Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of N-Containing Mesoporous Carbon and Mg and N Co-Doped Mesoporous Carbon
2.3. Steam Activation
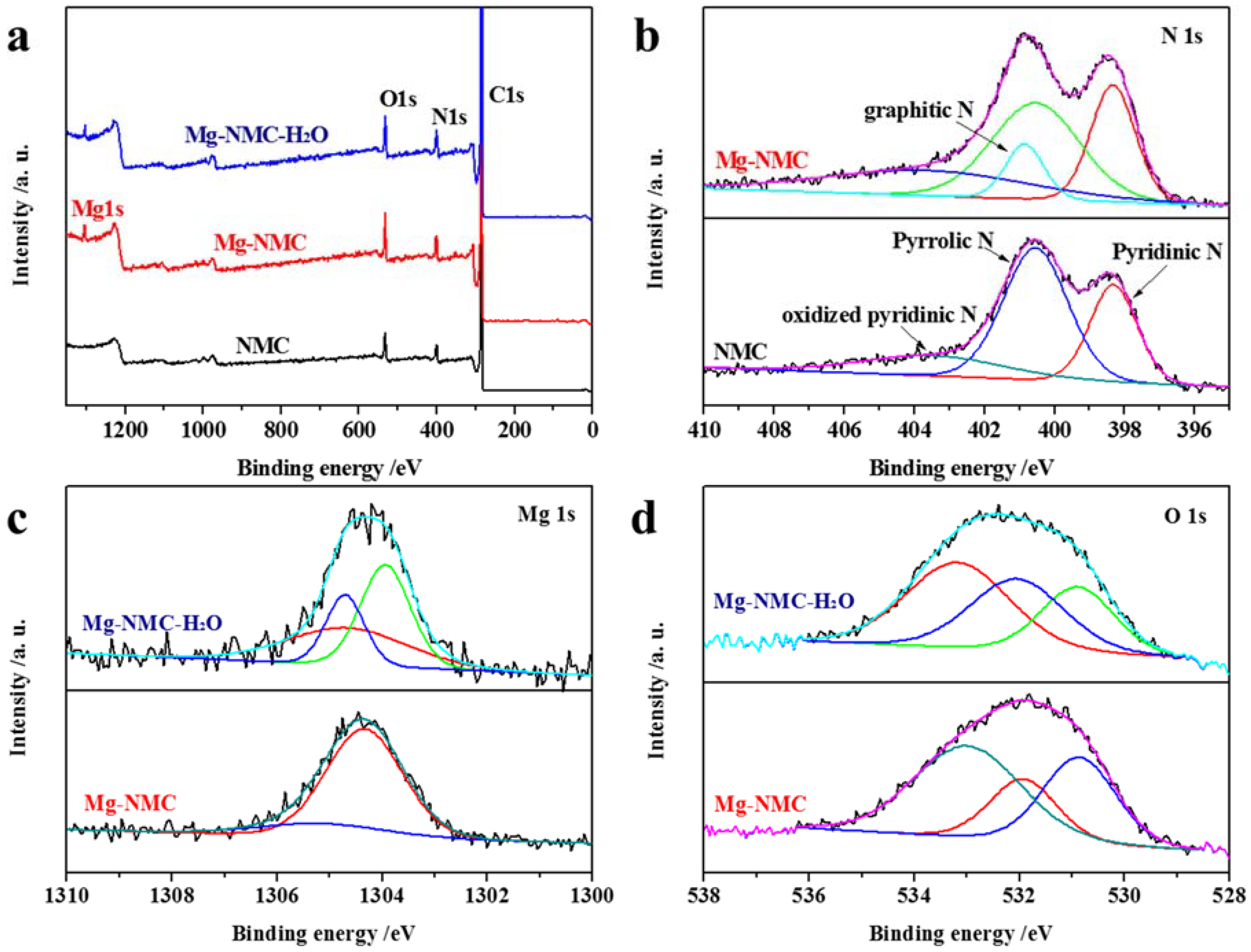
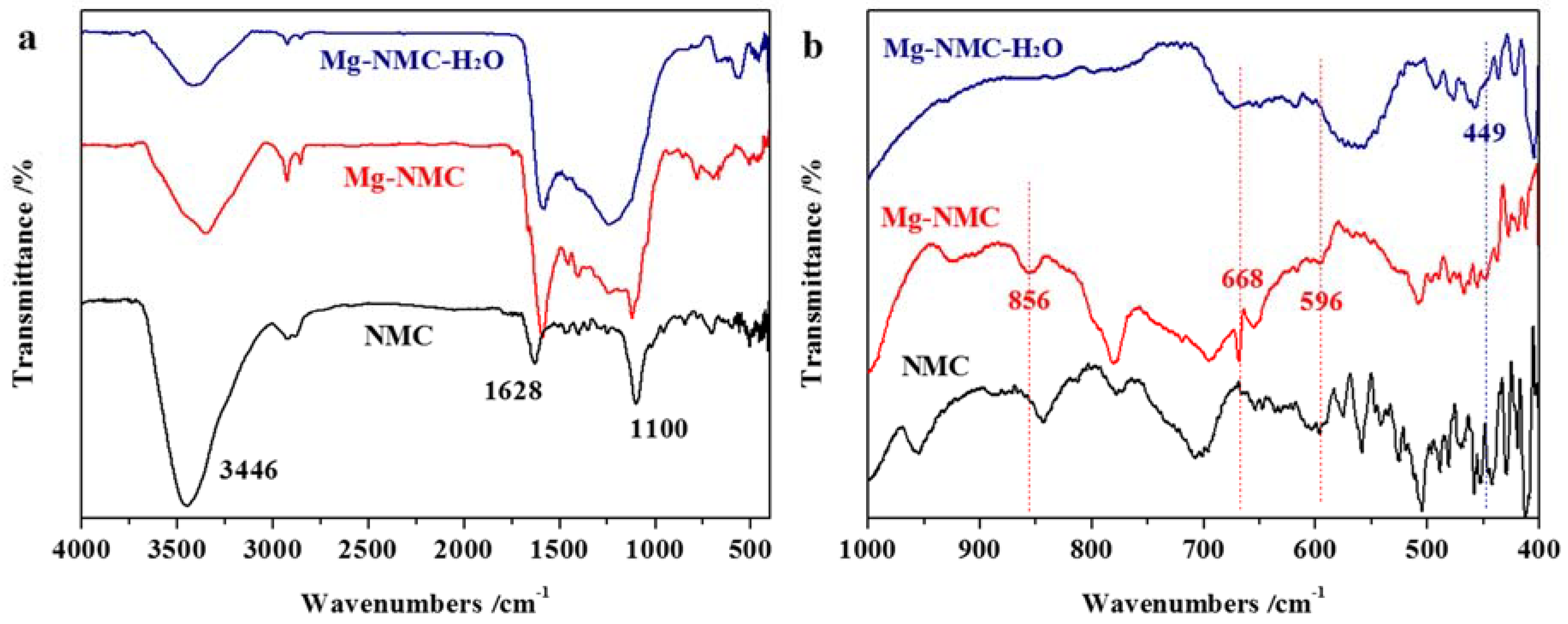
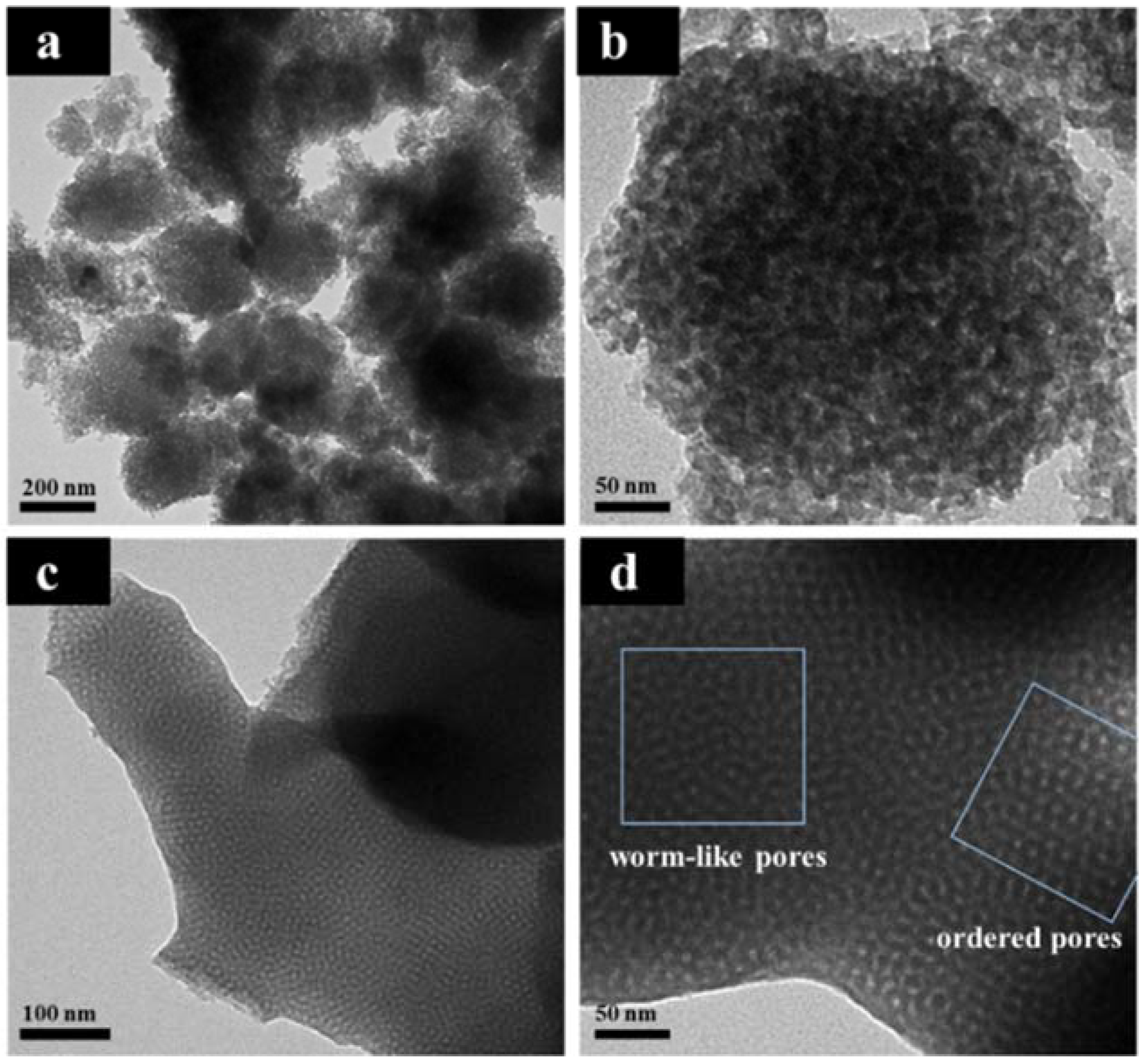
2.4. Characterization
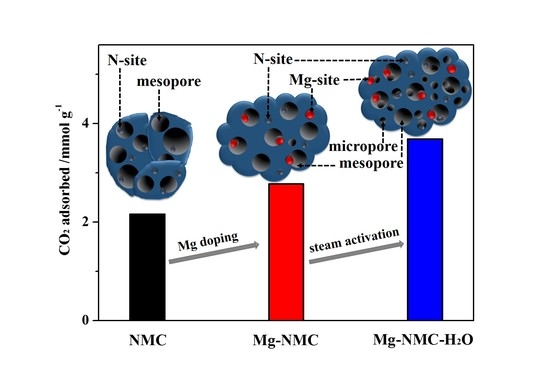
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liang, C.D.; Li, Z.J.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar]
- Tang, J.; Liu, J.; Li, C.L.; Li, Y.Q.; Tade, M.O.; Dai, S.; Yamauchi, Y. Synthesis of nitrogen-doped mesoporous carbon spheres with extra-large pores through assembly of diblock copolymer micelles. Angew. Chem. Int. Ed. 2015, 54, 588–593. [Google Scholar] [CrossRef]
- Asefa, T. Metal-free and noble metal-free heteroatom-doped nanostructured carbons as prospective sustainable electrocatalysts. Acc. Chem. Res. 2016, 49, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Shahtalebi, A.; Mar, M.; Guérin, K.; Bhatia, S.K. Effect of fluorine doping on structure and CO2 adsorption in silicon carbide-derived carbon. Carbon 2016, 96, 565–577. [Google Scholar] [CrossRef]
- Nabae, Y.; Nagata, S.; Ohnishi, K.; Liu, Y.Y.; Sheng, L.; Wang, X.L.; Hayakawa, T. Block copolymer templated carbonization of nitrogen containing polymer to create fine and mesoporous carbon for oxygen reduction catalyst. J. Polym. Sci. Polym. Chem. 2017, 55, 464–470. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Ohnishi, K.; Sugimoto, S.; Okuhara, K.; Maeda, R.; Nabae, Y.; Kakimoto, M.; Wang, X.L.; Hayakawa, T. Well-ordered mesoporous polymers and carbons based on imide-incorporated soft materials. Polym. Chem. 2014, 5, 6452–6460. [Google Scholar] [CrossRef]
