In-Situ Preparation of Aramid-Multiwalled CNT Nano-Composites: Morphology, Thermal Mechanical and Electric Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
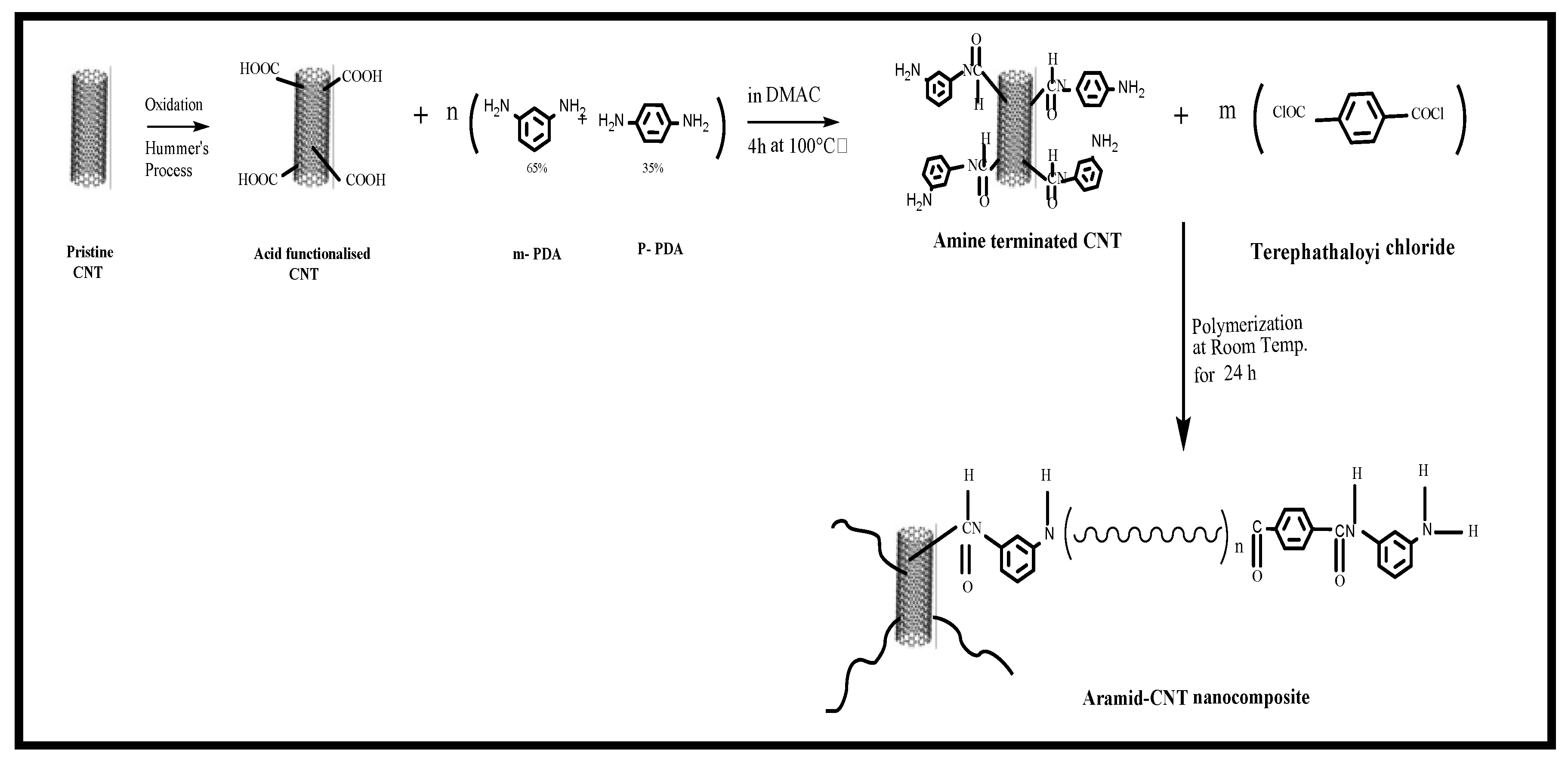
2.2. Preparation of Physically Linked AR-CNT Composite Films
2.3. Preparation of Chemically Bonded AR-CNT Composite Films
2.4. Characterization of Ar-CNT Composite Films
2.4.1. FTIR Spectroscopy
