Tailored Synthesis of Core-Shell Mesoporous Silica Particles—Optimization of Dye Sorption Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Procedures
2.3.1. Synthesis of SCMS Particles
2.3.2. Rhodamine 6G Adsorption Studies
2.3.3. Scanning Electron Microscopy Measurements
3. Results and Discussion
3.1. Synthesis and Microscopic Characterization of SCMS Particles
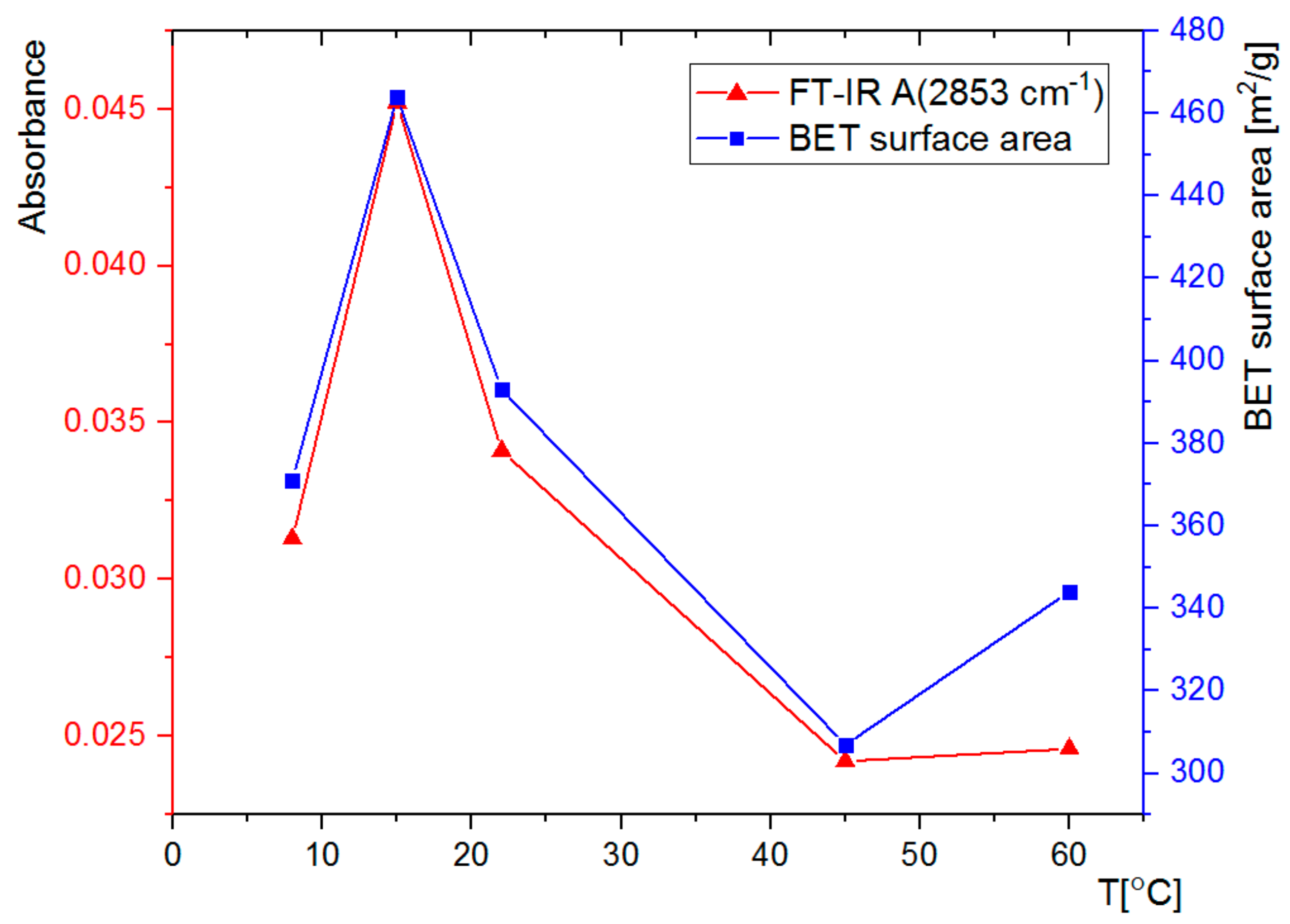
3.2. FT-IR Spectral Analysis
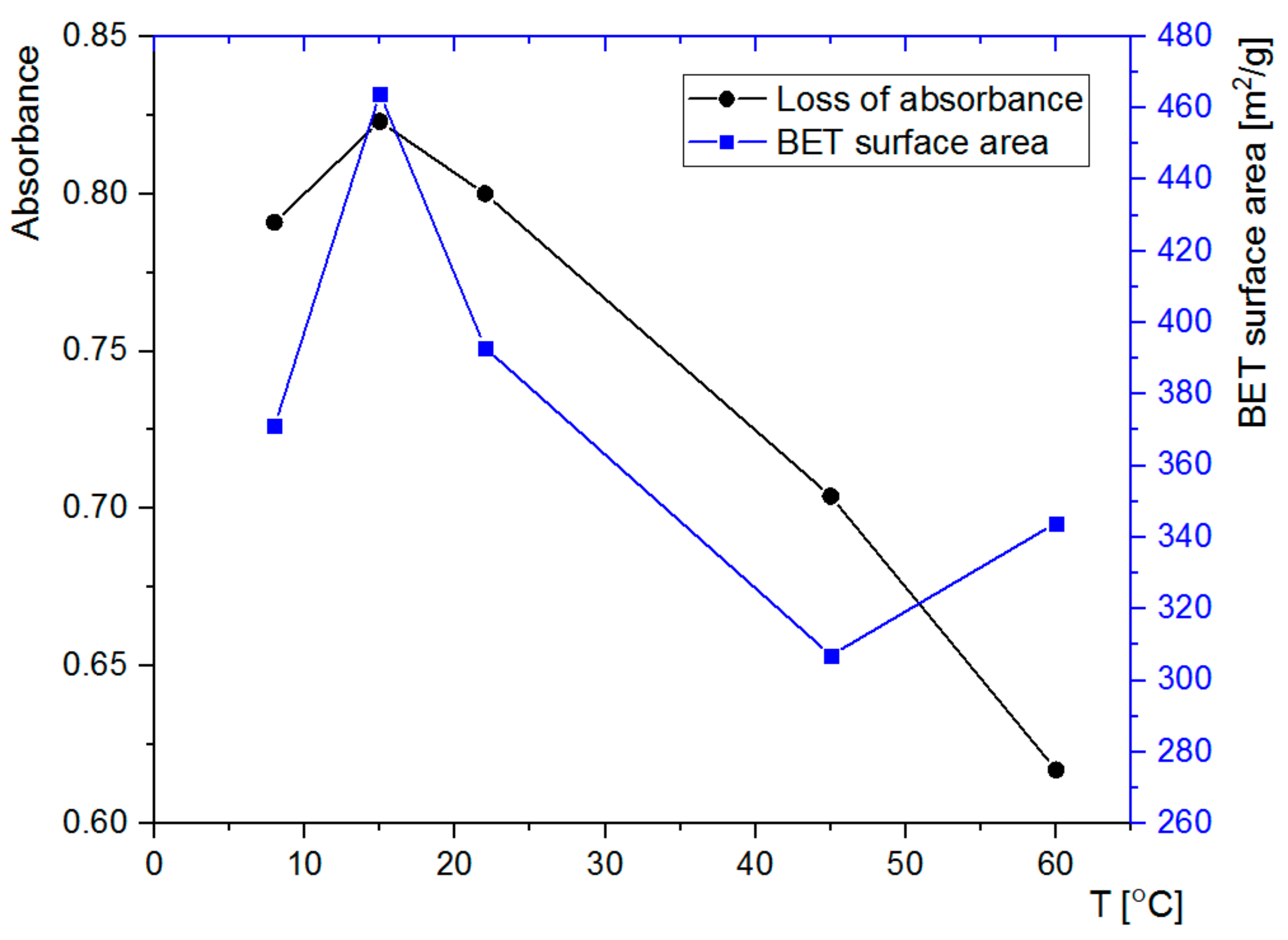
3.3. Nitrogen Adsorption Analysis
3.4. Adsorption of Rh6G within SCMS Particles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamamoto, E.; Kuroda, K. Colloidal mesoporous silica nanoparticles. Bull. Chem. Soc. Jpn. 2016, 89, 501–539. [Google Scholar] [CrossRef]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, E.Y.; Kurdyukov, D.A.; Yakovlev, S.A.; Kirilenko, D.A.; Yu, A.K.; Nashchekin, A.V.; Sitnikova, A.A.; Yagovkina, M.A.; Golubev, V.G. Monodisperse spherical mesoporous silica particles: Fast synthesis procedure and fabrication of photonic-crystal films. Nanotechnology 2013, 24, 155601. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: A review. Microporous Mesoporous Mater. 2016, 236, 141–157. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Moya, E.M.O.; Cavalcante, C.L.; Azevedo, D.C.S.; Rodríguez-Castellón, E. “Low cost” pore expanded SBA-15 functionalized with amine groups applied to CO2 adsorption. Materials 2015, 8, 2495–2513. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2017, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Luo, Z.S.; Pang, W.Q.; Fan, Y.W.; Chen, X.H.; Cui, F.Z. Dilute solution routes to various controllable morphologies of MCM-41 silica with a basic medium. Chem. Mater. 2001, 13, 258–263. [Google Scholar] [CrossRef]
- Fowler, C.E.; Khushalani, D.; Lebeau, B.; Mann, S. Nanoscale materials with mesostructured interiors. Adv. Mater. 2001, 13, 649–652. [Google Scholar] [CrossRef]
- Nooney, R.I.; Thirunavukkarasu, D.; Yimei, C.; Josephs, R.; Ostafin, A.E. Synthesis of nanoscale mesoporous silica spheres with controlled particle size. Chem. Mater. 2002, 14, 4721–4728. [Google Scholar] [CrossRef]
- Lai, C.Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Grün, M.; Lauer, I.; Unger, K.K. The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv. Mater. 1997, 9, 254–257. [Google Scholar] [CrossRef]
- Yano, K.; Fukushima, Y. Synthesis of mono-dispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactant. J. Mater. Chem. 2004, 14, 1579–1584. [Google Scholar] [CrossRef]
- Hao, Y.; Jiao, X.; Zou, H.; Yang, H.; Liu, J. Growing a hydrophilic nanoporous shell on a hydrophobic catalyst interface for aqueous reactions with high reaction efficiency and in situ catalyst recycling. J. Mater. Chem. A 2017, 5, 16162–16170. [Google Scholar] [CrossRef]
- Xia, H.; Wan, G.; Chen, G.; Bai, Q. Preparation of superficially porous core-shell silica particle with controllable mesopore by a dual-templating approach for fast HPLC of small molecules. Mater. Lett. 2017, 192, 5–8. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Bayindir, M. A porosity difference based selective dissolution strategy to prepare shape-tailored hollow mesoporous silica nanoparticles. J. Mater. Chem. A 2015, 3, 3839–3846. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-D.; Lian, H.-Y.; Leo, S.-Y.; Wang, S.-G.; Yamauchi, Y.; Wu, K.C.-W. Controlling Particle Size and Structural Properties of Mesoporous Silica Nanoparticles Using the Taguchi Method. J. Phys. Chem. C 2011, 115, 13158–13165. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, L.; Zhang, J.; Yuan, P.; Thorn, P.; Gu, W.; Yu, C. A simple approach to prepare monodisperse mesoporous silica nanospheres with adjustable sizes. J. Colloid Interface Sci. 2012, 376, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Allouche, J.; Dupin, J.-C.; Gonbeau, D. Generation of a mesoporous silica MSU shell onto solid core silica nanoparticles using a simple two-step sol-gel process. Chem. Commun. 2011, 47, 7476–7478. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, S.B.; Kim, J.Y.; Chae, Y.B.; Yu, J.S. Synthesis of monodisperse silica spheres with solid core and mesoporous shell: Morphological control of mesopores. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 313–314, 77–81. [Google Scholar] [CrossRef]
- Yoon, S.B.; Kim, J.-Y.; Kim, J.H.; Park, Y.J.; Yoon, K.R.; Park, S.-K.; Yu, J.-S. Synthesis of monodisperse spherical silica particles with solid core and mesoporous shell: Mesopore channels perpendicular to the surface. J. Mater. Chem. 2007, 17, 1758. [Google Scholar] [CrossRef]
- Stasiuk, E.N.B.; Schramm, L.L. The temperature dependence of the critical micelle concentrations of foam-forming surfactants. J. Colloid Interface Sci. 1996, 178, 324–333. [Google Scholar] [CrossRef]
- La Mesa, C.; Ranieri, G.A.; Terenzi, M. Studies on krafft point solubility in surfactant solutions. Thermochim. Acta 1988, 137, 143–150. [Google Scholar] [CrossRef]
- Boissière, C.; Larbot, A.; Prouzet, E. Synthesis of mesoporous MSU-x materials using inexpensive silica sources. Chem. Mater. 2000, 12, 1937–1940. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Di Renzo, F.; Ryoo, R.; Choi, M.; Fajula, F. Microporosity and connections between pores in SBA-15 mesostructured silicas as a function of the temperature of synthesis. New J. Chem. 2003, 27, 73–79. [Google Scholar] [CrossRef]