- Yu, J.; Guo, M.Y.; Muhammad, F.; Wang, A.F.; Zhang, F.; Li, Q.; Zhu, G.S. One-pot synthesis of highly ordered nitrogen-containing mesoporous carbon with resorcinol-urea-formaldehyde resin for CO2 capture. Carbon 2014, 69, 502–514. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, D.D.; Sun, Z.K.; Deng, Y.H.; Xia, Y.Y.; Zhao, D.Y. A controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for CO2 capture and supercapacitors. Adv. Funct. Mater. 2013, 23, 2322–2328. [Google Scholar] [CrossRef]
- Yang, J.P.; Zhai, Y.P.; Deng, Y.H.; Gu, D.; Li, Q.; Wu, Q.L.; Huang, Y.; Tu, B.; Zhao, D.Y. Direct triblock-copolymer-templating synthesis of ordered nitrogen-containing mesoporous polymers. J. Colloid Interface Sci. 2010, 342, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Zhu, C.M.; Sun, N.N.; Wang, H.; Tang, Z.Y.; Wei, W.; Sun, Y.H. One-pot solvent-free synthesis of nitrogen and magnesium codoped mesoporous carbon composites for CO2 Capture. J. Phys. Chem. C 2015, 119, 9302–9310. [Google Scholar] [CrossRef]
- Wang, G.H.; Deng, X.H.; Gu, D.; Chen, K.; Tüysüz, H.; Spliethoff, B.; Bongard, H.J.; Weidenthaler, C.; Schmidt, W.; Schüth, F. CO3O4 nanoparticles supported on mesoporous carbon for selective transfer hydrogenation of α,β-unsaturated aldehydes. Angew. Chem. Int. Ed. 2016, 55, 11267–11271. [Google Scholar] [CrossRef]