2.4.2. UV-Visible NIR Spectroscopy
2.4.3. Scanning Electron Microscopy
2.4.4. Atomic Force Microscopy (AFM)
2.4.5. Dynamic Mechanical Analysis (DMA)
2.4.6. Tensile Analysis
2.4.7. Electrical Properties
2.4.8. Water Absorption Measurements
2.4.9. Thermogravimetric Analysis (TGA)
3. Results and Discussion
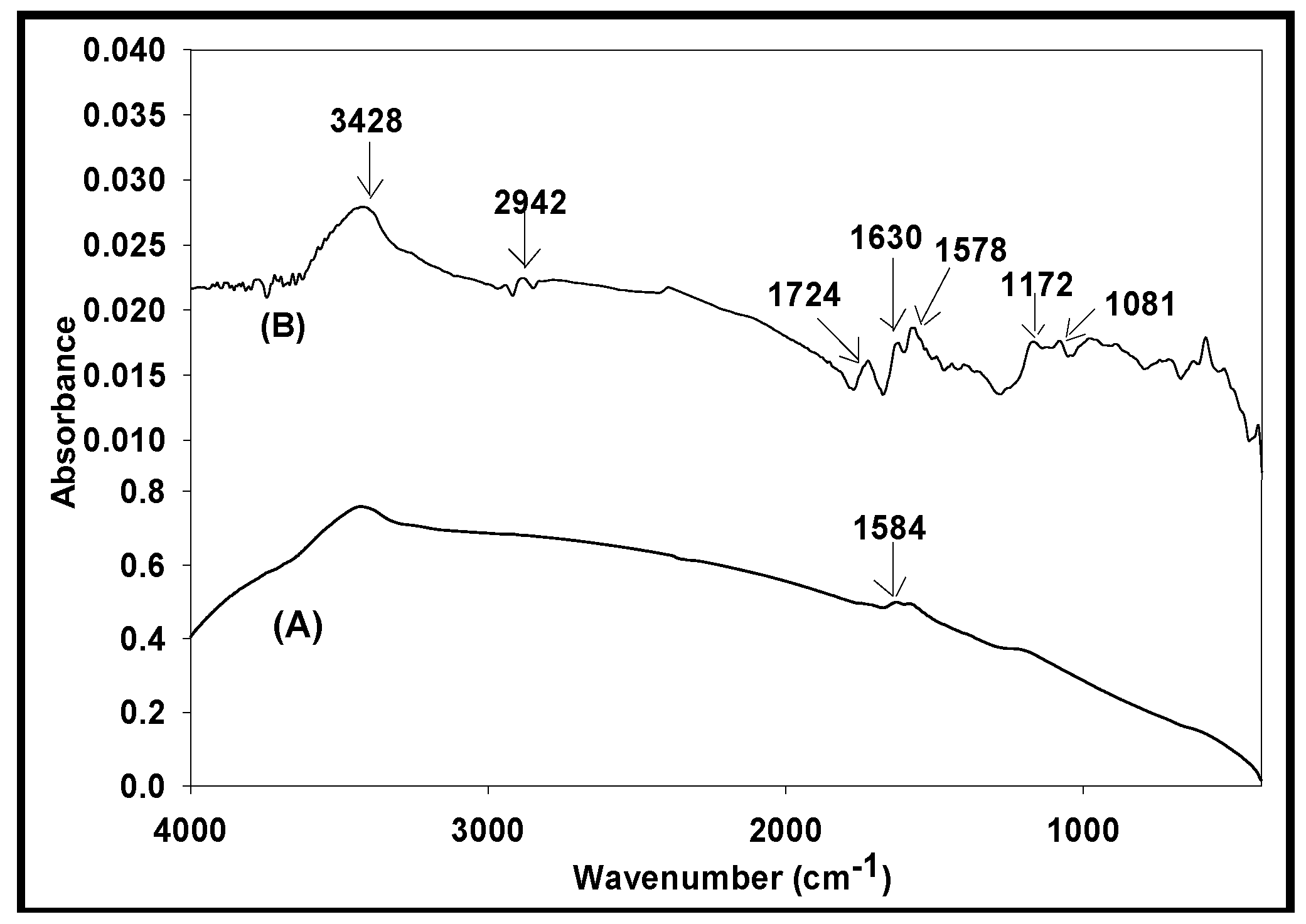
3.1. FTIR Analysis
3.2. UV-Visible NIR Analysis
