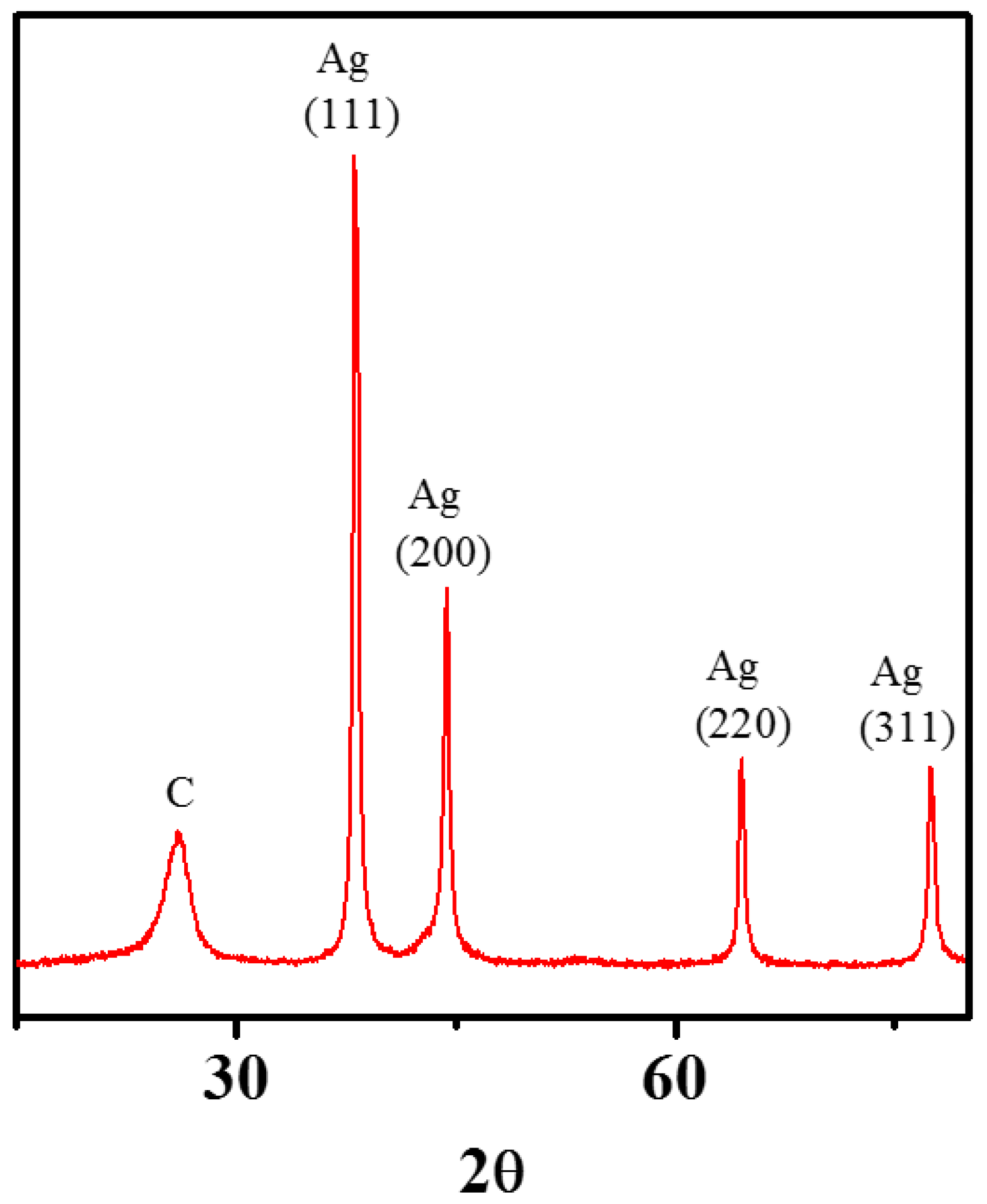
2.4. Analysis of Crystallinity
2.5. Measuring Oxidation-Reduction Potentials (ORPs)
3. Results and Discussion
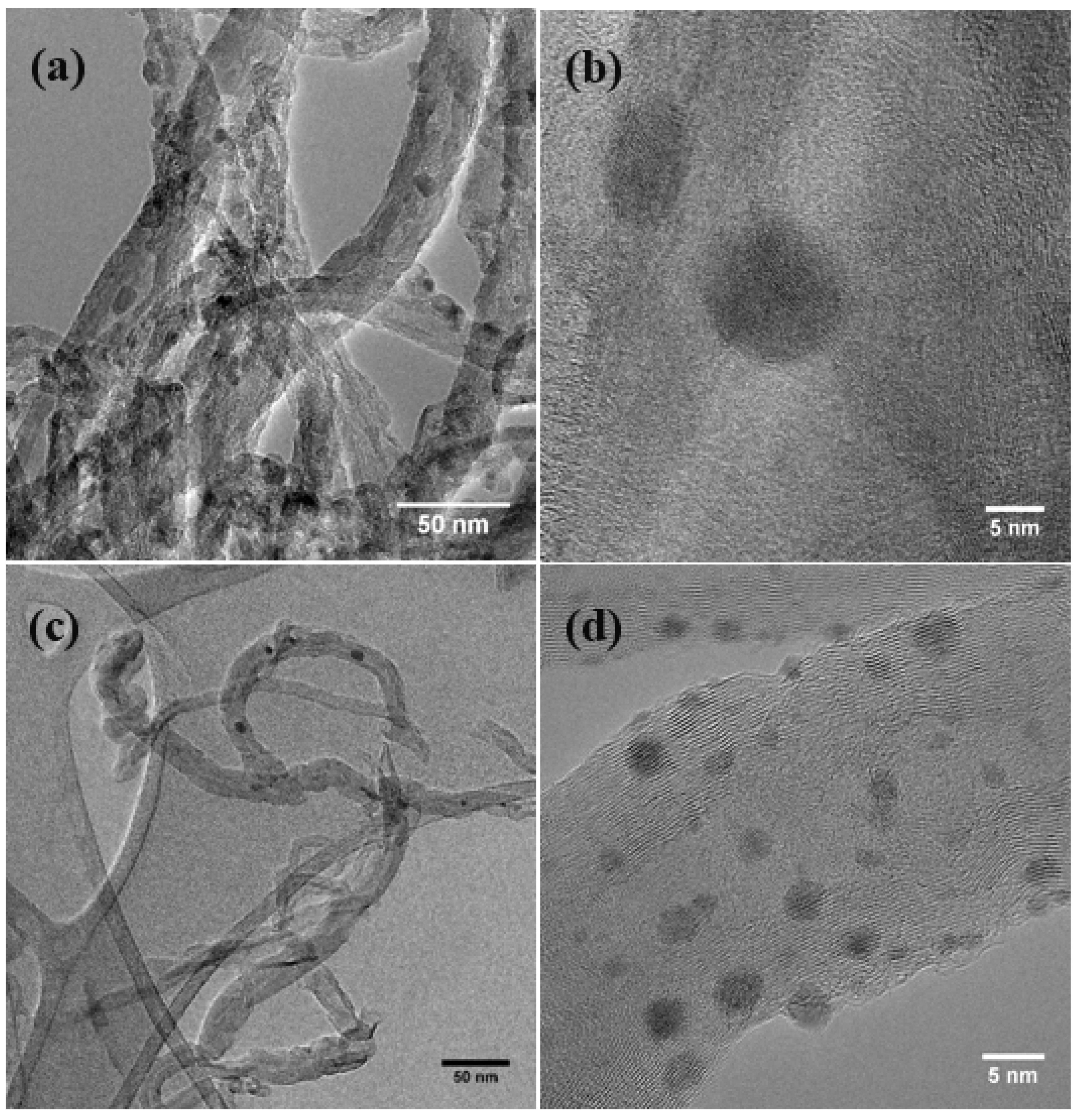
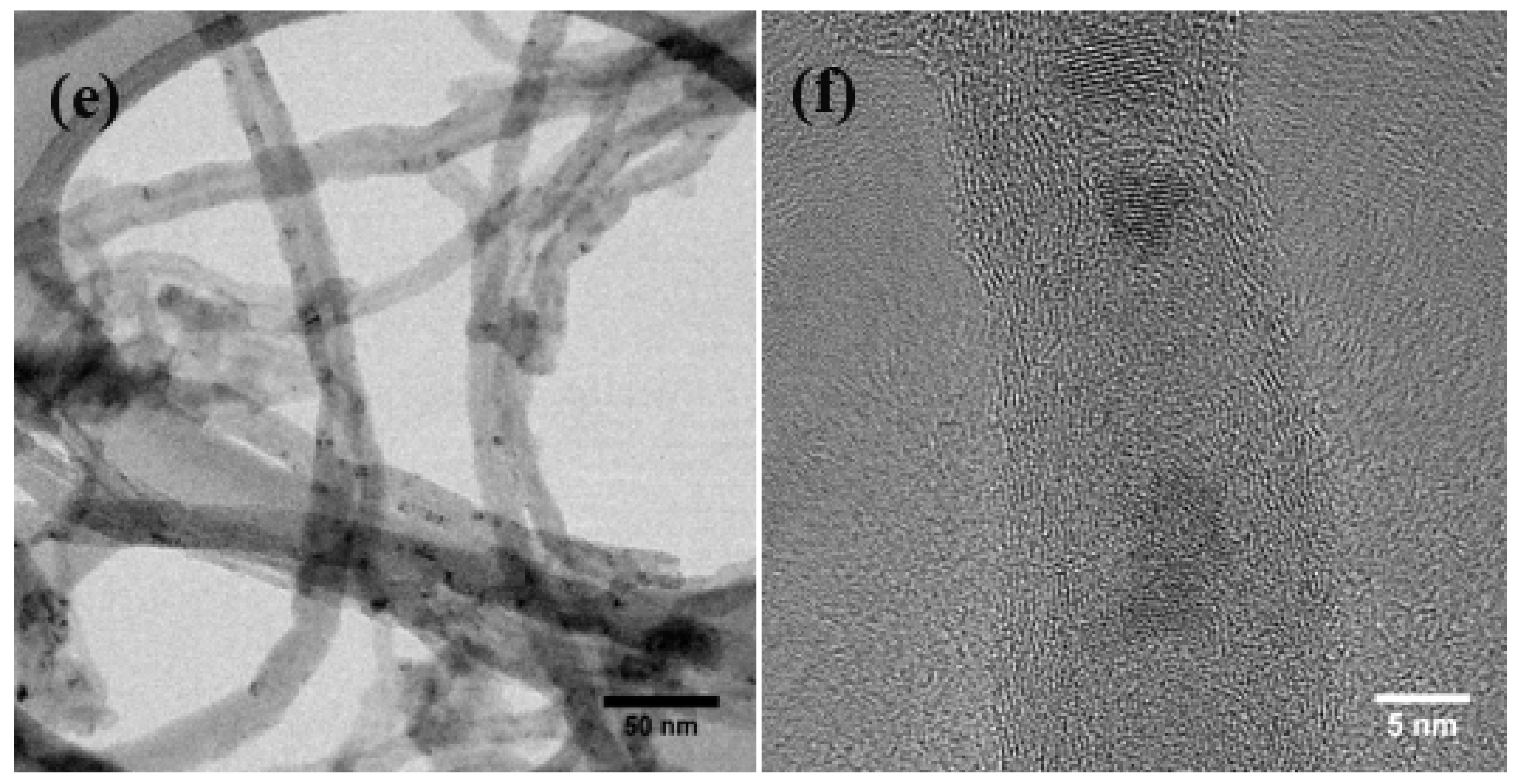
3.1. Physical Morphology and Composition
3.2. Chemical Attachment of Zn onto MWNTs: Hydroxide to Oxide Formation Pathway
3.3. Zero-Valent Metal Formation on MWNTs with no Reducing Agent
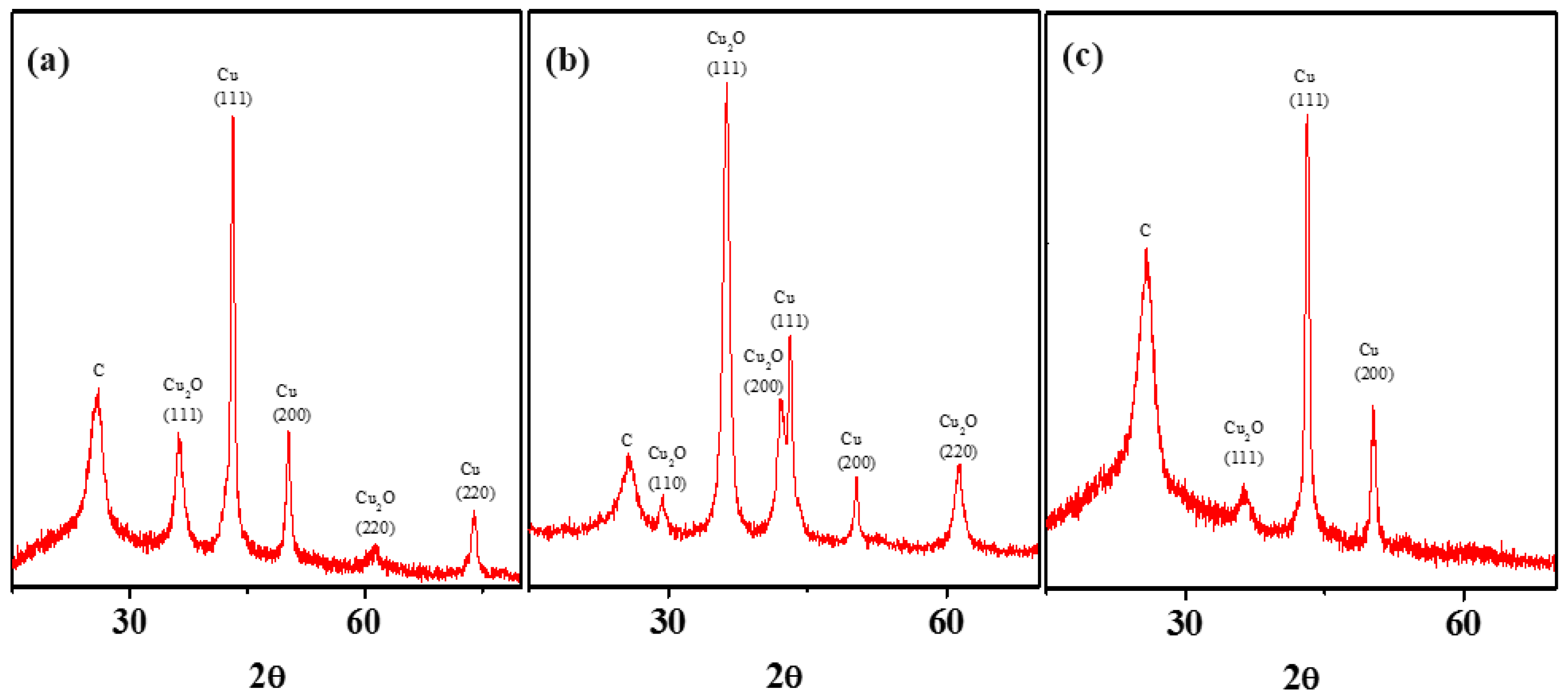
3.4. Intermediate SEP-Metal Cu: The Anomaly That Forces Oxide Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eder, D.; Windle, A.H. Carbon-inorganic hybrid materials: The carbon-nanotube/TiO2 interface. Adv. Mater. 2008, 20, 1787–1793. [Google Scholar] [CrossRef]
- Alley, N.J.; Liao, K.S.; Andreoli, E.; Dias, S.; Dillon, E.P.; Orbaek, A.W.; Barron, A.R.; Byrne, H.J.; Curran, S.A. Effect of carbon nanotube-fullerene hybrid additive on P3HT:PCBM bulk-heterojunction organic photovoltaics. Synth. Met. 2012, 162, 95–101. [Google Scholar] [CrossRef]
- Llobet, E.; Espinosa, E.; Sotter, E.; Ionescu, R.; Vilanova, X.; Torres, J.; Felten, A.; Pireaux, J.-J.; Ke, X.; Van Tendeloo, G. Carbon nanotube—TiO2 hybrid films for detecting traces of O2. Nanotechnology 2008, 19, 375501. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Xie, D.; Chen, G. Polyaniline-Coated Fe3O4 Nanoparticle—Carbon-Nanotube Composite and its Application in Electrochemical Biosensing. Small 2008, 4, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Elim, H.I.; Foo, Y.L.; Yu, T.; Liu, Y.; Ji, W.; Lee, J.Y.; Shen, Z.; Wee, A.T.-S.; Thong, J.T.-L. Multiwalled carbon nanotubes beaded with ZnO nanoparticles for ultrafast nonlinear optical switching. Adv. Mater. 2006, 18, 587–592. [Google Scholar] [CrossRef]
- Das, D.; Plazas-Tuttle, J.; Sabaraya, I.V.; Jain, S.S.; Sabo-Attwood, T.; Saleh, N.B. An elegant method for large scale synthesis of metal oxide-carbon nanotube nanohybrids for nano-environmental application and implication studies. Environ. Sci. Nano 2017, 4, 60–68. [Google Scholar] [CrossRef]