- Wang, X.Q.; Lee, J.S.; Zhu, Q.; Liu, J.; Wang, Y.; Dai, S. Ammonia-treated ordered mesoporous carbons as catalytic materials for oxygen reduction reaction. Chem. Mater. 2010, 22, 2178–2180. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Tian, K.; Ding, Y.W.; Yu, H.Q. Mesoporous carbon stabilized MgO nanoparticles synthesized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol. 2013, 47, 9397–9403. [Google Scholar] [CrossRef] [PubMed]
- She, L.; Li, J.; Wan, Y.; Yao, X.D.; Tu, B.; Zhao, D.Y. Synthesis of ordered mesoporous MgO/carbon composites by a one-pot assembly of amphiphilic triblock copolymers. J. Mater. Chem. 2011, 21, 795–800. [Google Scholar] [CrossRef]
- Fan, D.B.; Li, G.Y.; Qin, T.F.; Chu, F.X. Synthesis and structure characterization of phenol-urea-formaldehyde resins in the presence of magnesium oxide as catalyst. Polymers 2014, 6, 2221–2231. [Google Scholar] [CrossRef]
- Górka, J.; Jaroniec, M. Hierarchically porous phenolic resin-based carbons obtained by block copolymer-colloidal silica templating and post-synthesis activation with carbon dioxide and water vapor. Carbon 2011, 49, 154–160. [Google Scholar] [CrossRef]
- Ludwinowicz, J.; Jaroniec, M. Effect of activating agents on the development of microporosity in polymeric-based carbon for CO2 adsorption. Carbon 2015, 94, 673–679. [Google Scholar] [CrossRef]