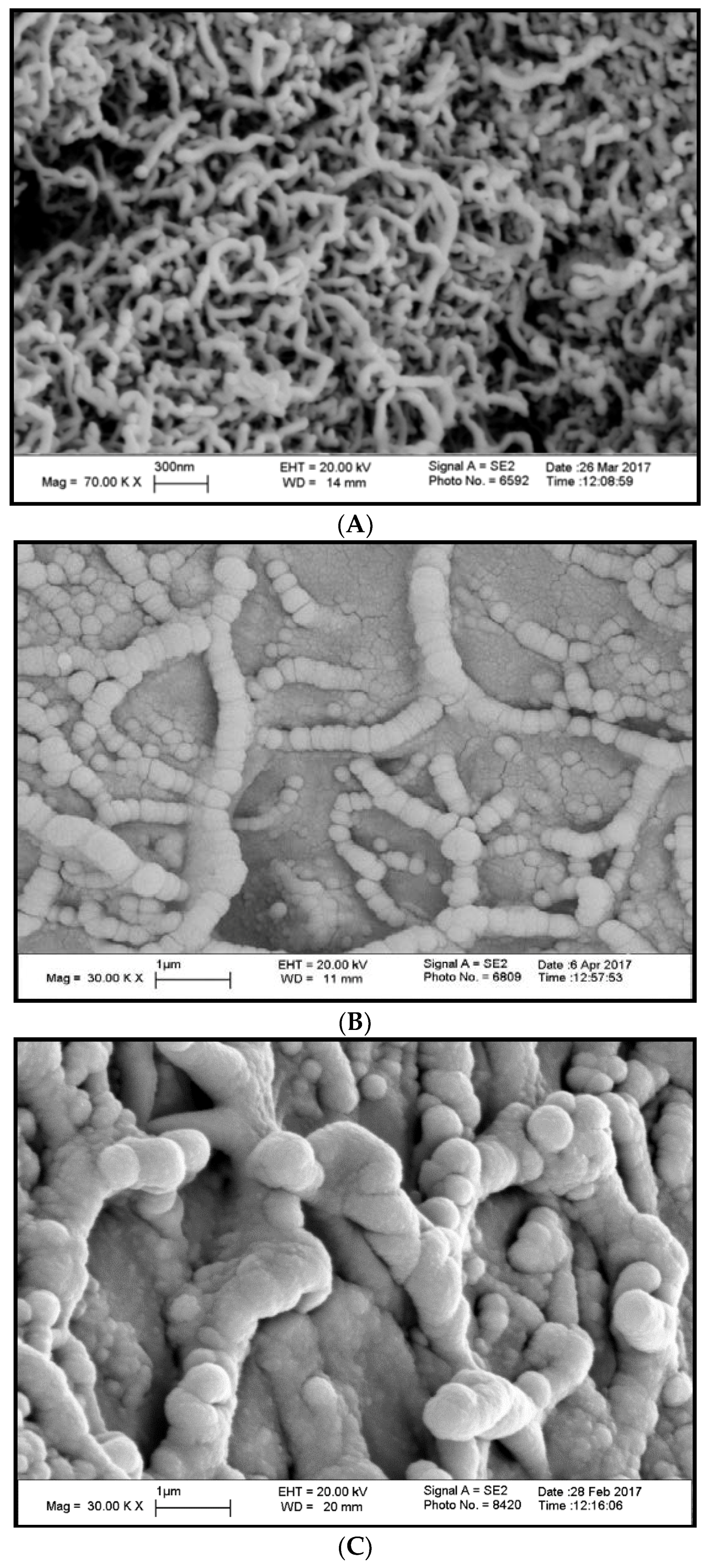
3.3. Scanning Electron Microscopy
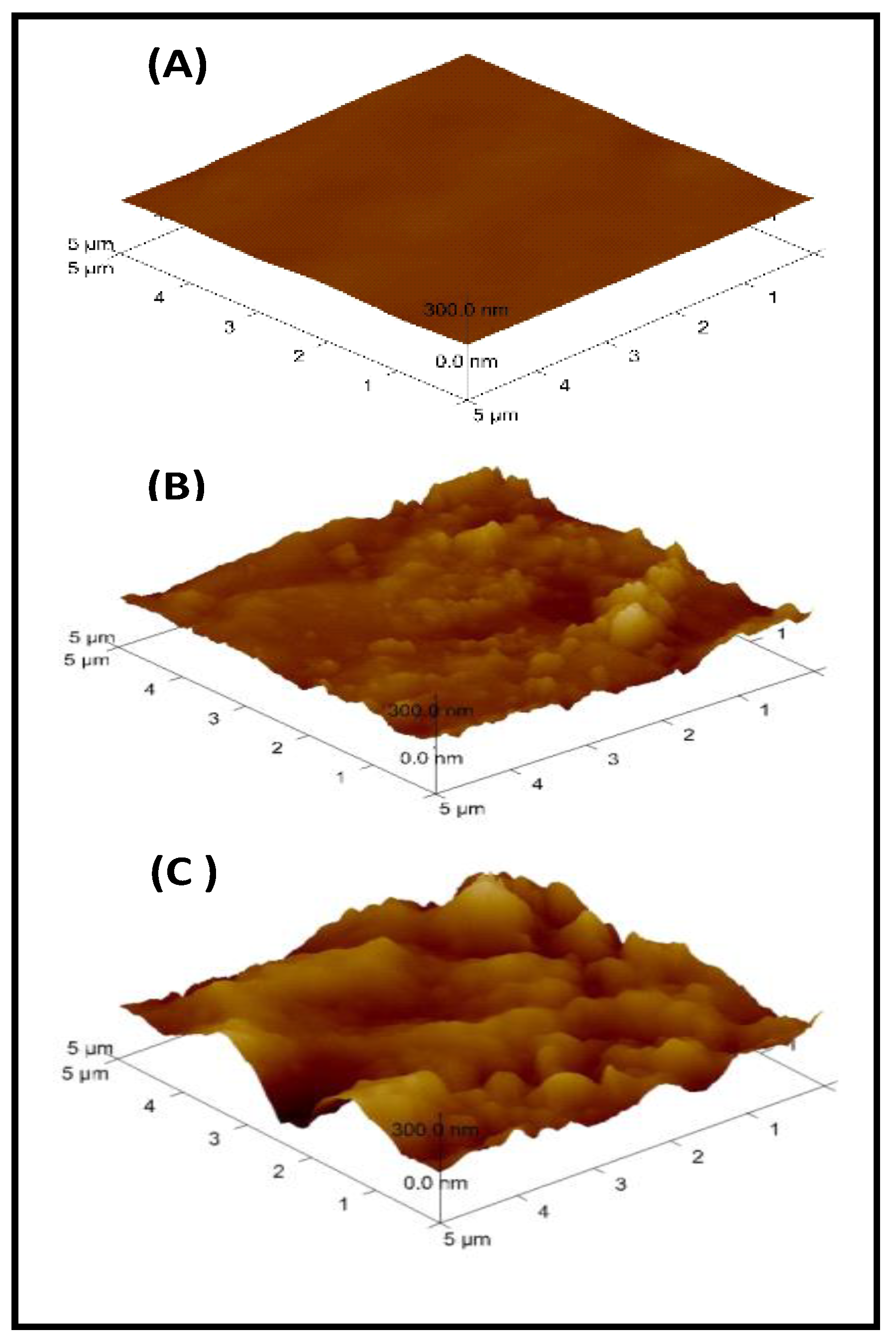
3.4. Atomic Force Microscopy
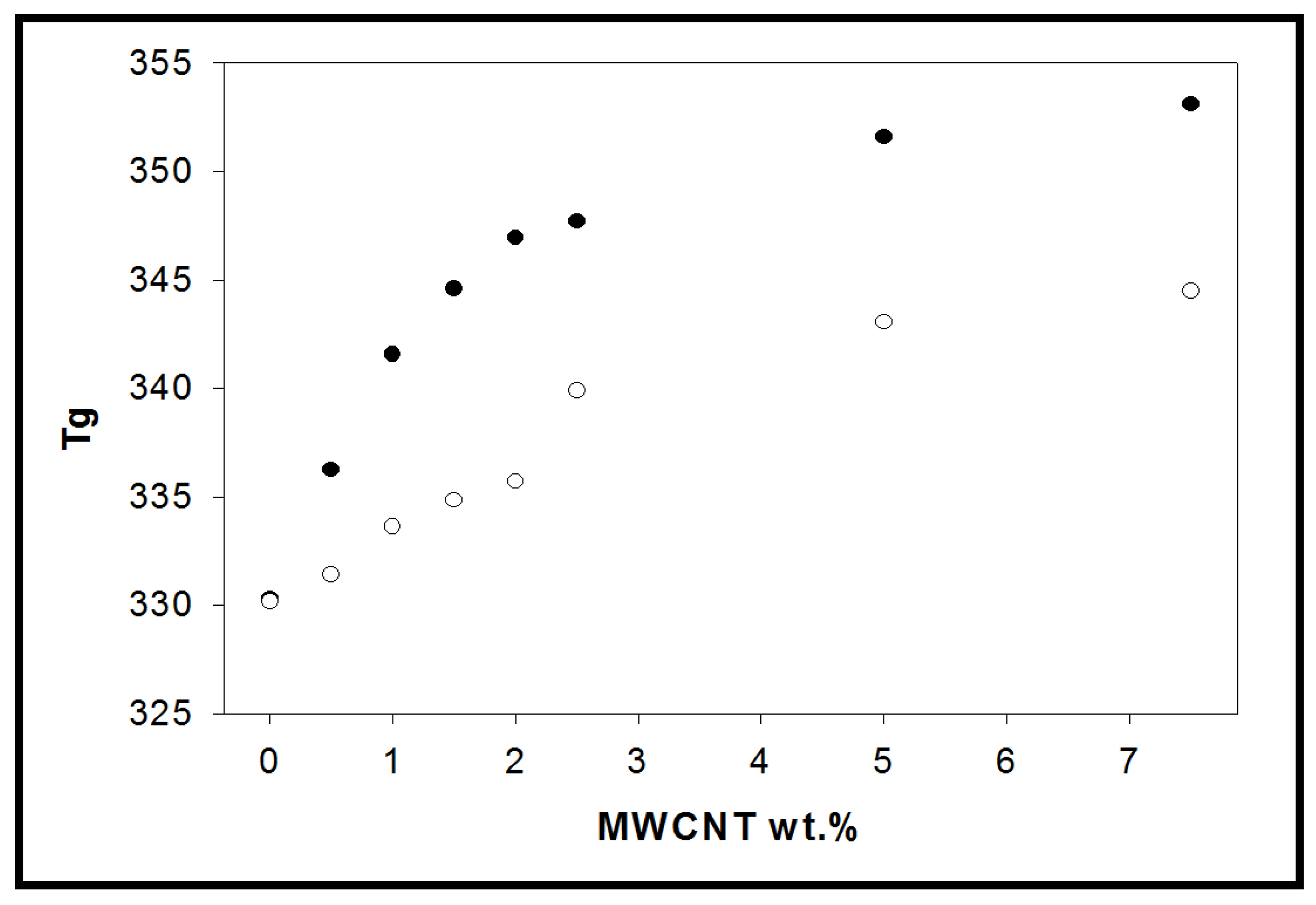
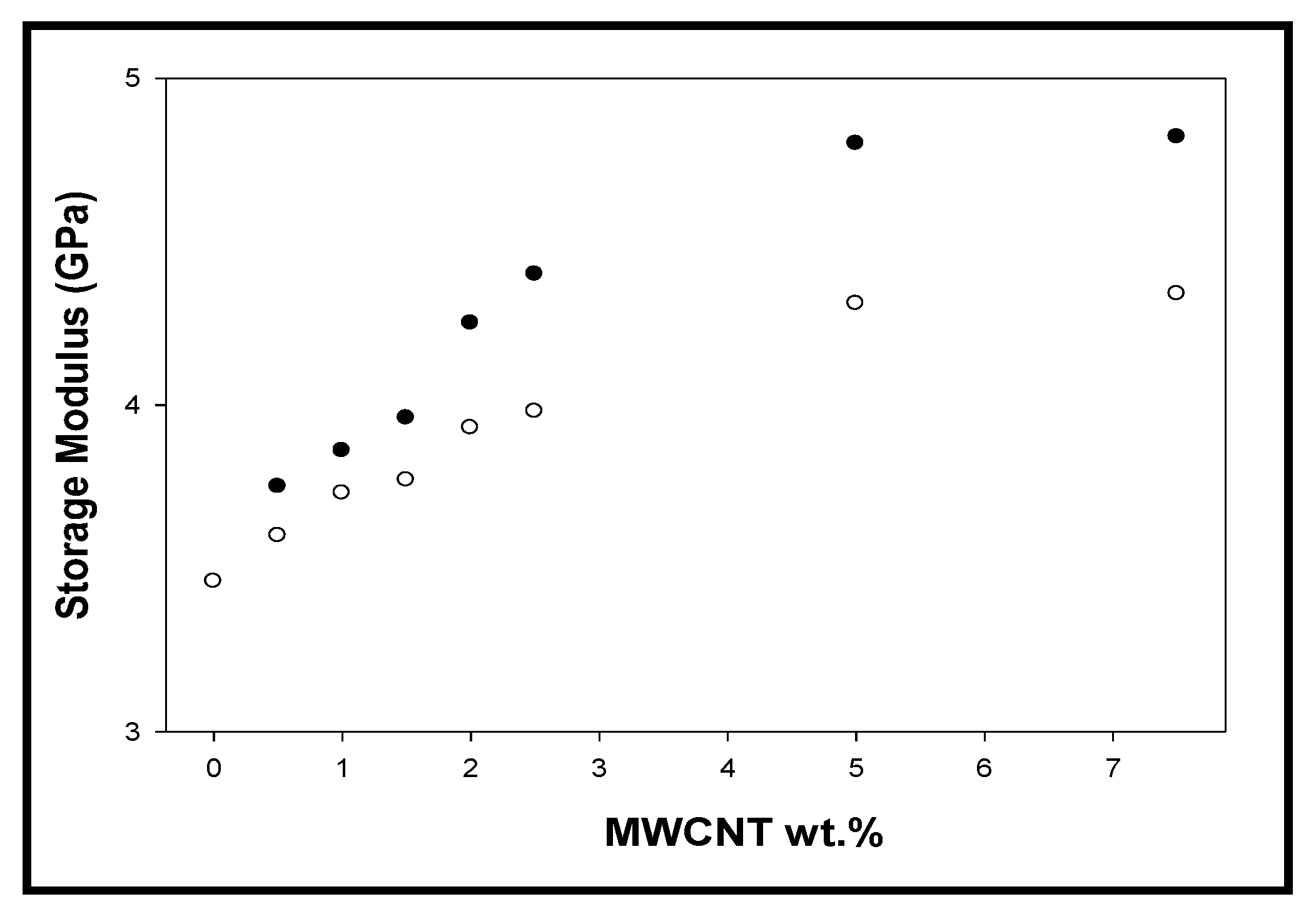
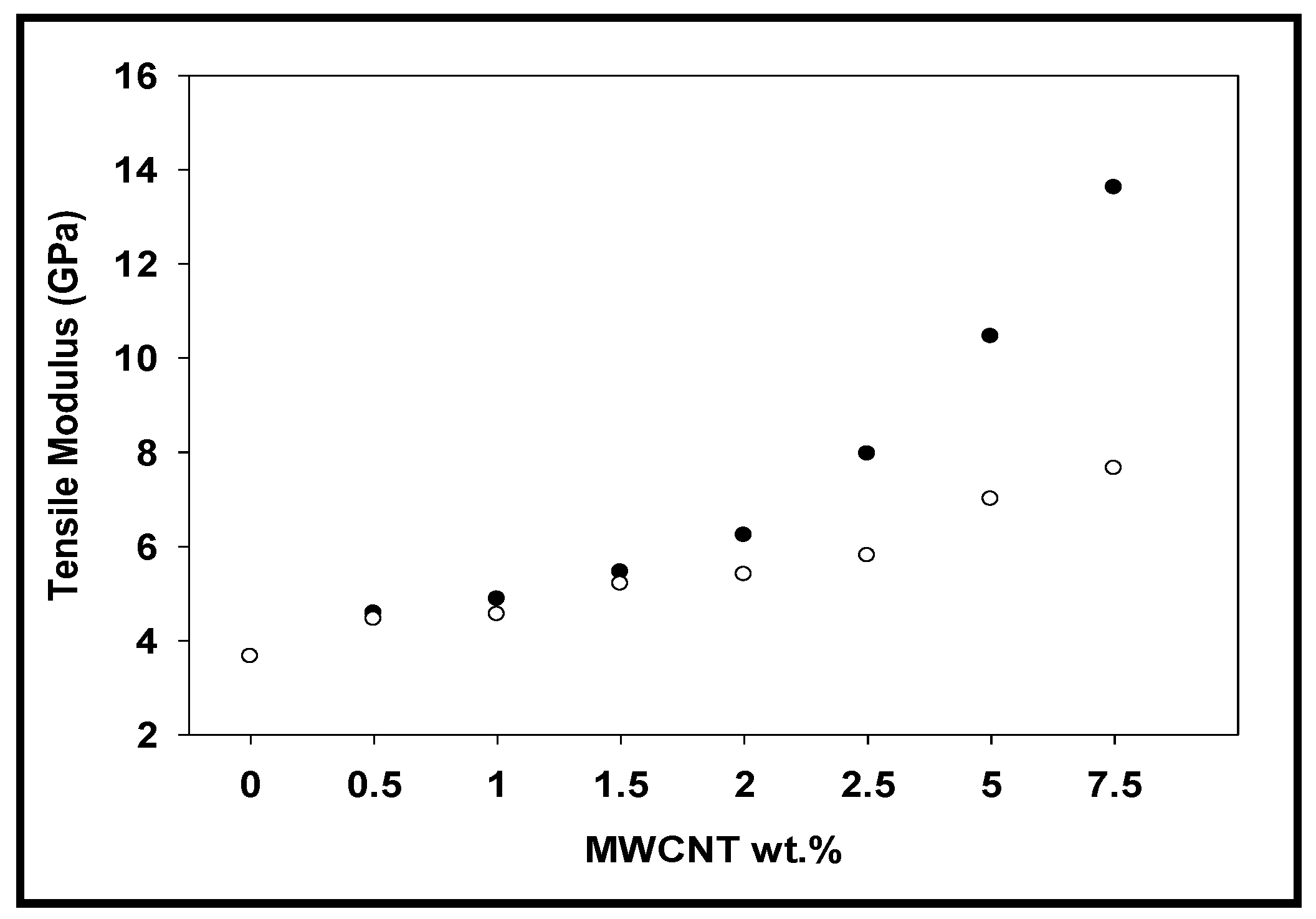
3.5. Dynamic Mechanical Analysis (DMA)
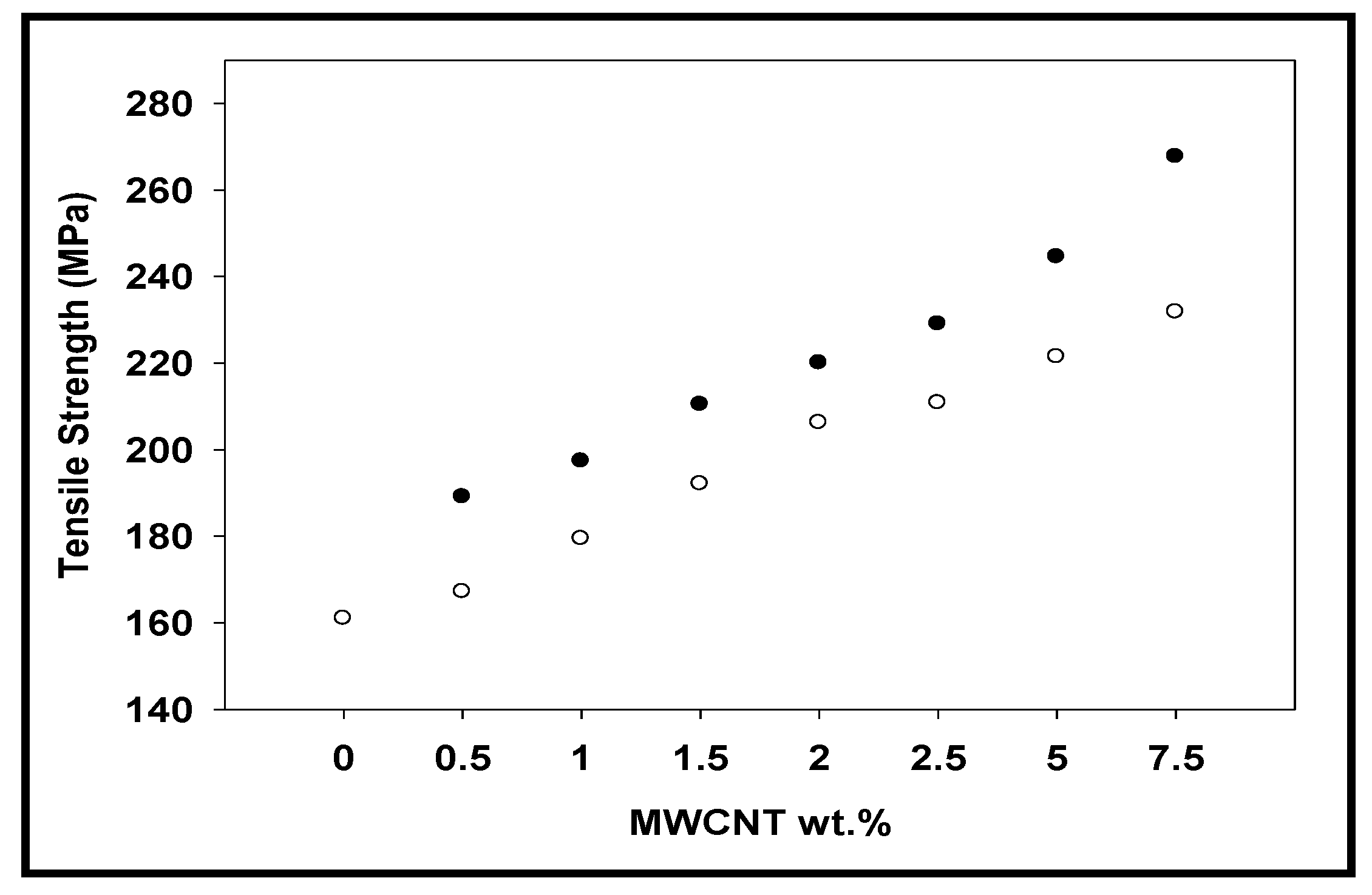
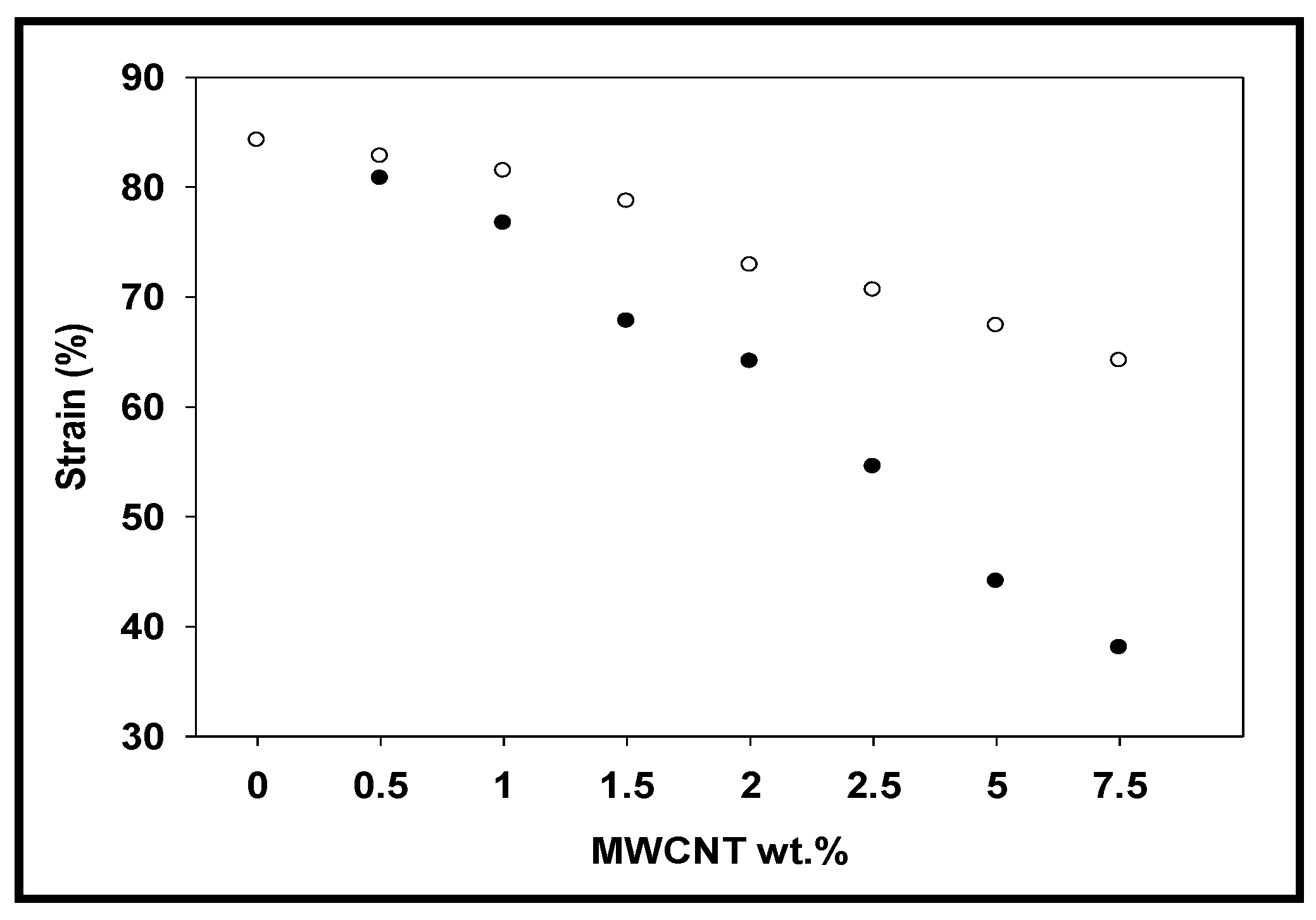