- Lv, X.; Xu, J.; Jiang, G.; Xu, X. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere 2011, 85, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Tang, B.Z.; Kim, J.-K. Effect of CNT decoration with silver nanoparticles on electrical conductivity of CNT-polymer composites. Carbon 2008, 46, 1497–1505. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Müller, A.; Cheetham, A.K. The Chemistry of Nanomaterials: Synthesis, Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue Calorimetric Studies of Blends of Poly(vinyl ester)s and Polyacrylates. Macromolecules 1996, 29, 1579–1583. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Analogue calorimetry of polymer blends: Poly(styrene-co-acrylonitrile) and poly(phenyl acrylate) or poly(vinyl benzoate). Polymer 1996, 37, 2439–2443. [Google Scholar] [CrossRef]
- Rana, D.; Mandal, B.M.; Bhattacharyya, S.N. Miscibility and phase diagrams of poly(phenyl acrylate) and poly(styrene-co-acrylonitrile) blends. Polymer 1993, 34, 1454–1459. [Google Scholar] [CrossRef]
- Fokin, V.M.; Zanotto, E.D.; Schmelzer, J.W.P. On the thermodynamic driving force for interpretation of nucleation experiments. J. Non-Cryst. Solids 2010, 356, 2185–2191. [Google Scholar] [CrossRef]
- Vorkapic, D.; Matsoukas, T. Effect of temperature and alcohols in the preparation of titania nanoparticles from alkoxides. J. Am. Ceram. Soc. 1998, 81, 2815–2820. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Ceram. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, D. Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers. Environ. Sci. Technol. 2007, 41, 6216–6221. [Google Scholar] [CrossRef] [PubMed]
- Bom, D.; Andrews, R.; Jacques, D.; Anthony, J.; Chen, B.; Meier, M.S.; Selegue, J.P. Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: Evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett. 2002, 2, 615–619. [Google Scholar] [CrossRef]
