Efficient Blue to Red Afterglow Tuning in a Binary Nanocomposite Plastic Film
Abstract
1. Introduction
2. Experimental
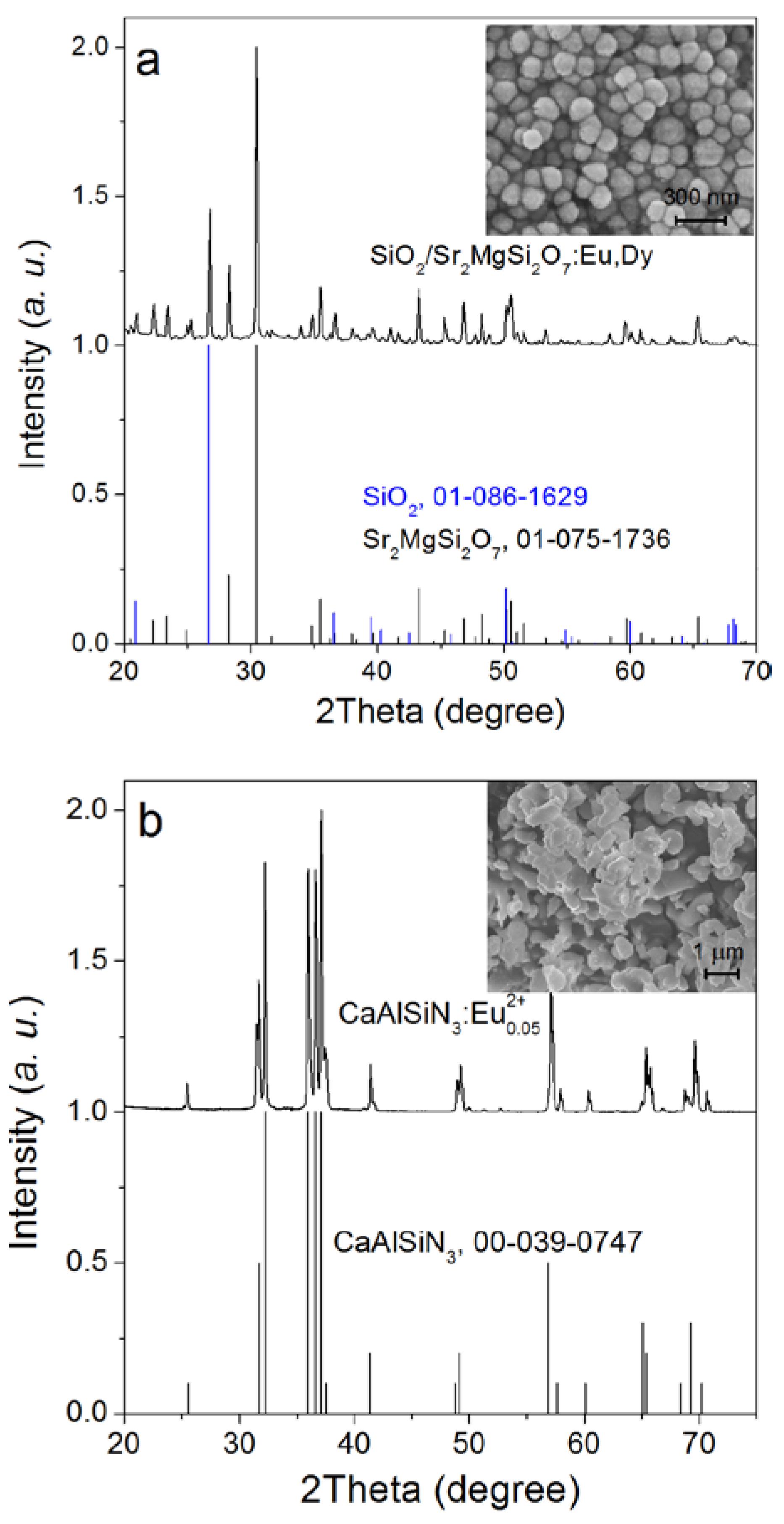
2.1. Synthesis of SMS
2.2. Synthesis of CASN
2.3. SMS/CASN Film Preparation
2.4. Characterization
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lecuyer, T.; Teston, E.; Ramirez-Garcia, G.; Maldiney, T.; Viana, B.; Seguin, J.; Mignet, N.; Scherman, D.; Richard, C. Chemically engineered persistent luminescence nanoprobes for bioimaging. Theranostics 2016, 6, 2488–2524. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gecevicius, M.; Qiu, J.R. Long persistent phosphors-from fundamentals to applications. Chem. Soc. Rev. 2016, 45, 2090–2136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Q.; Wang, Y.; Shen, H.; Yuan, Q. Recent progress in biomedical applications of persistent luminescence nanoparticles. Nanoscale 2017, 9, 6204–6218. [Google Scholar] [CrossRef] [PubMed]
- Chermont, Q.L.; Chaneac, C.; Seguin, J.; Pelle, F.; Maitrejean, S.; Jolivet, J.P.; Gourier, D.; Bessodes, M.; Scherman, D. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 9266–9271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, J.; Liu, J.; Ma, F.; Ju, H. Persistent luminescence nanoprobe for biosensing and lifetime imaging of cell apoptosis via time-resolved fluorescence resonance energy