- Sui, Z.Y.; Meng, Q.H.; Li, J.T.; Zhu, J.H.; Cui, Y.; Han, B.H. High surface area porous carbons produced by steam activation of graphene aerogels. J. Mater. Chem. A 2014, 2, 9891–9898. [Google Scholar] [CrossRef]
- Wang, G.H.; Cao, Z.W.; Gu, D.; Pfänder, N.; Swertz, A.-C.; Spliethoff, B.; Bongard, H.-J.; Weidenthaler, C.; Schmidt, W.; Rinaldi, R.; et al. Nitrogen-doped ordered mesoporous carbon supported bimetallic PtCo nanoparticles for upgrading of biophenolics. Angew. Chem. Int. Ed. 2016, 55, 8850–8855. [Google Scholar] [CrossRef] [PubMed]
- Daems, N.; Sheng, X.; Vankelecom, I.F.J.; Pescarmona, P.P. Metal-free doped carbon materials as electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 4085–4110. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, X.L.; Liang, Q.H.; Bai, Y.; Huang, Z.H.; Kang, F.Y. Nitrogen-doped hollow activated carbon nanofibers as high performance supercapacitor electrodes. J. Electroanal. Chem. 2015, 739, 84–88. [Google Scholar] [CrossRef]
- Guo, D.H.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, H.L.; Guo, S.J. Towards high-efficiency nanoelectrocatalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chem. Soc. Rev. 2016, 45, 1273–1307. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S.; Yano, T.; Kondo, S.; Kohno, Y.; Tomita, Y.; Maeda, Y.; Kobayashi, K. Electron emission from h-BN films codoped with Mg and O atoms. Thin Solid Films 2013, 546, 53–57. [Google Scholar] [CrossRef]
- Khairallah, F.; Glisenti, A. Synthesis, characterization and reactivity study of nanoscale magnesium oxide. J. Mol. Catal. A Chem. 2007, 274, 137–147. [Google Scholar] [CrossRef]