- Manojlović, J.Ž. The Krafft temperature of surfactant solutions. Therm. Sci. 2013, 16, 631–640. [Google Scholar] [CrossRef]
- Akbaş, H.; Kartal, Ç. Conductometric studies of hexadecyltrimethylammonium bromide in aqueous solutions of ethanol and ethylene glycol. Colloid J. 2006, 68, 125–130. [Google Scholar] [CrossRef]
- Fan, J.; Yu, C.; Lei, J.; Zhang, Q.; Li, T.; Tu, B.; Zhou, W.; Zhao, D. Low-temperature strategy to synthesize highly ordered mesoporous silicas with very large pores. J. Am. Chem. Soc. 2005, 127, 10794–10795. [Google Scholar] [CrossRef] [PubMed]
- Zana, R. Microviscosity of Aqueous Surfactant Micelles: Effect of Various Parameters. J. Phys. Chem. 1999, 103, 9117–9125. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Thomassen, L.C.J.; Aerts, A.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Napierska, D.; Hoet, P.H.; Kirschhock, C.E.A.; Martenset, J.A. Synthesis and Characterization of Stable Monodisperse Silica Nanoparticle Sols for in Vitro Cytotoxicity Testing. Langmuir 2010, 26, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, C.; Liu, Z.; Liu, P.; Zheng, N. A cationic surfactant assisted selective etching strategy to hollow mesoporous silica spheres. Nanoscale 2011, 3, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Bain, A.J.; Chandna, P.; Butcher, G.; Bryant, J. Picosecond polarized fluorescence studies of anisotropic fluid media. II.Experimental studies of molecular order and motion in jet aligned rhodamine 6G and resorufin solutions. J. Chem. Phys. 2000, 23, 10435–10449. [Google Scholar] [CrossRef]
- Naumov, S.; Valiullin, R.; Kärger, J. Understanding adsorption and desorption processes in mesoporous materialswith independent disordered channels. Phys. Rev. E 2009, 80, 031607. [Google Scholar] [CrossRef] [PubMed]
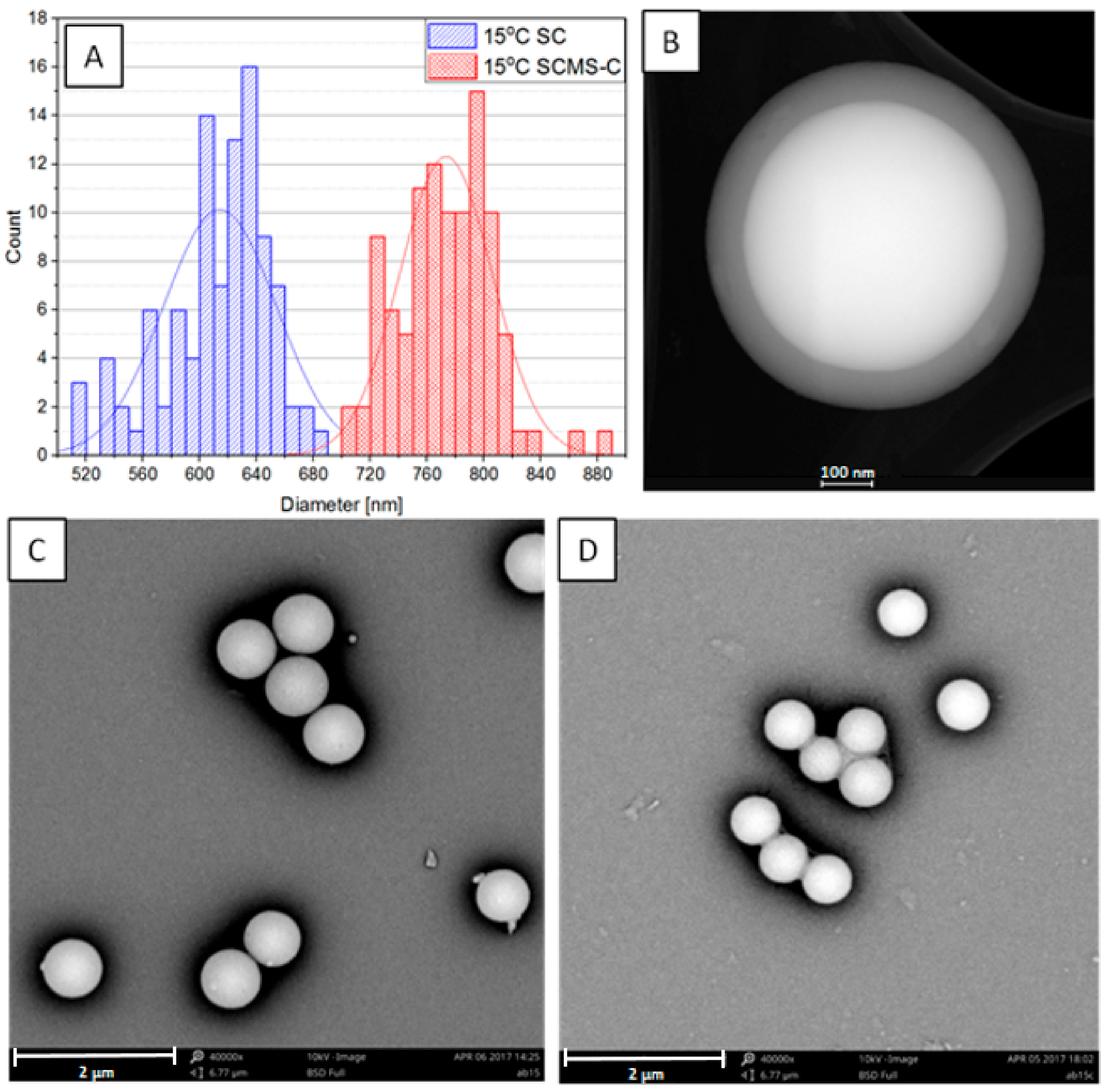
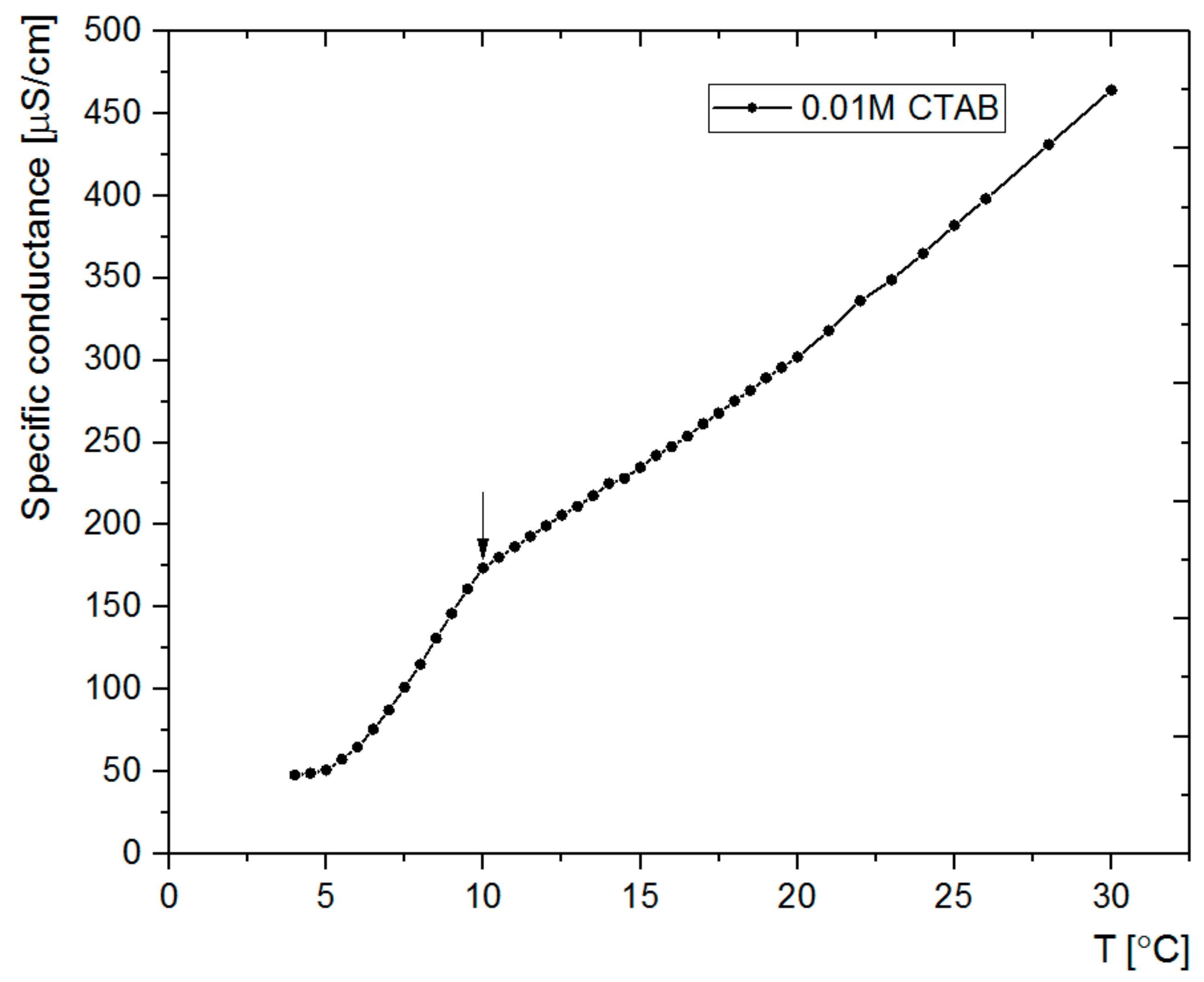

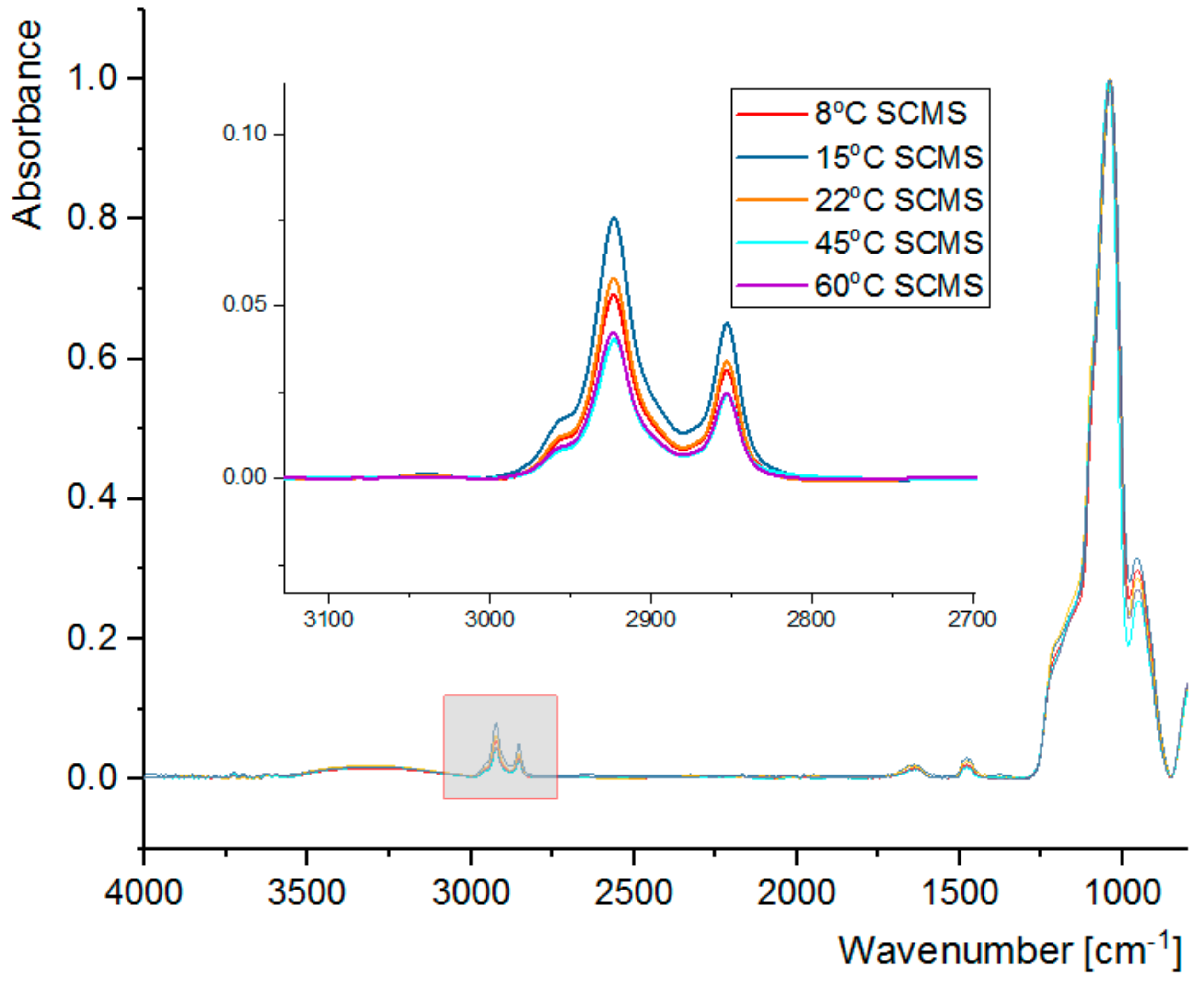
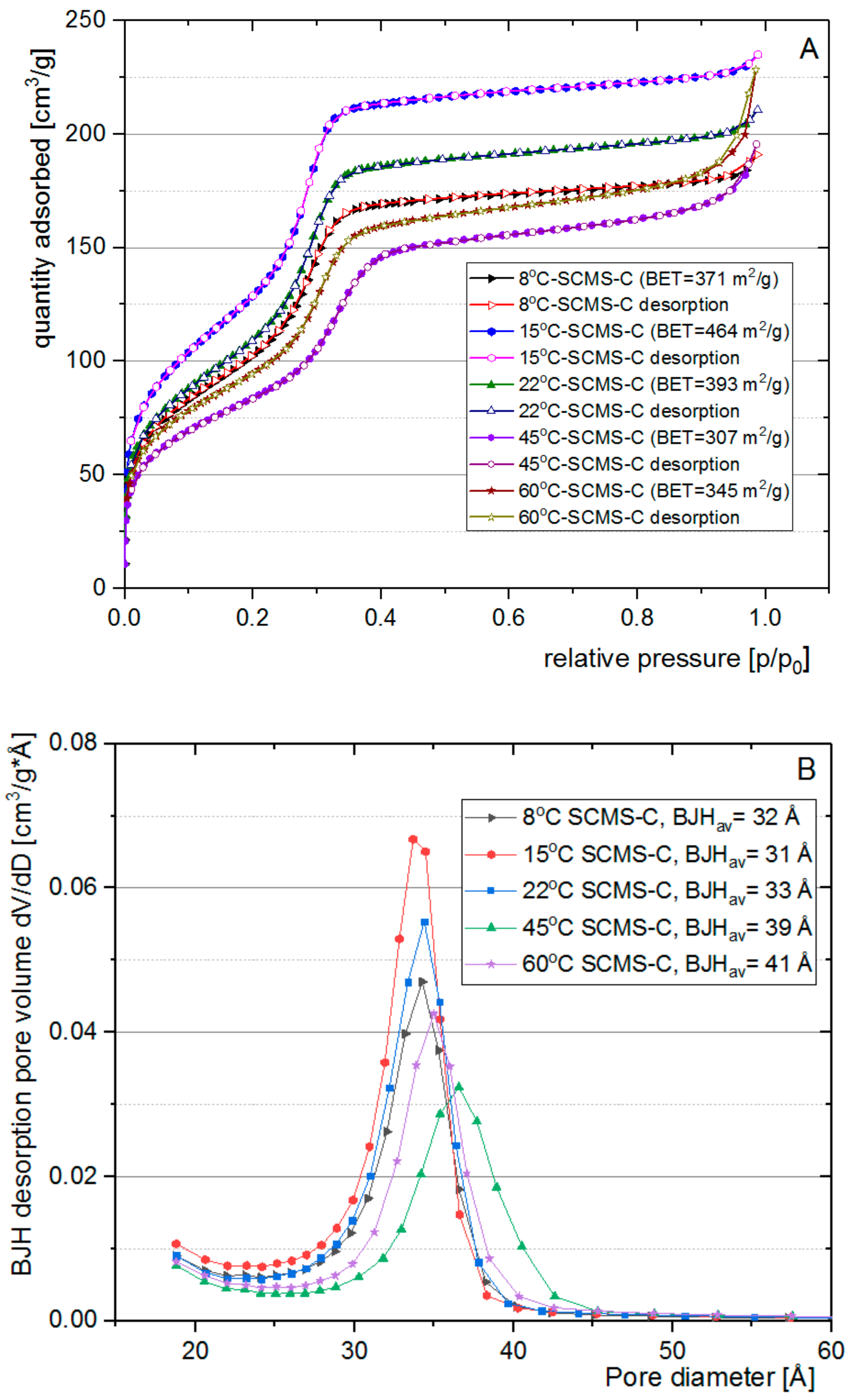

| Temp [°C] | dsc [nm] | dscms-c [nm] | Shell Thickness a [nm] |
|---|---|---|---|