transfer. Biomaterials 2015, 67, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.W.; Lu, Y.Y.; Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 2012, 11, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Bessiere, A.; Seguin, J.; Teston, E.; Sharma, S.K.; Viana, B.; Bos, A.J.J.; Dorenbos, P.; Bessodes, M.; Gourier, D.; et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 2014, 13, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Zhang, Y.W.; Wu, X.; Huang, L.; Li, D.S.; Fan, W.; Han, G. Direct Aqueous-Phase Synthesis of Sub-10 nm “Luminous Pearls” with Enhanced in Vivo Renewable Near-Infrared Persistent Luminescence. J. Am. Chem. Soc. 2015, 137, 5304–5307. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.P.; Sun, M.; Sun, X.; Zhang, H.W. Near-infrared persistent luminescence hollow mesoporous nanospheres for drug delivery and in vivo renewable imaging. J. Mater. Chem. B 2016, 4, 7845–7851. [Google Scholar] [CrossRef]
- Chen, L.J.; Yang, C.X.; Yan, X.P. Liposome-Coated Persistent Luminescence Nanoparticles as Luminescence Trackable Drug Carrier for Chemotherapy. Anal. Chem. 2017, 89, 6936–6939. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, X.; Li, Y.; Jiang, M.; Liu, H.; Zeng, S.; Hao, J. X-ray-Activated Near-Infrared Persistent Luminescent Probe for Deep-Tissue and Renewable in Vivo Bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 22132–22142. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Kuroishi, K.; Tanabe, S. Bright persistent ceramic phosphors of Ce3+-Cr3+-codoped garnet able to store by blue light. Appl. Phys. Lett. 2014, 104, 101904. [Google Scholar] [CrossRef]
- Liu, F.; Liang, Y.J.; Chen, Y.F.; Pan, Z.W. Divalent Nickel-Activated Gallate-Based Persistent Phosphors in the Short-Wave Infrared. Adv. Opt. Mater. 2016, 4, 562–566. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.Y.; Sharafudeen, K.; Dong, G.P.; Zhou, S.F.; Ma, Z.J.; Peng, M.Y.; Qiu, J.R. A strategy for developing near infrared long-persistent phosphors: Taking MAlO3:Mn4+,Ge4+ (M = La, Gd) as an example. J. Mater. Chem. C 2014, 2, 2019–2027. [Google Scholar] [CrossRef]
- Guo, H.J.; Wang, Y.H.; Li, G.; Liu, J.; Feng, P.; Liu, D.W. Cyan emissive super-persistent luminescence and thermoluminescence in BaZrSi3O9: Eu2+, Pr3+ phosphors. J. Mater. Chem. C 2017, 5, 2844–2851. [Google Scholar] [CrossRef]
- Sun, W.Z.; Pang, R.; Li, H.M.; Li, D.; Jiang, L.H.; Zhang, S.; Fu, J.P.; Li, C.Y. Investigation of a novel color tunable long afterglow phosphor KGaGeO4:Bi3+: Luminescence properties and mechanism. J. Mater. Chem. C 2017, 5, 1346–1355. [Google Scholar] [CrossRef]