3.6. Tensile Analysis
3.7. Electrical Properties
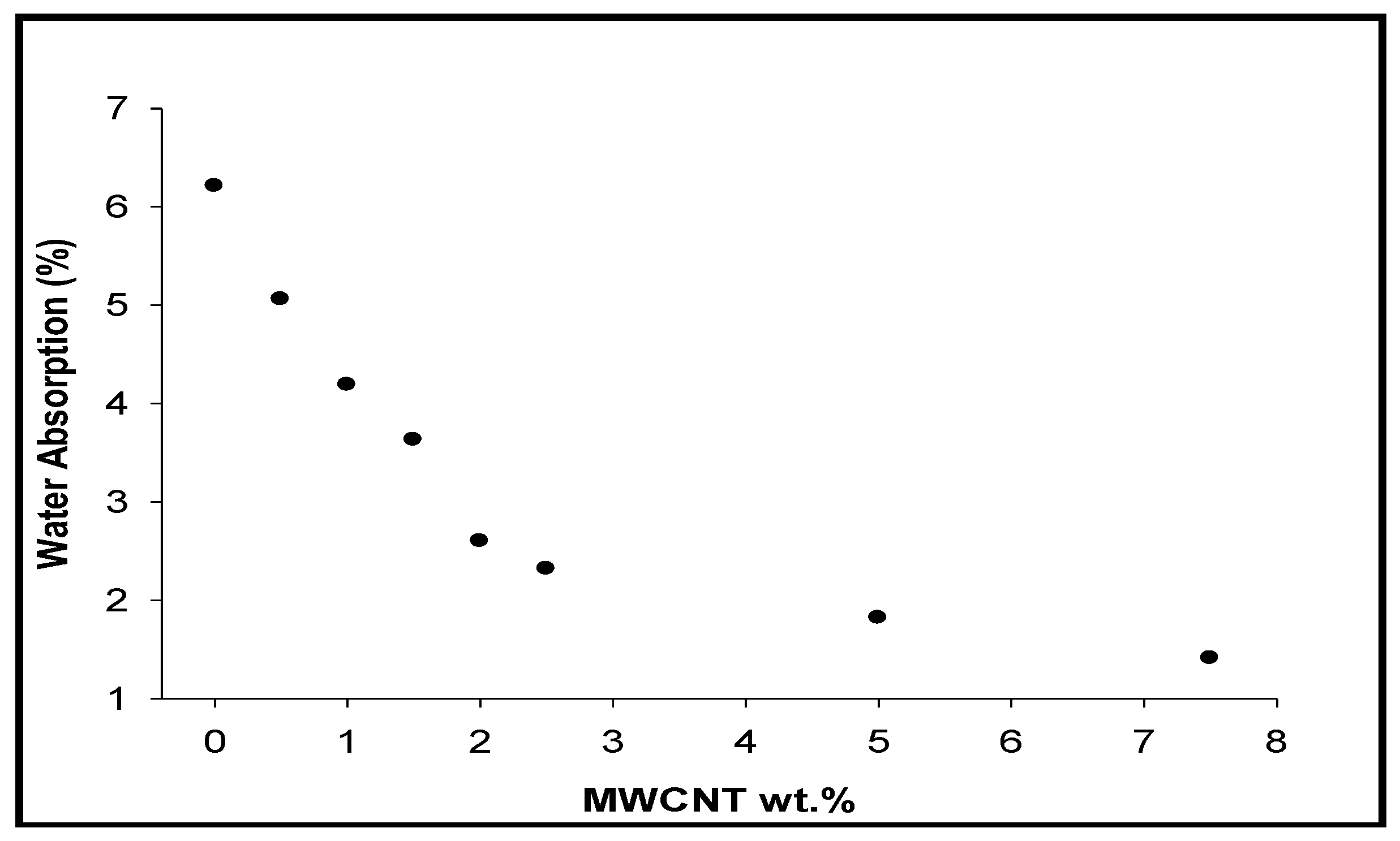
3.8. Water Absorption
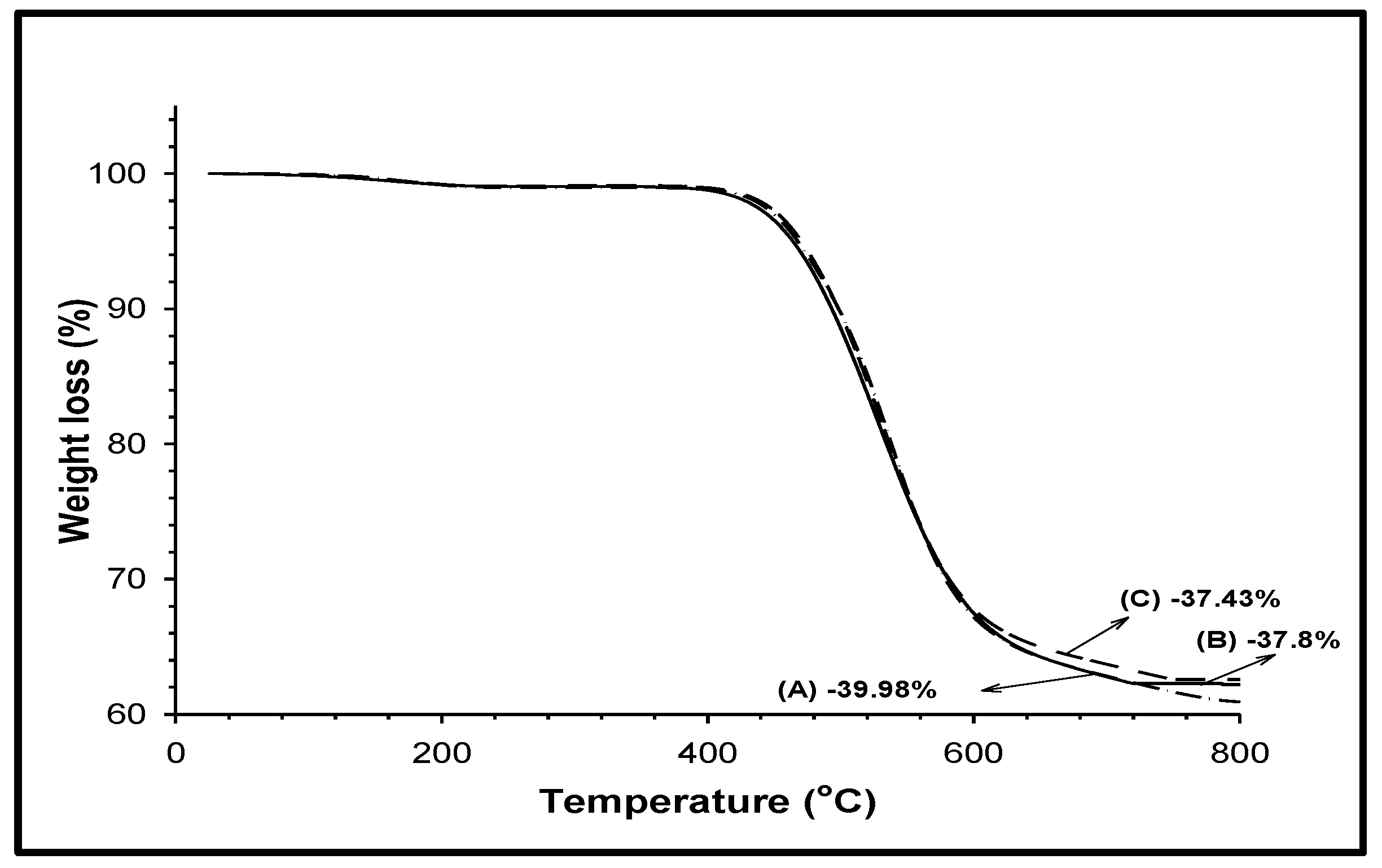
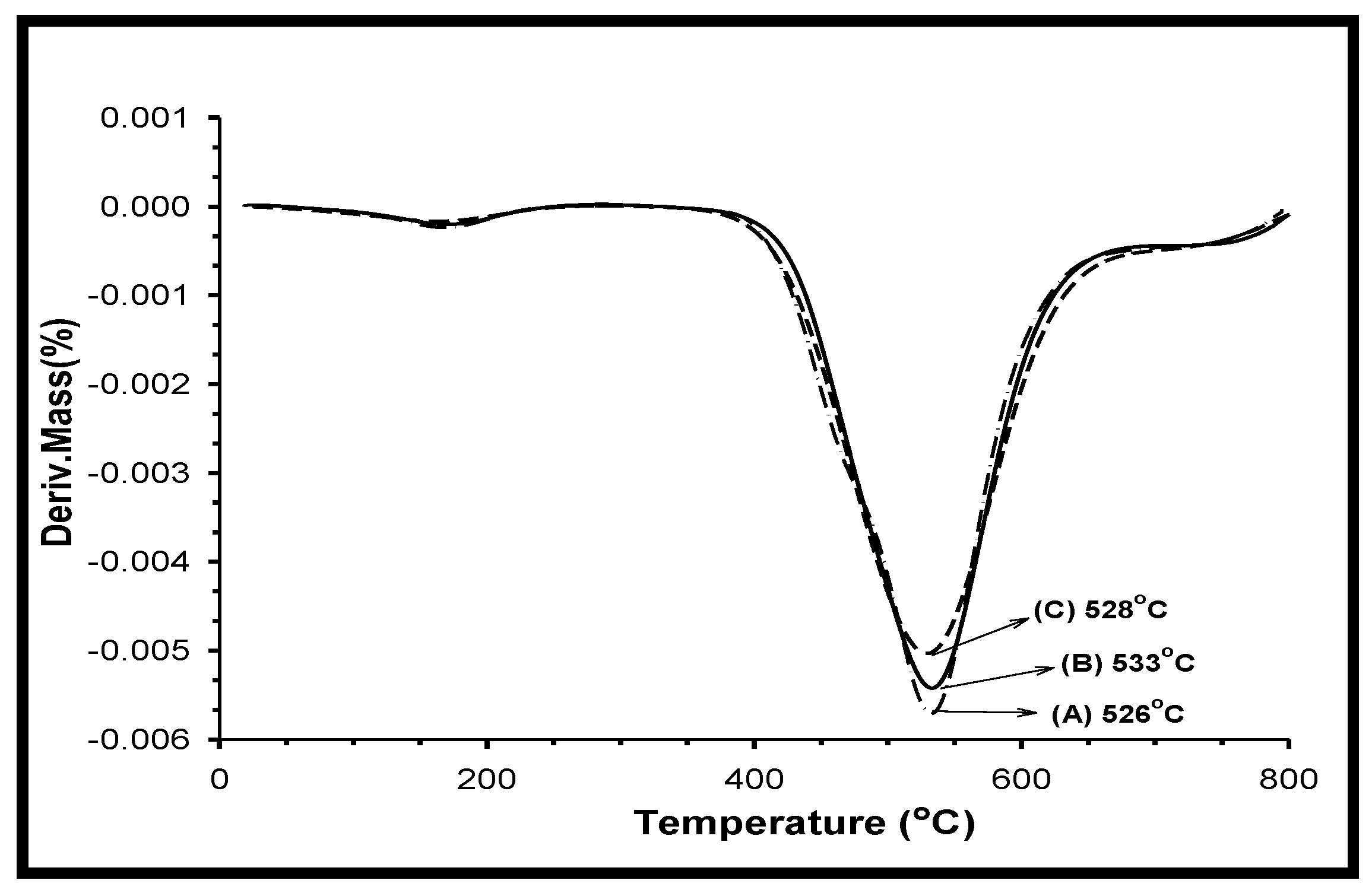
3.9. Thermogravimetric Analysis
4. Conclusions
- With a careful choice of matrix, having aromatic rings like the aramid chains, it is possible to achieve physical bonding with the conjugated surface on the pristine CNTs through π-π stacking.
- The interfacial interactions can further be increased by developing a chemical linkage between the matrix and the CNTs using in situ polymerization of the monomers and the functionalized CNTs.
- Chemically bonded composites result in higher mechanical properties in comparison to the physically associated composites using pristine CNTs.