- Mulfinger, L.; Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322. [Google Scholar] [CrossRef]
- Mo, C.B.; Cha, S.I.; Kim, K.T.; Lee, K.H.; Hong, S.H. Fabrication of carbon nanotube reinforced alumina matrix nanocomposite by sol-gel process. Mater. Sci. Eng. A 2005, 395, 124–128. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Li, W. Colloidal processing of carbon nanotube/alumina composites. Chem. Mater. 2002, 14, 5169–5172. [Google Scholar] [CrossRef]
- Kumari, L.; Zhang, T.; Du, G.; Li, W.; Wang, Q.; Datye, A.; Wu, K. Synthesis, microstructure and electrical conductivity of carbon nanotube—Alumina nanocomposites. Ceram. Int. 2009, 35, 1775–1781. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, L.; Fu, H.; Fang, J. Ultrasonic-assisted preparation of carbon nanotube/cerium oxide composites. Carbon 2006, 44, 2853–2855. [Google Scholar] [CrossRef]
- Kalubarme, R.S.; Kim, Y.-H.; Park, C.-J. One step hydrothermal synthesis of a carbon nanotube/cerium oxide nanocomposite and its electrochemical properties. Nanotechnology 2013, 24, 365401. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Yan, X.; Xue, Q. Facile preparation and electrochemical characterization of cobalt oxide/multi-walled carbon nanotube composites for supercapacitors. J. Power Source 2011, 196, 7841–7846. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Yao, J.; Wexler, D.; Ahn, J.-H. Hydrothermal synthesis of carbon nanotube/cobalt oxide core-shell one-dimensional nanocomposite and application as an anode material for lithium-ion batteries. Electrochem. Commun. 2009, 11, 546–549. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, D.; Shi, L.; Fang, J. Synthesis and strong red photoluminescence of europium oxide nanotubes and nanowires using carbon nanotubes as templates. Acta Mater. 2008, 56, 955–967. [Google Scholar] [CrossRef]