- Palner, M.; Pu, K.Y.; Shao, S.; Rao, J.H. Semiconducting Polymer Nanoparticles with Persistent Near-Infrared Luminescence for In Vivo Optical Imaging. Angew. Chem. Int. Ed. 2015, 54, 11477–11480. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.G.; Yan, D.P. Long-afterglow metal-organic frameworks: Reversible guest-induced phosphorescence tunability. Chem. Sci. 2016, 7, 4519–4526. [Google Scholar] [CrossRef]
- Van den Eeckhout, K.; Smet, P.F.; Poelman, D. Persistent Luminescence in Eu2+-Doped Compounds: A Review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef]
- Zhuang, Y.X.; Katayama, Y.; Ueda, J.; Tanabe, S. A brief review on red to near-infrared persistent luminescence in transition-metal-activated phosphors. Opt. Mater. 2014, 36, 1907–1912. [Google Scholar] [CrossRef]
- Smet, P.F.; Parmentier, A.B.; Poelman, D. Selecting Conversion Phosphors for White Light-Emitting Diodes. J. Electrochem. Soc. 2011, 158, R37–R54. [Google Scholar] [CrossRef]
- Palazon, F.; Di Stasio, F.; Akkerman, Q.A.; Krahne, R.; Prato, M.; Manna, L. Polymer-Free Films of Inorganic Halide Perovskite Nanocrystals as UV-to-White Color-Conversion Layers in LEDs. Chem. Mater. 2016, 28, 2902–2906. [Google Scholar] [CrossRef] [PubMed]
- Pust, P.; Weiler, V.; Hecht, C.; Tucks, A.; Wochnik, A.S.; Henss, A.K.; Wiechert, D.; Scheu, C.; Schmidt, P.J.; Schnick, W. Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material. Nat. Mater. 