- Wu, Z.X.; Meng, Y.; Zhao, D.Y. Nanocasting fabrication of ordered mesoporous phenol–formaldehyde resins with various structures and their adsorption performances for basic organic compounds. Microporous Mesoporous Mat. 2010, 128, 165–179. [Google Scholar] [CrossRef]
- Wu, Z.X.; Hao, N.; Xiao, G.K.; Liu, L.Y.; Webley, P.; Zhao, D.Y. One-pot generation of mesoporous carbon supported nanocrystalline calcium oxides capable of efficient CO2 capture over a wide range of temperatures. Phys. Chem. Chem. Phys. 2011, 13, 2495–2503. [Google Scholar] [CrossRef] [PubMed]
- Neville, A.; Morizot, A.P. Calcareous scales formed by cathodic protection-an assessment of characteristics and kinetics. J. Cryst. Growth 2002, 243, 490–502. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, R.F.; Li, W.J.; Wang, J.; Maitz, M.F.; Wang, J.; Wan, G.J.; Chen, Y.Q.; Sun, H.; Jiang, C.X.; et al. Epigallocatechin gallate (EGCG) induced chemical conversion coatings for corrosion protection of biomedical MgZnMn alloys. Corros. Sci. 2015, 94, 305–315. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.Q.; Wang, L.; Zhang, Y.W.; Qin, X.Y.; Luo, Y.L.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X.P. Hydrothermal treatment of grass: A low-cost, green route to nitrogen-doped, carbon-rich, photoluminescent polymer nanodots as an effective fluorescent sensing platform for label-free detection of Cu(II) ions. Adv. Mater. 2012, 24, 2037–2041. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.; Quarto, F.D.; Zanna, S.; Marcus, P. Initial surface film on magnesium metal: A characterization by X-ray photoelectron spectroscopy (XPS) and photocurrent spectroscopy (PCS). Electrochim. Acta 2007, 53, 1314–1324. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Wu, F.-C.; Juang, R.-S. Adsorption of CO2 at atmospheric pressure on activated carbons prepared from melamine-modified phenol-formaldehyde resins. Sep. Purif. Technol. 2015, 140, 53–60. [Google Scholar] [CrossRef]
- Li, Y.B.; Zhang, H.M.; Wang, Y.; Liu, P.R.; Yang, H.G.; Yao, X.D.; Wang, D.; Tang, Z.Y.; Zhao, H.J. A self-sponsored doping approach for controllable synthesis of S and N co-doped trimodal-porous structured graphitic carbon electrocatalysts. Energy Environ. Sci. 2014, 7, 3720–3726. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, M.H.; Liu, Y.; He, F.J.; Gao, F.; Su, Y.J.; Wei, H.; Zhang, Y.F. Nitrogen-doped, carbon-rich, highly photoluminescent carbon dots from ammonium citrate. Nanoscale 2014, 6, 1890–1895. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Ali, M.A.; Krishnan, S.; Agrawal, V.V.; Al Kheraif, A.A.; Fouad, H.; Ansari, Z.A.; Ansari, S.G.; Malhotra, B.D. A label-free photoluminescence genosensor using nanostructured magnesium oxide for cholera detection. Sci. Rep. UK 2015, 5, 17384. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Min, Y.J.; Ahn, S.H.; Hong, S.-M.; Shin, J.-S.; Kim, J.H.; Lee, K.B. Graft copolymer templated synthesis of mesoporous MgO/TiO2 mixed oxide nanoparticles and their CO2 adsorption capacities. Colloids Surf. A Physicochem. Eng. Asp. 2012, 414, 75–81. [Google Scholar] [CrossRef]