- The low electrical percolation threshold is observed at 3.5 wt % of CNTs in the case of the composites when the pristine CNTs were used, whereas the chemical functionalization of CNTs with the matrix seems to have disrupted the electrical paths.
- Water absorption capacity of aramid matrix decreases with the loading of pristine CNTs due to their low mass density and their hydrophobic nature.
- Depending on the nature of application, both types of light-weight polymeric nano-composites with a high glass transition temperature, tensile strength and thermal stability, can be useful for various applications including EMI shielding and antistatic materials for low-observable applications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, H. Carbon Nanotubes: Synthesis, Integration, and Properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. (Ed.) Carbon Nanotubes: Science and Applications; Taylor and Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. Rev. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Hone, J.; Llaguno, M.C.; Biercuk, M.J.; Johnson, A.T.; Batlogg, B.; Benes, Z.; Fischer, J.E. Thermal properties of carbon nanotubes and nanotube-based materials. Appl. Phys. A 2002, 74, 339–343. [Google Scholar] [CrossRef]
- Bal, S.; Samal, S.S. Carbon Nanotube Reinforced Polymer Composites—A State of Art. Bull. Mater. Sci. 2007, 30, 379–386. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’ko, Y.K. Small but Strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Deng, F. Investigation of the Interfacial Bonding and Deformation Mechanism of the Nano Composites Containing Carbon Nanotubes. Ph.D. Dissertation, Tokyo University, Tokyo, Japan, 2008. [Google Scholar]
- Tsai, J.; Lu, T. Investigating the Load Transfer Efficiency in Carbon Nanotubes Reinforced Nano-composites. Compos. Struct. 2009, 90, 172–179. [Google Scholar] [CrossRef]
- Du, J.H.; Bai, J.; Cheng, H.M. The present status and key problems of carbon nanotube based polymer composites. Express Polym. Lett. 2007, 1, 253–273. [Google Scholar] [CrossRef]
- Green, M.J. Analysis and measurement of carbon nanotube dispersions: Nanodispersion versus macro-dispersion. Polym. Int. 2010, 59, 1319–1322. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion and rheology of carbon nanotubes in polymers. Int. J. Mater. Form. 2008, 1, 63–74. [Google Scholar] [CrossRef]
- Masuda, J.; Torkelson, J.M. Dispersion and major property enhancement in polymer/multiwall carbon nanotube nanocomposites via solid-state shear pulverization followed by melt mixing. Macromolecules 2008, 41, 5974–5977. [Google Scholar] [CrossRef]
- Socher, R.; Krause, B.; Müller, M.T.; Boldt, R.; Pötschke, P. The influence of matrix viscosity on MWCNT dispersion and electrical properties in different thermoplastic nanocomposites. Polymer 2012, 53, 495–504. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Minot, M.; Rantel, T. Fabrication of Carbon Multiwall Nanotube/Polymer Composites by Shear Mixing. Macromol. Mater. Eng. 2002, 287, 395–403. [Google Scholar] [CrossRef]
- Alig, I.; Pötschke, P.; Lellinger, D.; Skipa, T.; Pegel, S.; Kasaliwal, G.R.; Villmow, T. Establishment, morphology and properties of carbon nanotube networks in polymer melts. Polymer 2012, 53, 4–28. [Google Scholar] [CrossRef]
- Kasaliwal, G.R.; Göldel, A.; Pötschke, P.; Heinrich, G. Influences of polymer matrix viscosity and molecular weight on MWCNT agglomerate dispersion. Polymer 2011, 52, 1027–1036. [Google Scholar] [CrossRef]
- Bose, S.; Khare, R.A.; Moldenaers, P. Assessing the strengths and weaknesses of various types of pre-treatments of carbon nanotubes on the properties of polymer/carbon nanotubes composites: A critical review. Polymer 2010, 51, 975–993. [Google Scholar] [CrossRef]
- Ahmad, Z.; Al-Sagheer, F.; Shiju, J. Aramid–multiwalled carbon nanotube nanocomposites: Effect of compatibilization through oligomer wrapping of the nanotube. Polym. Int. 2016, 65, 1204–1213. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, H.S. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Touhara, H.; Inahara, J.; Mizuno, T. Fluorination of cup-stacked carbon nanotubes, structure and properties. J. Fluor. Chem. 2002, 114, 181–188. [Google Scholar]
- Stevens, J.L.; Huang, A.Y.; Peng, H. Sidewall amino-functionalization of SWNTs through fluorination and subsequent reactions with terminal diamines. Nano Lett. 2003, 3, 331–336. [Google Scholar] [CrossRef]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Dyke, C.A.; Tour, J.M. Covalent functionalization of single-walled carbon nanotubes for materials applications. J. Phys. Chem. A 2008, 105, 11151–11159. [Google Scholar] [CrossRef]
- Shen, J.; Huang, W.; Wu, L.; Hua, Y.; Ye, M. Study on amino-functionalized multiwalled carbon nanotubes. Mater. Sci. Eng. A 2007, 464, 151–156. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lin, W.H.; Chang, Y.C. The influence of treatment duration on multi-walled carbon nanotubes functionalized by H2 SO4/HNO3 oxidation. Appl. Surf. Sci. 2011, 257, 2401–2410. [Google Scholar] [CrossRef]
- Sandler, J.K.W.; Pegel, S.; Cadek, M.; Gojny, F.; Vanes, M.; Lohmar, J.; Blau, W.J.; Schulte, K.; Windle, A.H.; Shaffer, M.S.P. A comparative study of melt spun polyamide-12 fibres reinforced with carbon nanotubes and nanofibers. Polymer 2004, 45, 2001–2015. [Google Scholar] [CrossRef]
- Liu, T.; Phang, I.Y.; Shen, L.; Chow, S.Y.; Zhang, W.D. Morphology and mechanical properties of multiwalled carbon nanotubes reinforced nylon-6 composites. Macromolecules 2004, 37, 7214–7222. [Google Scholar] [CrossRef]
- Mahmood, N.; Islam, M.; Hameed, A.; Saeed, S.; Khan, A.N. Polyamide based composites reinforced with pristine or functionalized multiwalled carbon nanotubes produced using melt extrusion technique. J. Compos. Mater. 2013, 48, 1197–1207. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nuesch, F.A.; Chu, B.T.T. Comparing carbon nanotubes and graphene nanoplatelets as reinforcements in polyamide-12 composites. Nanotechnology 2011, 22, 275714–275722. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.D.; Kumar, R.P.; Chandra, R.B. Mechanical, thermomechanical, and creep performance of CNT embedded epoxy at elevated temperatures: An emphasis on the role of carboxyl functionalization. J. Appl. Polym. Sci. 2017, 134, 44851. [Google Scholar]