| 8 | 793 ± 30 | 941 ± 27 | 74 |
| 15 | 614 ± 40 | 773 ± 33 | 80 |
| 22 | 451 ± 23 | 531 ± 30 | 40 |
| 45 | 236 ± 13 | 257 ± 15 | 11 |
| 60 | 173 ± 10 | 194 ± 11 | 11 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baliś, A.; Zapotoczny, S. Tailored Synthesis of Core-Shell Mesoporous Silica Particles—Optimization of Dye Sorption Properties. Nanomaterials 2018, 8, 230. https://doi.org/10.3390/nano8040230
Baliś A, Zapotoczny S. Tailored Synthesis of Core-Shell Mesoporous Silica Particles—Optimization of Dye Sorption Properties. Nanomaterials. 2018; 8(4):230. https://doi.org/10.3390/nano8040230
Chicago/Turabian StyleBaliś, Andrzej, and Szczepan Zapotoczny. 2018. "Tailored Synthesis of Core-Shell Mesoporous Silica Particles—Optimization of Dye Sorption Properties" Nanomaterials 8, no. 4: 230. https://doi.org/10.3390/nano8040230
APA StyleBaliś, A., & Zapotoczny, S. (2018). Tailored Synthesis of Core-Shell Mesoporous Silica Particles—Optimization of Dye Sorption Properties. Nanomaterials, 8(4), 230. https://doi.org/10.3390/nano8040230