- Botha, A.; Strydom, C.A. DTA and FT-IR analysis of the rehydration of basic magnesium carbonate. J. Therm. Anal. Calorim. 2003, 71, 987–995. [Google Scholar] [CrossRef]
- Ghanbari, D.; Salavati-Niasari, M.; Sabet, M. Preparation of flower-like magnesium hydroxide nanostructure and its influence on the thermal stability of poly vinyl acetate and poly vinyl alcohol. Compos. Part B Eng. 2013, 45, 550–555. [Google Scholar] [CrossRef]
- Huang, J.Y.; Xu, M.Q.; Ge, Q.; Lin, M.H.; Lin, Q.; Chen, Y.H.; Chu, J.Y.; Dai, L.Z.; Zou, Y.S. Controlled synthesis of high-ortho-substitution phenol-formaldehyde resins. J. Appl. Polym. Sci. 2005, 97, 652–658. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liang, C.D.; Dai, S. Facile synthesis of ordered mesoporous carbons with high thermal stability by self-assembly of resorcinol-formaldehyde and block copolymers under highly acidic conditions. Langmuir 2008, 24, 7500–7505. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Yoo, J.C.; Hayhurst, D.T. Synthesis of hydrothermally stable MCM-41 with initial adjustment of pH and direct addition of NaF. Microporous Mesoporous Mat. 2000, 39, 177–186. [Google Scholar] [CrossRef]
- Deng, Q.F.; Liu, L.; Lin, X.Z.; Du, G.H.; Liu, Y.P.; Yuan, Z.Y. Synthesis and CO2 capture properties of mesoporous carbon nitride materials. Chem. Eng. J. 2012, 203, 63–70. [Google Scholar] [CrossRef]