2014, 13, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, B.K.; Wang, Z.G.; Hung, T.F.; Susha, A.S.; Zhong, H.Z.; Rogach, A.L. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 2016, 7, 5699–5703. [Google Scholar] [CrossRef]
- Oh, J.H.; Kang, H.; Eo, Y.J.; Park, H.K.; Do, Y.R. Synthesis of narrow-band red-emitting K2SiF6:Mn4+ phosphors for a deep red monochromatic LED and ultrahigh color quality warm-white LEDs. J. Mater. Chem. C 2015, 3, 607–615. [Google Scholar] [CrossRef]
- Chen, W.B.; Wang, Y.H.; Zeng, W.; Han, S.C.; Li, G.; Guo, H.J.; Li, Y.Y.; Qiang, Q.P. Long persistent composite phosphor CaAl2O4:Eu2+,Nd3+/Y3Al5O12:Ce3+: A novel strategy to tune the colors of persistent luminescence. New J. Chem. 2016, 40, 485–491. [Google Scholar] [CrossRef]
- Pan, W.; Ning, G.L.; Zhang, X.; Wang, J.; Lin, Y.; Ye, J.W. Enhanced luminescent properties of long-persistent Sr2MgSi2O7:Eu2+, Dy3+ phosphor prepared by the co-precipitation method. J. Lumin. 2008, 128, 1975–1979. [Google Scholar] [CrossRef]
- Shi, X.D.; Sho, L.; Li, M.Z.; Hou, J.; Chen, L.F.; Ye, C.Q.; Shen, W.Z.; Jiang, L.; Song, Y.L. Efficient Luminescence of Long Persistent Phosphor Combined with Photonic Crystal. ACS Appl. Mater. Interfaces 2014, 6, 6317–6321. [Google Scholar] [CrossRef] [PubMed]