- Chuansheng, C.; Tiangui, L.; Xiaohua, C.; Bin, Y.; Zhenwu, N.; Zhenwu, N.; Can, Z.; Chenchong, H. Preparation of Multi-Walled Carbon Nanotubes/Europium Oxide Composite. Rare Met. Mater. Eng. 2009, 38, 477–480. [Google Scholar]
- Chen, C.; Hu, J.; Shao, D.; Li, J.; Wang, X. Adsorption behavior of multiwall carbon nanotube/iron oxide magnetic composites for Ni (II) and Sr (II). J. Hazard. Mater. 2009, 164, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shao, D.; Chen, C.; Sheng, G.; Li, J.; Wang, X.; Nagatsu, M. Plasma-induced grafting of cyclodextrin onto multiwall carbon nanotube/iron oxides for adsorbent application. J. Phys. Chem. B 2010, 114, 6779–6785. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, X.; Nagatsu, M. Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ. Sci. Technol. 2009, 43, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Chen, C.; Ren, X.; Hu, J.; Wang, X. Removal of cobalt from aqueous solution by magnetic multiwalled carbon nanotube/iron oxide composites. Chem. Eng. J. 2011, 174, 126–133. [Google Scholar] [CrossRef]
- Ikuno, T.; Yasuda, T.; Honda, S.-I.; Oura, K.; Katayama, M.; Lee, J.-G.; Mori, H. Coating carbon nanotubes with inorganic materials by pulsed laser deposition. J. Appl. Phys. 2005, 98, 114305. [Google Scholar] [CrossRef]
- Ikuno, T.; Katayama, M.; Kamada, K.; Honda, S.-I.; Lee, J.-G.; Mori, H.; Oura, K. Insulator-coated carbon nanotubes synthesized by pulsed laser deposition. Jpn. J. Appl. Phys. 2003, 42, L1356. [Google Scholar] [CrossRef]
- Pan, L.; Konishi, Y.; Tanaka, H.; Chakrabarti, S.; Hokushin, S.; Akita, S.; Nakayama, Y. Effect of MgO coating on field emission of a stand-alone carbon nanotube. J. Vacuum Sci. Technol. B 2007, 25, 1581–1583. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, Z.H.; Li, J.H. A hybrid supercapacitor fabricated with a carbon nanotube cathode and a TiO2-B nanowire anode. Adv. Funct. Mater. 2006, 16, 2141–2146. [Google Scholar] [CrossRef]