- Dehghan, M.; Al-Mahaidi, R.; Sbarski, I. Investigation of CNT modification of epoxy resin in CFRP strengthening systems. Polym. Compos. 2016, 37, 1021–1033. [Google Scholar] [CrossRef]
- Asad, H.; Mohammad, I.; Iftikhar, A.; Nasir, M.; Shaukat, S.; Hassan, J. Thermal and mechanical properties of carbon nanotube/epoxy nanocomposites reinforced with pristine and functionalized multi-walled carbon nanotubes. Polym. Compos. 2015, 36, 1891–1898. [Google Scholar]
- Zhou, Y.X.; Wu, P.X.; Cheng, Z.-Y.; Ingram, J.; Jeelani, S. Improvement in electrical, thermal and mechanical properties of epoxy by filling carbon nanotube. Express Polym. Lett. 2008, 2, 40–48. [Google Scholar] [CrossRef]
- Yepez, C.F.; Robert, S.; Beate, K.; Robert, H.; Brian, P.; Ricardo, P.-S.; Petra, P. Electrical, mechanical, and glass transition behavior of polycarbonate-based nanocomposites with different multi-walled carbon nanotubes. Polymer 2011, 52, 3835–3845. [Google Scholar]
- Babal, A.S.; Gupta, R.; Singh, B.P.; Sanjay, R. Depression in glass transition temperature of multiwalled carbon nanotubes reinforced polycarbonate composites: Effect of functionalization. RSC Adv. 2015, 5, 43462–43472. [Google Scholar] [CrossRef]
- Tripathi, S.N.; Singh, S.; Malik, R.S.; Choudhary, V. Effect of Multiwalled Carbon Nanotubes on the Properties of Poly(methyl methacrylate) in PMMA/CNT Nanocomposites. Macromol. Symp. 2014, 341, 75–89. [Google Scholar] [CrossRef]
- Thaher, A.I.; Al-Amer, A.; Selvin, T.P.; Al-Harthi, M.; Adamu, G.S.; Rachid, S.; Ali, A.M. Effect of acid treated carbon nanotubes on mechanical, rheological and thermal properties of polystyrene nanocomposites. Compos. Part B Eng. 2011, 42, 1554–1561. [Google Scholar]
- Marc, J.M.; Abadie, L.; Rusanov, A. Practical Guide to Polyimides; Smithers Rapra Technology Limited: Shropshire, UK, 2007; pp. 45–77. [Google Scholar]
- Garcia, J.M.; Garcia, F.C.; Serna, F.; Jose, J.; de la Pena, J.L. High performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Kwolek, S.; Mera, H.; Takata, T. High-Performance Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Gremany, 2002. [Google Scholar]
- Yang, Y.L.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Electromagnetic interference shielding characteristics of carbon fiber-polymer composites. J. Nanosci. Nanotechnol. 2007, 7, 549–554. [Google Scholar] [PubMed]
- Rashid, E.S.A.; Ariffin, K.; Akil, H.M.; Kooi, C.C. Mechanical and Thermal Properties of Polymer Composites for Electronic Packaging Application. J. Reinf. Plast. Compos. 2008, 27, 1573–1584. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, G.; Huang, Y.; Li, F.; Ma, Y.; Guo, T.; He, X.; Lin, X.; Gao, H.; Chen, Y. Microwave Absorption of Single-Walled Carbon Nanotubes/Soluble Cross-Linked Polyurethane Composites. J. Phys. Chem. C 2007, 111, 13696–13700. [Google Scholar] [CrossRef]
- Connor, I.O.; Hayden, H.; Coleman, J.N.; Gun’ko, Y.K. High-Strength, High-Toughness Composite Fibers by Swelling Kevlar in Nanotube Suspensions. Small 2009, 5, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, T.; Erickson, K.; Okawa, D.; Zonte, C.S.; Fréchet, J.M.J.; Zettl, A. Kevlar Functionalized Carbon Nanotubes for Next-Generation Composites. Chem. Mater. 2010, 22, 2164–2171. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papgelis, K.; Partenois, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Liu, G.L.; Wu, H.H.; Lee, C.C. Effects of polarity and pH on the solubility of acid-treated carbon nanotubes in different media. Carbon 2007, 45, 1880–1890. [Google Scholar] [CrossRef]
- Pavia, D.; Lampman, G.; Kriz, G.; Vyvyan, J. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008. [Google Scholar]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Titus, E.; Ali, N.; Cabral, G.; Gracio, J.; Ramesh, P.B.; Jackson, M.J. Chemically Functionalized Carbon Nanotubes and Their Characterization Using Thermogravimetric Analysis, Fourier Transform Infrared, and Raman Spectroscopy. J. Mater. Eng. Perform. 2006, 15, 182–186. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 16, 3712–3718. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y. Optical properties of carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Ryabenko, G.; Dorofeeva, T.V.; Zvereva, G.I. UV-vis-NIR Spectroscopy Study of Sensitivity of Single Wall Carbon Nanotubes to Chemical Processing and Van-der-Waals SWNT/SWNT Interaction. Verification of the SWNT Content Measurements by Absorption Spectroscopy. Carbon 2004, 42, 1523–1535. [Google Scholar] [CrossRef]
- Meincke, O.; Kaempfer, D.; Weickmann, H.; Friedrich, C.; Vathauer, M.; Warth, H. Mechanical properties and electrical conductivity of carbon-nanotube filled polyamide-6 and its blends with acrylonitrile/butadiene/styrene. Polymer 2004, 45, 739–748. [Google Scholar] [CrossRef]
- Nasir, M.; Mohammad, I.; Asad, H.; Shaukat, S. Polyamide 6/Multiwalled Carbon Nanotubes Nanocomposites with Modified Morphology and Thermal Properties. Polymers 2013, 5, 1380–1391. [Google Scholar]
- Vankayala, R.R.; Lai, W.J.; Cheng, K.C.; Hwang, K.C. Enhanced electrical conductivity of Nylon-6 using polyaniline coated multiwalled carbon nanotubes as additives. Polymer 2011, 52, 3337–3343. [Google Scholar] [CrossRef]
- Huang, J.C. Carbon black filled conducting polymers and polymer blends. Adv. Polym. Technol. 2002, 21, 229–3132. [Google Scholar] [CrossRef]
- Hamdi, H.; Aboura, Z.; Hariz, W. Improvement of the electrical conductivity of carbon fiber reinforced polymer by incorporation of nanofillers and the resulting thermal and mechanical behavior. J. Compos. Mater. 2017. [CrossRef]

 ), 1.5 (
), 1.5 ( ), 2 (■), 2.5 (
), 2 (■), 2.5 ( ), 5 (
), 5 ( ), 7.5 (*).
), 7.5 (*).
 ), 1.5 (
), 1.5 ( ), 2 (■), 2.5 (
), 2 (■), 2.5 ( ), 5 (
), 5 ( ), 7.5 (*).
), 7.5 (*).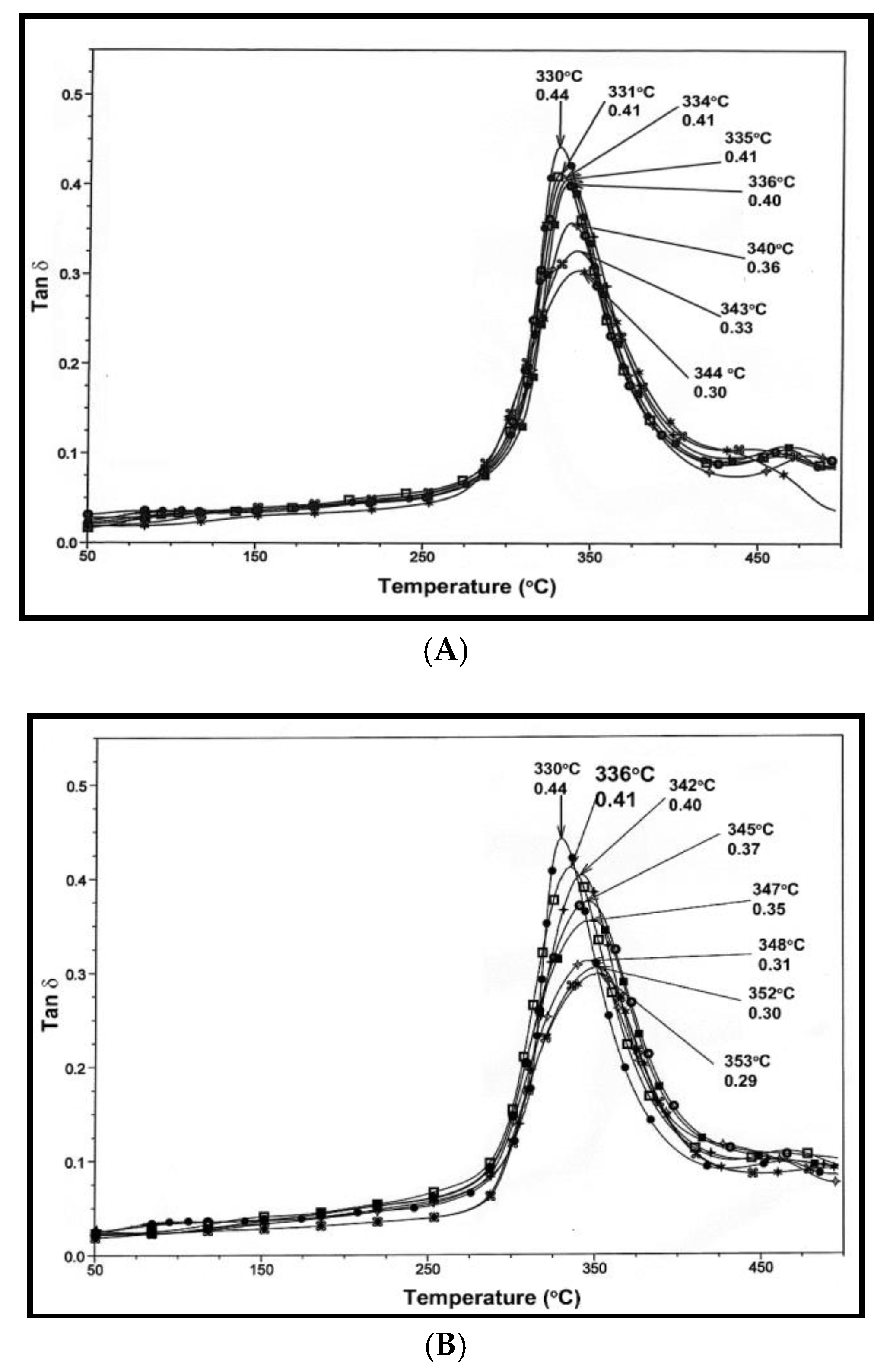
 ), 1.5 (
), 1.5 ( ), 2 (■), 2.5 (
), 2 (■), 2.5 ( ), 5 (
), 5 ( ) and 7.5 (*).
) and 7.5 (*).
 ), 1.5 (
), 1.5 ( ), 2 (■), 2.5 (
), 2 (■), 2.5 ( ), 5 (
), 5 ( ) and 7.5 (*).