- Homayoni, H.; Ma, L.; Zhang, J.Y.; Sahi, S.K.; Rashidi, L.H.; Bui, B.; Chen, W. Synthesis and conjugation of Sr2MgSi2O7:Eu2+, Dy3+ water soluble afterglow nanoparticles for photodynamic activation. Photodiagn. Photodyn. Ther. 2016, 16, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Sohn, K.S. Effect of inhomogeneous broadening on time-resolved photoluminescence in CaAlSiN3:Eu2+. Opt. Lett. 2010, 35, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Machida, K.; Horikawa, T.; Hanzawa, H. Synthesis and Photoluminescence Properties of CaAlSiN3:Eu2+ Nanocrystals. Chem. Lett. 2010, 39, 104–105. [Google Scholar] [CrossRef]
- Piao, X.; Machida, K.; Horikawa, T.; Hanzawa, H.; Shimomura, Y.; Kijima, N. Preparation of CaAlSiN3:Eu2+ Phosphors by the Self-Propagating High-Temperature Synthesis and Their Luminescent Properties. Chem. Mater. 2007, 19, 4592–4599. [Google Scholar] [CrossRef]
- Li, Z.J.; Zhang, H.W.; Sung, M.; Shen, J.S.; Fu, H.X. A facile and effective method to prepare long-persistent phosphorescent nanospheres and its potential application for in vivo imaging. J. Mater. Chem. 2012, 22, 24713–24720. [Google Scholar]
- Li, Z.; Shi, J.; Zhang, H.; Sun, M. Highly controllable synthesis of near-infrared persistent luminescence SiO2/CaMgSi2O6 composite nanospheres for imaging in vivo. Opt. Express 2014, 22, 10509–10518. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.G.; Liu, Q.L. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Fresta, E.; Costa, R.D. Beyond traditional light-emitting electrochemical cells—A review of new device designs and emitters. J. Mater. Chem. C 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.W.; Huang, W.; Bettinelli, M.; Liu, X.G. Lanthanide-Activated Phosphors Based on 4f-5d Optical Transitions: Theoretical and Experimental Aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Tang, Z.L.; Zhang, Z.T.; Wang, X.X.; Zhang, J.Y. Preparation of a new long afterglow blue-emitting Sr2MgSi2O7-based photoluminescent phosphor. J. Mater. Sci. Lett. 2001, 20, 1505–1506. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, C.K.; Mao, D.L. Luminescent properties of Sr2MgSi2O7 and Ca2MgSi2O7 long lasting phosphors activated by Eu2+, Dy3+. J. Alloy Compd. 2005, 390, 133–137. [Google Scholar] [CrossRef]
- Lin, Y.H.; Nan, C.W.; Zhou, X.S.; Wu, J.B.; Wang, H.F.; Chen, D.P.; Xu, S.M. Preparation and characterization of long afterglow M2MgSi2O7-based (M: Ca, Sr, Ba) photoluminescent phosphors. Mater. Chem. Phys. 2003, 82, 860–863. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Ai, P.; Chen, X. Synthesis and characterization of Y2O2S:Eu3+, Mg2+, Ti4+ nanorods via a solvothermal routine. J. Rare Earth 2009, 27, 895–899. [Google Scholar] [CrossRef]
- Jia, D. Enhancement of long-persistence by CeCo-doping in CaS:Eu2+, Tm3+ red phosphor. J. Electrochem. Soc. 2006, 153, H198–H201. [Google Scholar] [CrossRef]