- Roro, K.T.; Tile, N.; Mwakikunga, B.; Yalisi, B.; Forbes, A. Solar absorption and thermal emission properties of multiwall carbon nanotube/nickel oxide nanocomposite thin films synthesized by sol-gel process. Mater. Sci. Eng. B 2012, 177, 581–587. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.-S.; Hou, Z.-L.; Song, W.-L.; Zhang, L.; Lu, M.-M.; Jin, H.-B.; Fang, X.-Y.; Wang, W.-Z.; Yuan, J. Temperature dependent microwave attenuation behavior for carbon-nanotube/silica composites. Carbon 2013, 65, 124–139. [Google Scholar] [CrossRef]
- Sivakumar, R.; Guo, S.; Nishimura, T.; Kagawa, Y. Thermal conductivity in multi-wall carbon nanotube/silica-based nanocomposites. Scr. Mater. 2007, 56, 265–268. [Google Scholar] [CrossRef]
- Bottini, M.; Tautz, L.; Huynh, H.; Monosov, E.; Bottini, N.; Dawson, M.I.; Bellucci, S.; Mustelin, T. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chem. Commun. 2005, 6, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Wongchoosuk, C.; Wisitsoraat, A.; Tuantranont, A.; Kerdcharoen, T. Portable electronic nose based on carbon nanotube-SnO2 gas sensors and its application for detection of methanol contamination in whiskeys. Sens. Actuators B Chem. 2010, 147, 392–399. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube–TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Ding, M.; Sorescu, D.C.; Star, A. Photoinduced Charge Transfer and Acetone Sensitivity of Single-Walled Carbon Nanotube—Titanium Dioxide Hybrids. J. Am. Chem. Soc. 2013, 135, 9015–9022. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-M.; Wang, A.-R.; Zhang, M.-X.; Yang, H.-Y.; Zhou, B.; Shen, J. Investigation on properties of V2O5–MWCNTs composites as cathode materials. J. Sol Gel Sci. Technol. 2008, 46, 79–85. [Google Scholar] [CrossRef]