- Verziu, M.; Cojocaru, B.; Hu, J.C.; Richards, R.; Ciuculescu, C.; Filip, P.; Parvulescu, V.I. Sunflower and rapeseed oil transesterification to biodiesel over different nanocrystalline MgO catalysts. Green Chem. 2008, 10, 373–381. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
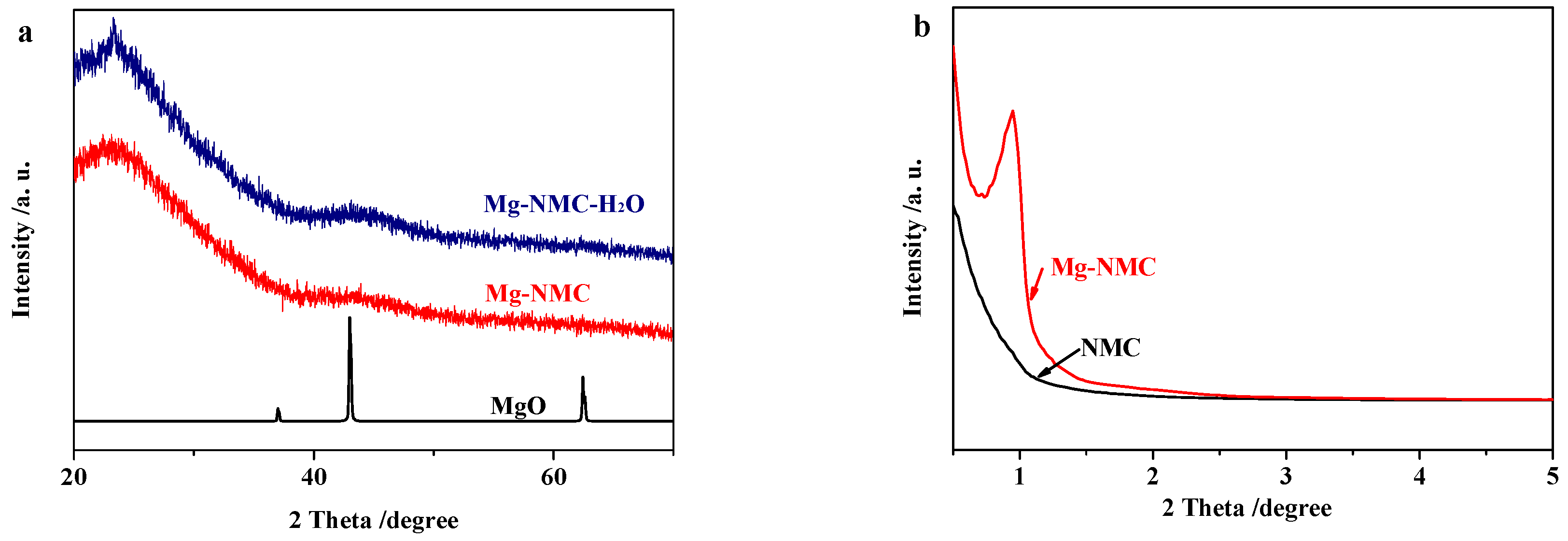
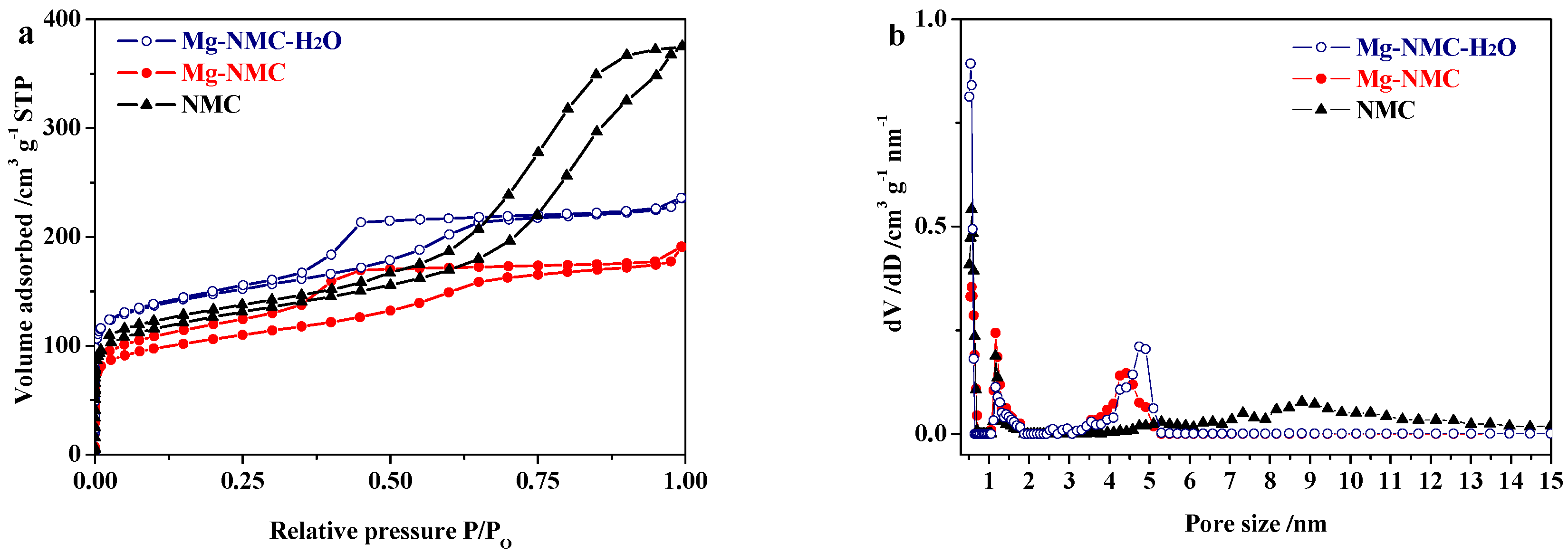
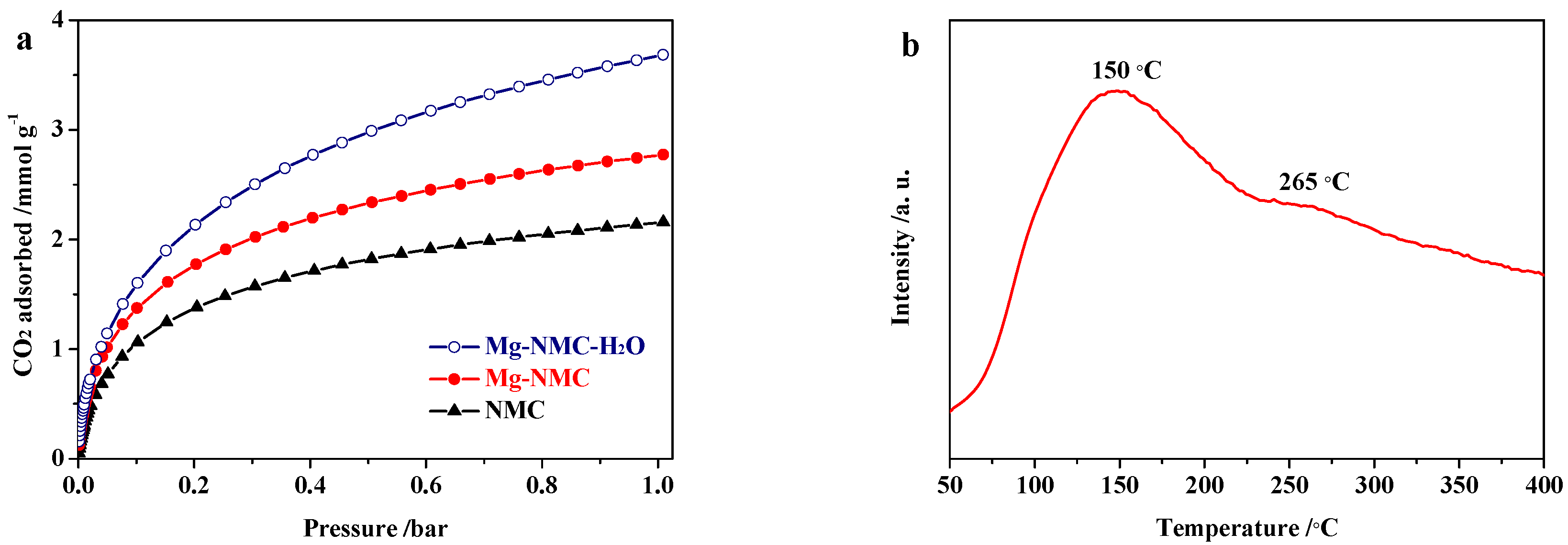
| Sample | SBET/m2 g−1 | Vtotal/cm3 g−1 | Vmicro/cm3 g−1 | Vmeso/cm3 g−1 | Microporosity/% |
|---|---|---|---|---|---|
| NMC | 457 | 0.58 | 0.106 | 0.476 | 18.3 |
| Mg-NMC | 385 | 0.30 | 0.088 | 0.212 | 29.8 |
| Mg-NMC-H2O | 541 | 0.36 | 0.136 | 0.224 | 37.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Jiao, C.; Majeed, Z.; Jiang, H. Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption. Nanomaterials 2018, 8, 275. https://doi.org/10.3390/nano8050275
Lu J, Jiao C, Majeed Z, Jiang H. Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption. Nanomaterials. 2018; 8(5):275. https://doi.org/10.3390/nano8050275
Chicago/Turabian StyleLu, Jingting, Chengli Jiao, Zeeshan Majeed, and Heqing Jiang. 2018. "Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption" Nanomaterials 8, no. 5: 275. https://doi.org/10.3390/nano8050275
APA StyleLu, J., Jiao, C., Majeed, Z., & Jiang, H. (2018). Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption. Nanomaterials, 8(5), 275. https://doi.org/10.3390/nano8050275