) and 7.5 (*).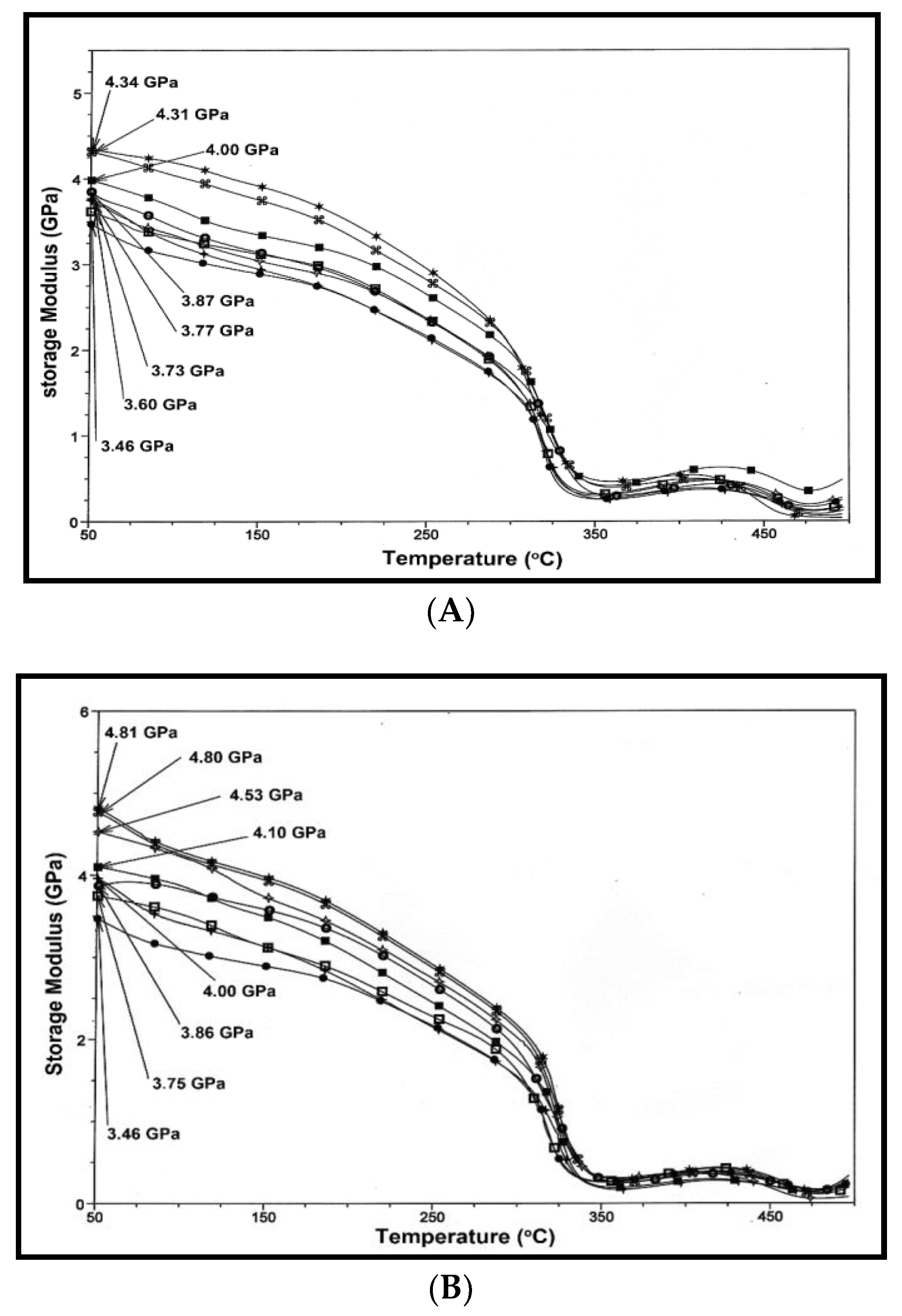
 ), 7.5 (− − −).
), 7.5 (− − −).
 ), 7.5 (− − −).
), 7.5 (− − −).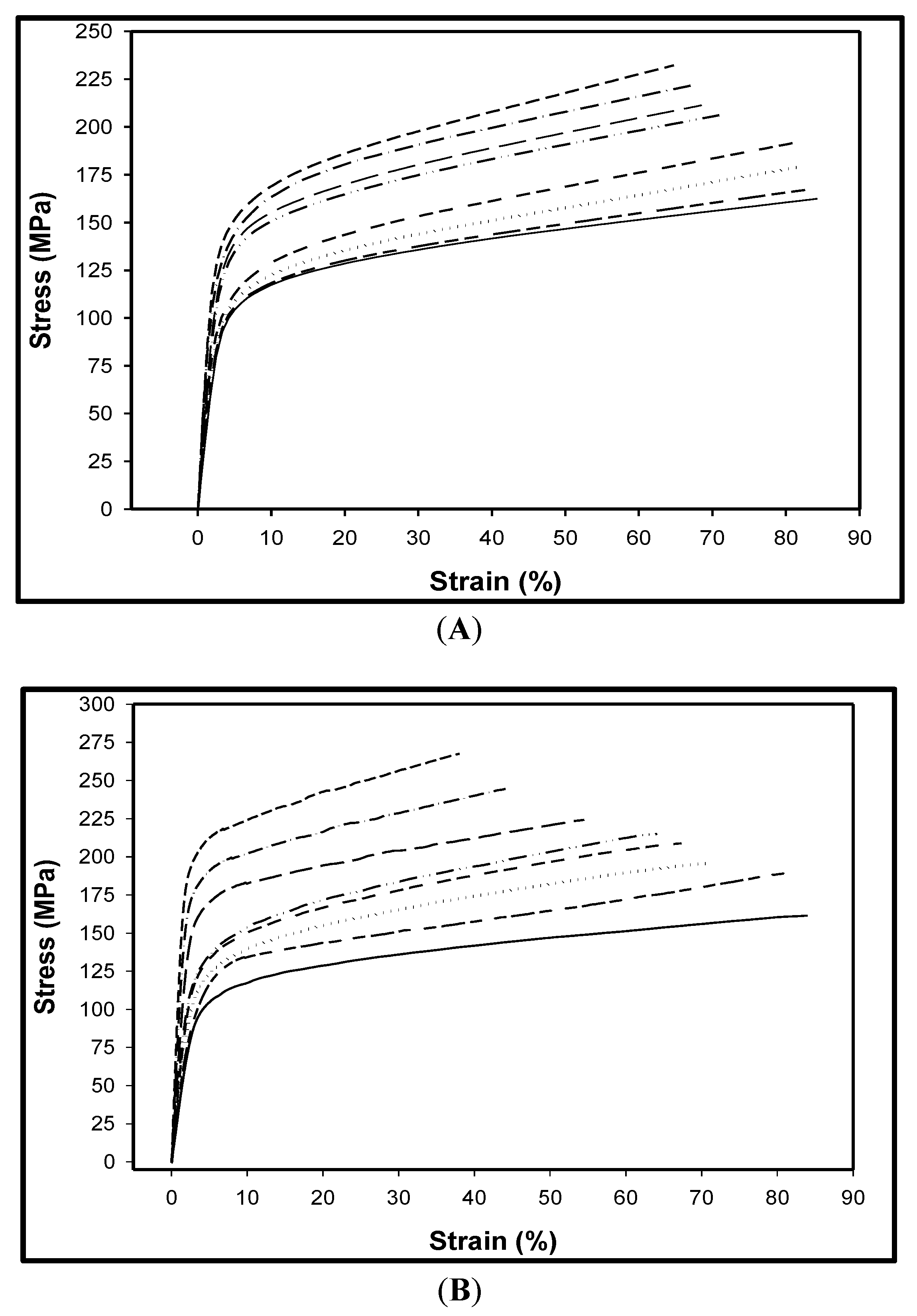
| Sample | MWCNT wt % | Tensile Modulus (GPa) | Tensile Strength (MPa) | Strain at Break (%) | |||
|---|---|---|---|---|---|---|---|
| - | - | Ar-CNT-P | Ar-CNT-C | Ar-CNT-P | Ar-CNT-C | Ar-CNT-P | Ar-CNT-C |
| Ar neat | 0 | 3.65 | 3.65 | 161.10 | 161.00 | 83.85 | 83.85 |
| Ar-CNT-0.5% | 0.5 | 4.40 | 4.58 | 167.42 | 189.03 | 82.90 | 80.77 |
| Ar-CNT-1% | 1 | 4.50 | 4.87 | 179.42 | 197.32 | 81.61 | 73.20 |
| Ar-CNT-1.5% | 1.5 | 5.30 | 5.45 | 192.05 | 210.38 | 81.02 | 67.78 |
| Ar-CNT-2% | 2 | 5.36 | 6.23 | 206.19 | 216.18 | 70.40 | 64.11 |
| Ar-CNT-2.5% | 2.5 | 5.70 | 7.96 | 211.74 | 224.35 | 68.52 | 54.53 |
| Ar-CNT-5% | 5 | 7.37 | 10.45 | 221.42 | 244.50 | 67.10 | 44.08 |
| Ar-CNT-7.5% | 7.5 | 7.65 | 13.61 | 232.45 | 267.67 | 64.00 | 38.05 |
| CNT wt % in the Aramid Matrix | Electrical Conductivity (S cm−1) |
|---|---|
| 3.5 | 5.12 × 10−5 |
| 5.0 | 9.78 × 10−5 |
| 7.5 | 2.78 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiju, J.; Al-Sagheer, F.; Bumajdad, A.; Ahmad, Z. In-Situ Preparation of Aramid-Multiwalled CNT Nano-Composites: Morphology, Thermal Mechanical and Electric Properties. Nanomaterials 2018, 8, 309. https://doi.org/10.3390/nano8050309
Shiju J, Al-Sagheer F, Bumajdad A, Ahmad Z. In-Situ Preparation of Aramid-Multiwalled CNT Nano-Composites: Morphology, Thermal Mechanical and Electric Properties. Nanomaterials. 2018; 8(5):309. https://doi.org/10.3390/nano8050309
Chicago/Turabian StyleShiju, Jessy, Fakhreia Al-Sagheer, Ali Bumajdad, and Zahoor Ahmad. 2018. "In-Situ Preparation of Aramid-Multiwalled CNT Nano-Composites: Morphology, Thermal Mechanical and Electric Properties" Nanomaterials 8, no. 5: 309. https://doi.org/10.3390/nano8050309
APA StyleShiju, J., Al-Sagheer, F., Bumajdad, A., & Ahmad, Z. (2018). In-Situ Preparation of Aramid-Multiwalled CNT Nano-Composites: Morphology, Thermal Mechanical and Electric Properties. Nanomaterials, 8(5), 309. https://doi.org/10.3390/nano8050309