- Green, J.M.; Dong, L.; Gutu, T.; Jiao, J.; Conley, J.F., Jr.; Ono, Y. ZnO-nanoparticle-coated carbon nanotubes demonstrating enhanced electron field-emission properties. J. Appl. Phys. 2006, 99, 094308. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Na, N.; Liu, Z.; Han, B.; An, G. Synthesis of ZrO2-Carbon Nanotube Composites and Their Application as Chemiluminescent Sensor Material for Ethanol. J. Phys. Chem. B 2006, 110, 13410–13414. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Li, W.; Jin, Y.Z.; Kong, H. Facile and large-scale synthesis and characterization of carbon nanotube/silver nanocrystal nanohybrids. Nanotechnology 2006, 17, 2882. [Google Scholar] [CrossRef]
- Rahman, G.; Guldi, D.M.; Zambon, E.; Pasquato, L.; Tagmatarchis, N.; Prato, M. Dispersable carbon nanotube/gold nanohybrids: Evidence for strong electronic interactions. Small 2005, 1, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Kuang, Y.; Zhang, X.; Chen, J. Noble metal nanoparticles/carbon nanotubes nanohybrids: Synthesis and applications. Nano Today 2011, 6, 75–90. [Google Scholar] [CrossRef]
- Jawale, D.V.; Gravel, E.; Boudet, C.; Shah, N.; Geertsen, V.; Li, H.; Namboothiri, I.N.; Doris, E. Room temperature Suzuki coupling of aryl iodides, bromides, and chlorides using a heterogeneous carbon nanotube-palladium nanohybrid catalyst. Catal. Sci. Technol. 2015, 5, 2388–2392. [Google Scholar] [CrossRef]
- Kecsenovity, E.; Endrődi, B.; Pápa, Z.; Hernádi, K.; Rajeshwar, K.; Janáky, C. Decoration of ultra-long carbon nanotubes with Cu2O nanocrystals: A hybrid platform for enhanced photoelectrochemical CO2 reduction. J. Mater. Chem. A 2016, 4, 3139–3147. [Google Scholar] [CrossRef]
- Chen, L.; Tsang, S.C. Ag doped WO 3-based powder sensor for the detection of NO gas in air. Sens. Actuators B Chem. 2003, 89, 68–75. [Google Scholar] [CrossRef]