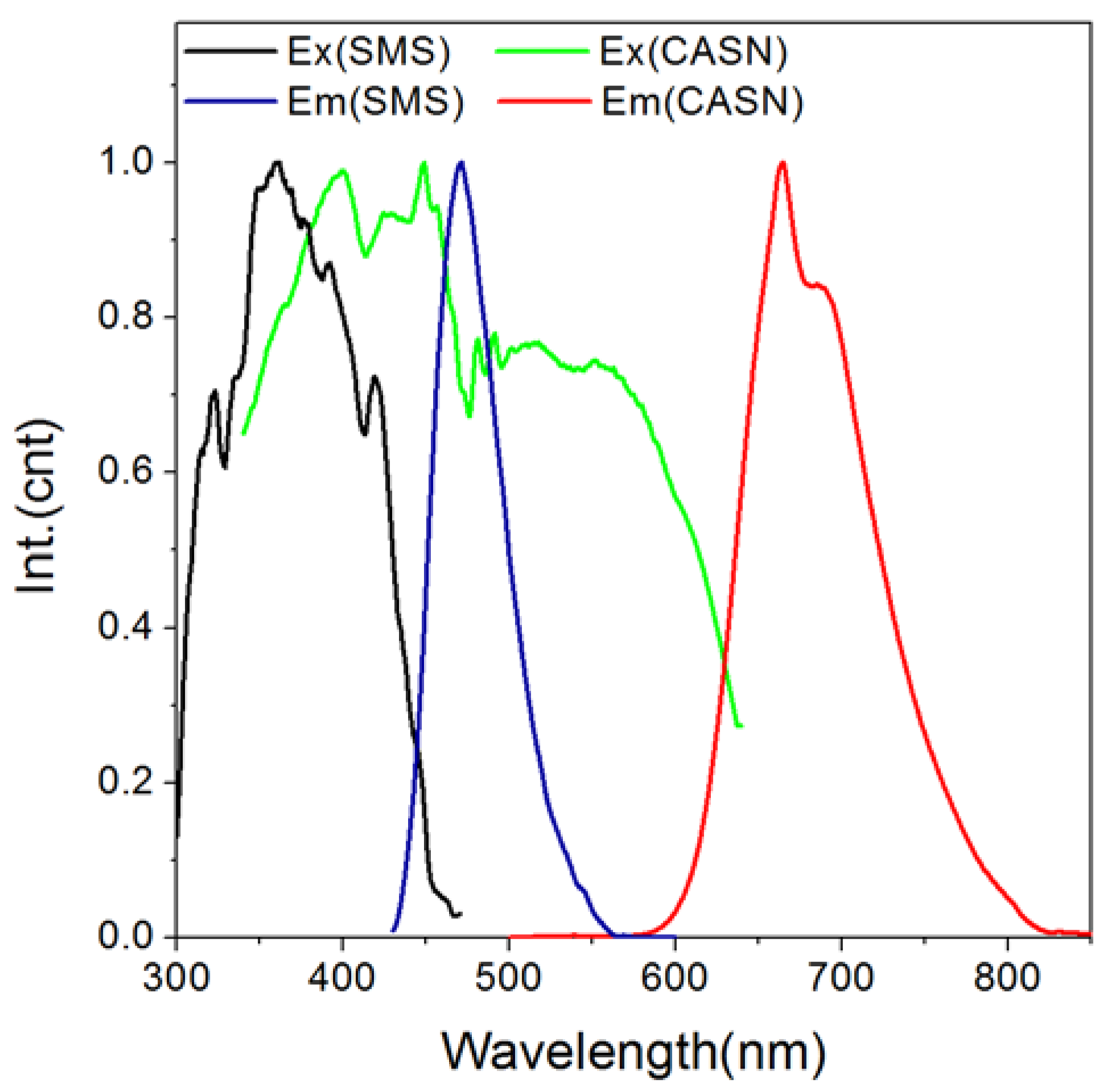
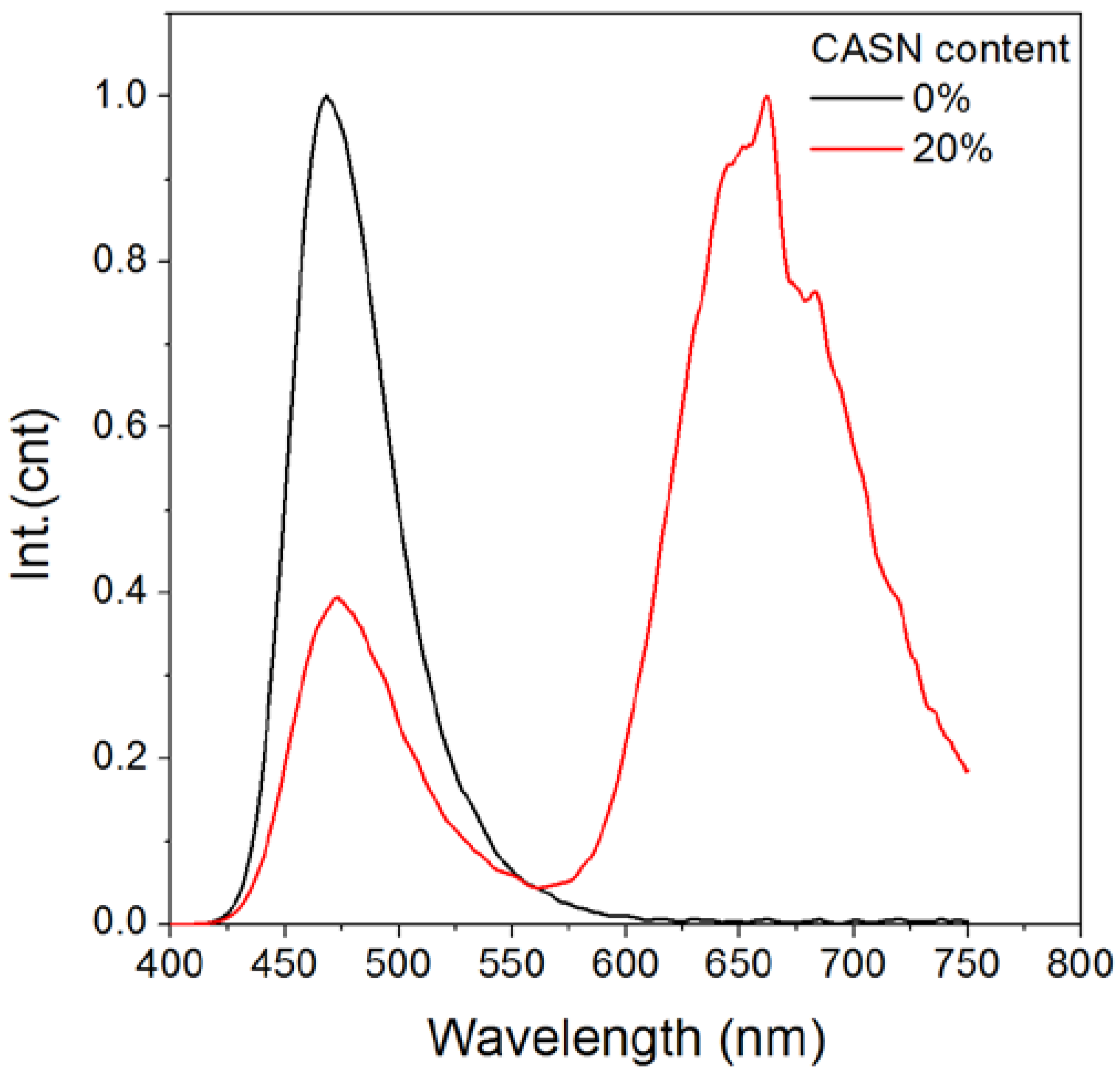
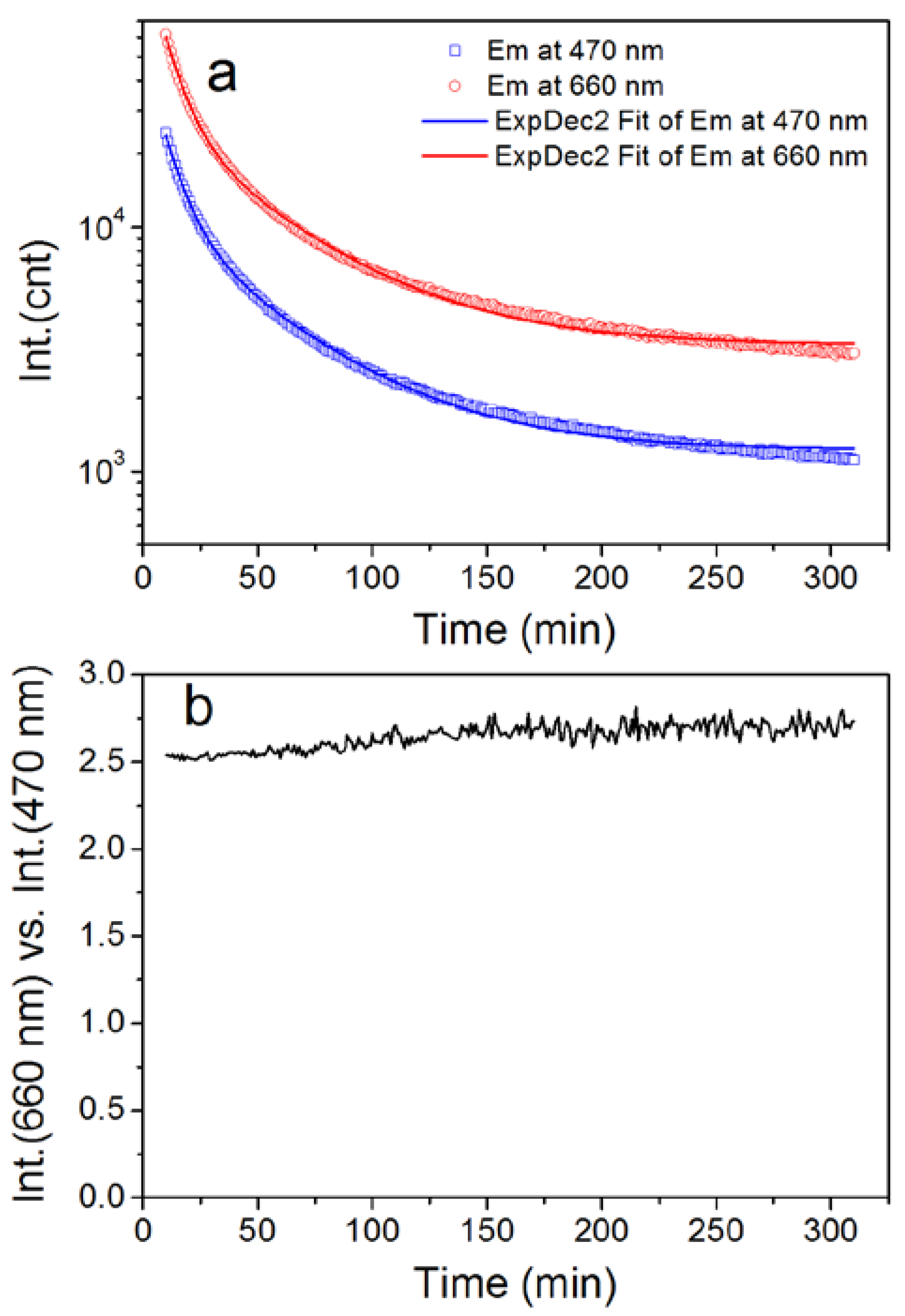
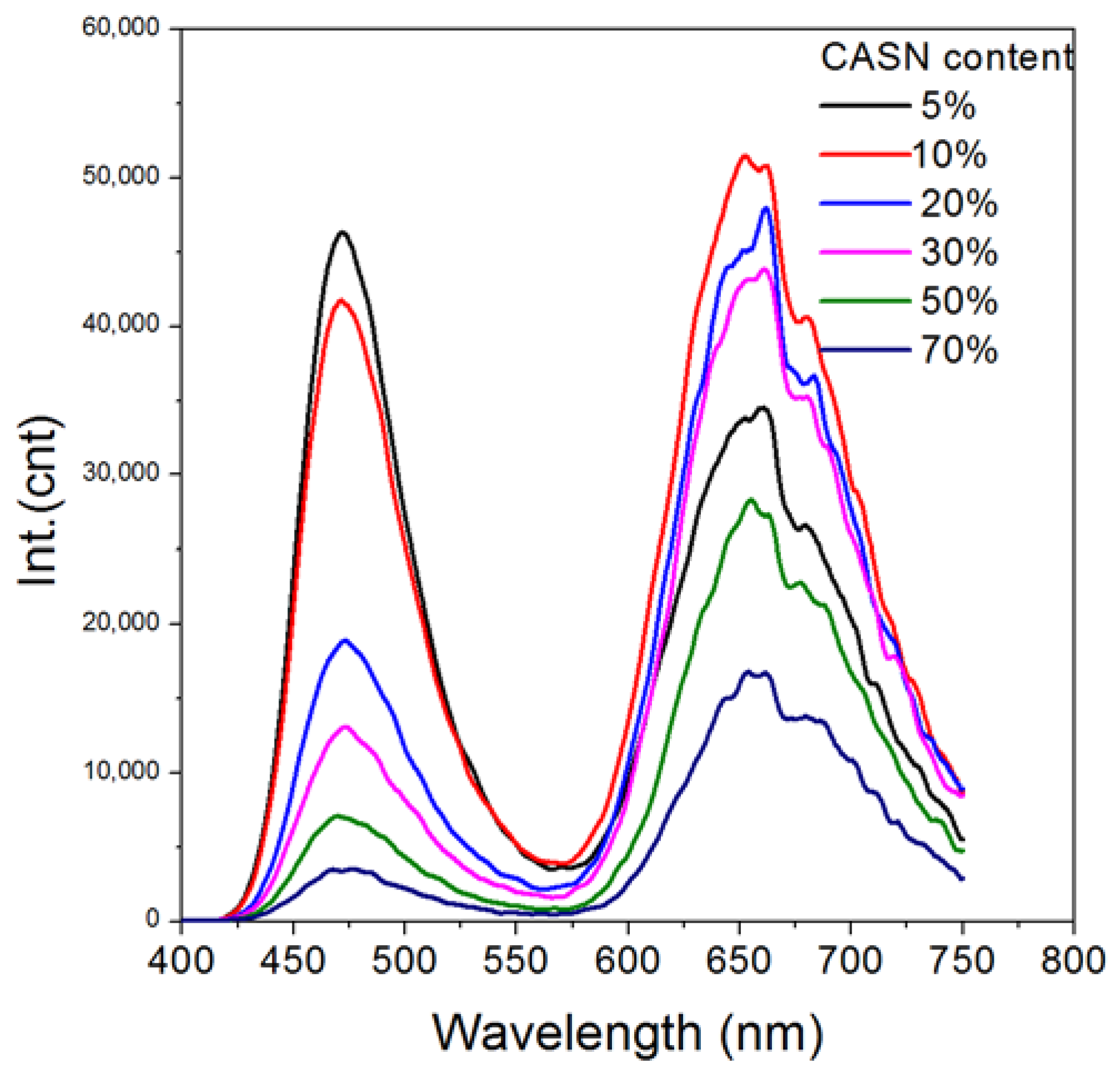
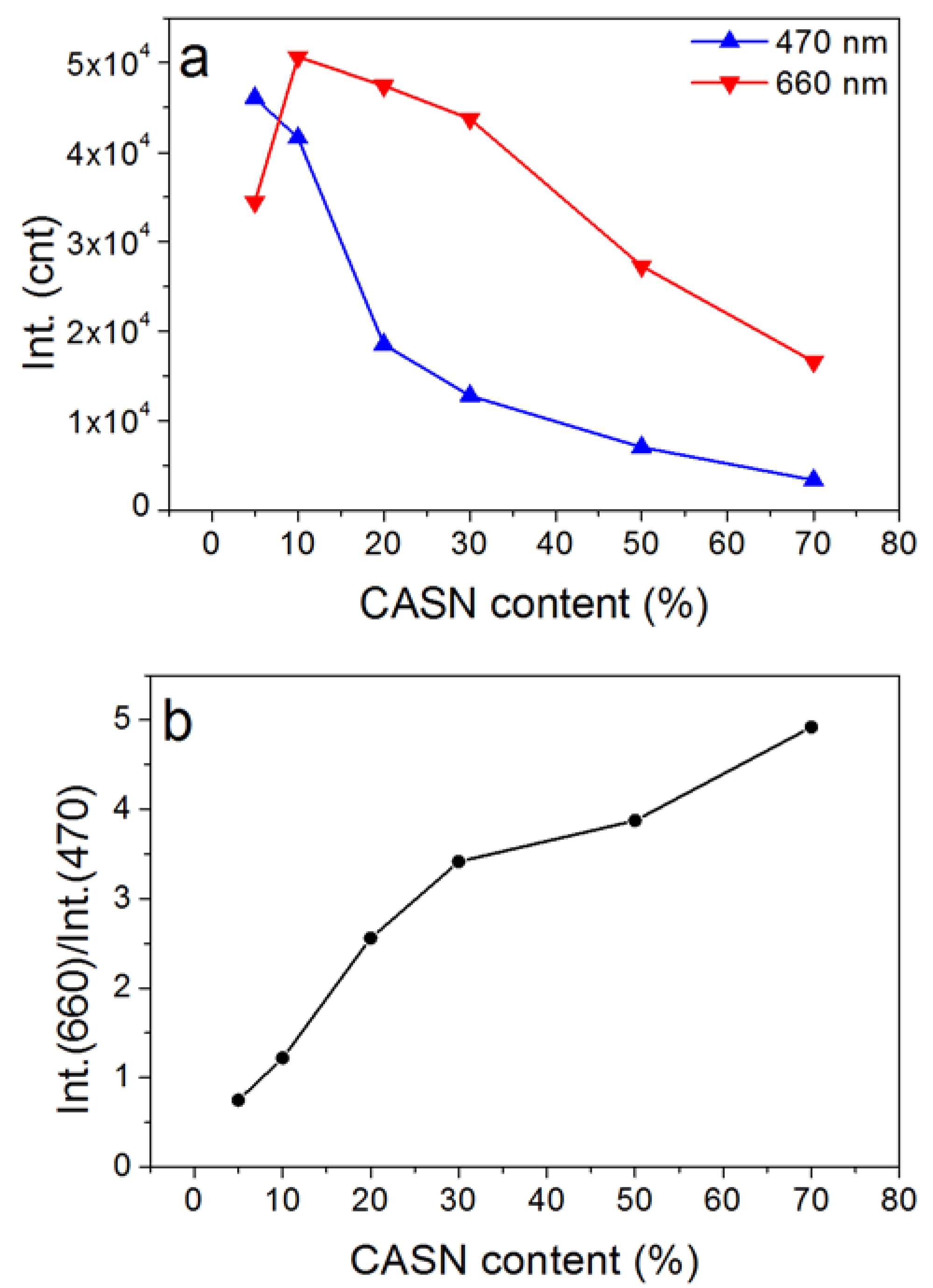
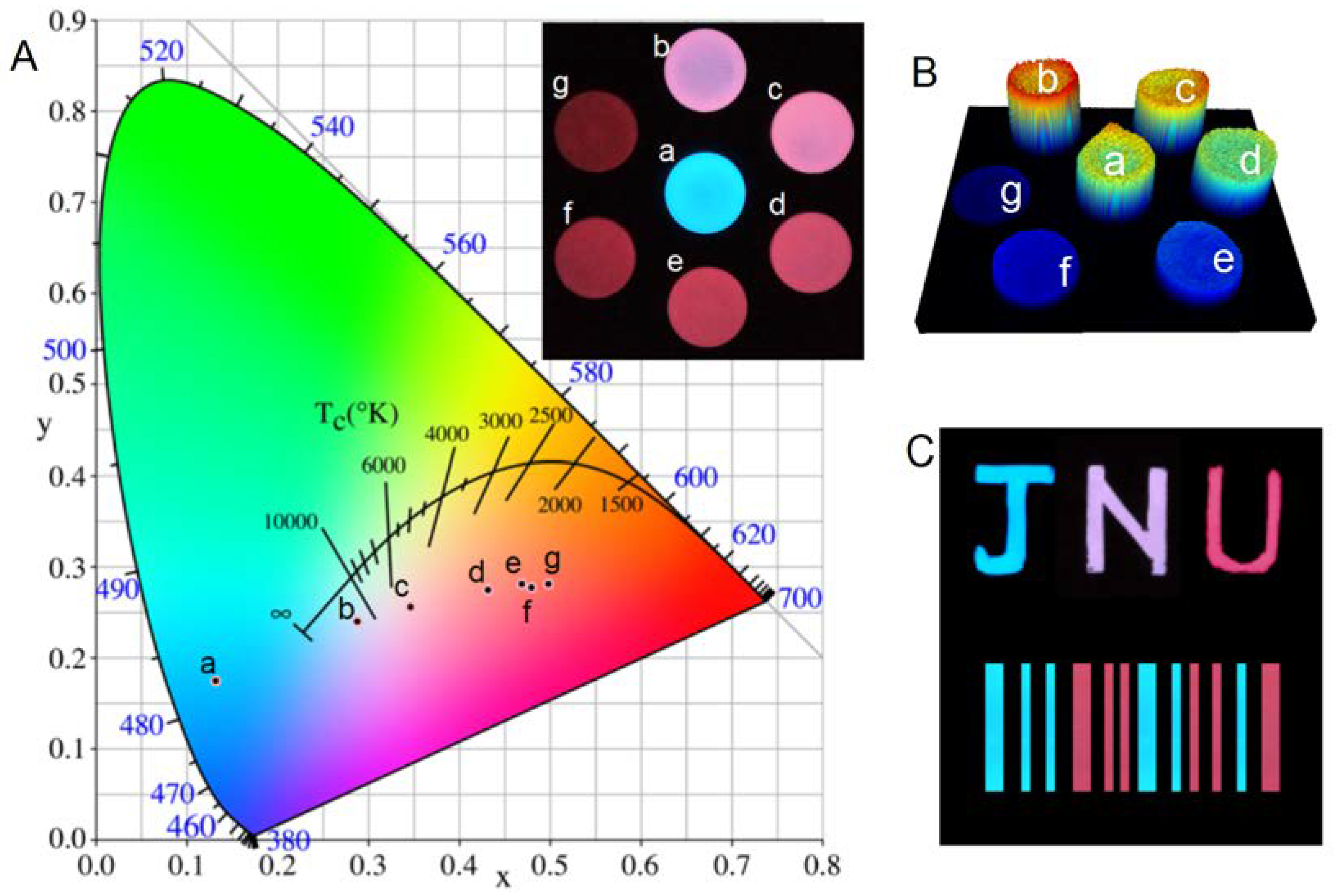
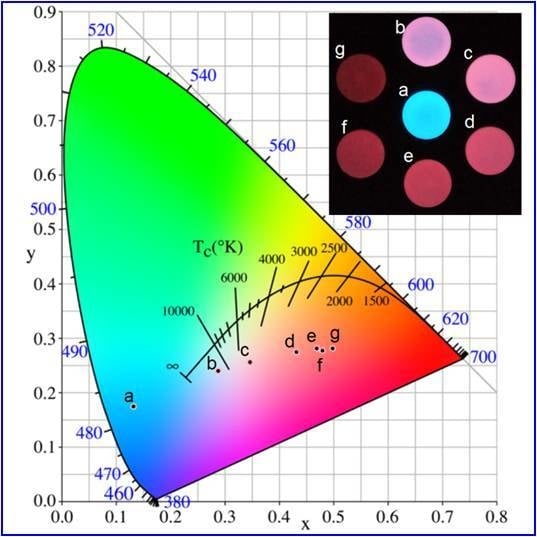
| Wavelength/nm | t1/min | t2/min | A1 | A2 | R2 |
|---|---|---|---|---|---|
| 470 | 8.8 | 48.1 | 42,980 | 10,797 | 0.9994 |
| 660 | 8.9 | 48.8 | 111,110 | 26,721 | 0.9993 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Ou, H.; Li, W.; Han, G.; Li, Z. Efficient Blue to Red Afterglow Tuning in a Binary Nanocomposite Plastic Film. Nanomaterials 2018, 8, 260. https://doi.org/10.3390/nano8040260
Xia Y, Ou H, Li W, Han G, Li Z. Efficient Blue to Red Afterglow Tuning in a Binary Nanocomposite Plastic Film. Nanomaterials. 2018; 8(4):260. https://doi.org/10.3390/nano8040260
Chicago/Turabian StyleXia, Yan, Huase Ou, Wanbin Li, Gang Han, and Zhanjun Li. 2018. "Efficient Blue to Red Afterglow Tuning in a Binary Nanocomposite Plastic Film" Nanomaterials 8, no. 4: 260. https://doi.org/10.3390/nano8040260
APA StyleXia, Y., Ou, H., Li, W., Han, G., & Li, Z. (2018). Efficient Blue to Red Afterglow Tuning in a Binary Nanocomposite Plastic Film. Nanomaterials, 8(4), 260. https://doi.org/10.3390/nano8040260