- Sohrabi, M.R.; Mansouriieh, N.; Khosravi, M.; Zolghadr, M. Removal of diazo dye Direct Red 23 from aqueous solution using zero-valent iron nanoparticles immobilized on multi-walled carbon nanotubes. Water Sci. Technol. 2015, 71, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Afrooz, A.N.; Das, D.; Murphy, C.J.; Vikesland, P.; Saleh, N.B. Co-transport of gold nanospheres with single-walled carbon nanotubes in saturated porous media. Water Res. 2016, 99, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Aich, N.; Boateng, L.K.; Sabaraya, I.V.; Das, D.; Flora, J.R.; Saleh, N.B. Aggregation kinetics of higher order fullerene clusters in aquatic systems. Environ. Sci. Technol. 2016, 50, 3562–3571. [Google Scholar] [CrossRef] [PubMed]
- Afrooz, A.N.; Khan, I.A.; Hussain, S.M.; Saleh, N.B. Mechanistic heteroaggregation of gold nanoparticles in a wide range of solution chemistry. Environ. Sci. Technol. 2013, 47, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Afrooz, A.R.M.N.; Flora, J.R.V.; Schierz, P.A.; Ferguson, P.L.; Sabo-Attwood, T.; Saleh, N.B. Chirality affects aggregation kinetics of single-walled carbon nanotubes. Environ. Sci. Technol. 2013, 47, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Aich, N.; Afrooz, A.R.M.N.; Flora, J.R.V.; Ferguson, L.; Sabo-Attwood, T.; Saleh, N.B. Fractal structures of single-walled carbon nanotubes in biologically relevant conditions: Role of chirality vs. media conditions. Chemosphere 2013, 93, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Flora, J.R.V.; Afrooz, A.R.M.N.; Aich, N.; Schierz, P.A.; Ferguson, P.L.; Sabo-Attwood, T.; Saleh, N.B. Change in chirality of semiconducting single-walled carbon nanotubes can overcome anionic surfactant stabilisation: A systematic study of aggregation kinetics. Environ. Chem. 2015, 12, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.B.; Pfefferle, L.D.; Elimelech, M. Aggregation kinetics of multiwalled carbon nanotubes in aquatic systems: Measurements and environmental implications. Environ. Sci. Technol. 2008, 42, 7963–7969. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Chen, H.B.; Liu, C.W.; Chen, H.; Huang, Q.Y. Photocatalytic Degradation of Sulfonamides over the Short Multi-walled Carbon Nanotube-TiO2 Hybrid. In Frontier of Nanoscience and Technology II; Kao, J.C.M., Hou, M., Chen, R., Eds.; Trans Tech Publications: Hong Kong, China, 2012; Volume 528, pp. 259–262. [Google Scholar]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Ghodselahi, T.; Vesaghi, M.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@ Cu2O core-shell nanoparticles. Appl. Surface Sci. 2008, 255, 2730–2734. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation and stabilization of silver nanoparticles through reduction by N,N-dimethylformamide. Langmuir 1999, 15, 948–951. [Google Scholar] [CrossRef]
- Ashby, M.T. Hypothiocyanite. In Advances in Inorganic Chemistry; Elsevier: New York, NY, USA, 2012; pp. 263–303. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, D.; Sabaraya, I.V.; Sabo-Attwood, T.; Saleh, N.B. Insights into Metal Oxide and Zero-Valent Metal Nanocrystal Formation on Multiwalled Carbon Nanotube Surfaces during Sol-Gel Process. Nanomaterials 2018, 8, 403. https://doi.org/10.3390/nano8060403
Das D, Sabaraya IV, Sabo-Attwood T, Saleh NB. Insights into Metal Oxide and Zero-Valent Metal Nanocrystal Formation on Multiwalled Carbon Nanotube Surfaces during Sol-Gel Process. Nanomaterials. 2018; 8(6):403. https://doi.org/10.3390/nano8060403
Chicago/Turabian StyleDas, Dipesh, Indu V. Sabaraya, Tara Sabo-Attwood, and Navid B. Saleh. 2018. "Insights into Metal Oxide and Zero-Valent Metal Nanocrystal Formation on Multiwalled Carbon Nanotube Surfaces during Sol-Gel Process" Nanomaterials 8, no. 6: 403. https://doi.org/10.3390/nano8060403
APA StyleDas, D., Sabaraya, I. V., Sabo-Attwood, T., & Saleh, N. B. (2018). Insights into Metal Oxide and Zero-Valent Metal Nanocrystal Formation on Multiwalled Carbon Nanotube Surfaces during Sol-Gel Process. Nanomaterials, 8(6), 403. https://doi.org/10.3390/nano8060403




