One-Step Green Hydrothermal Synthesis of Few-Layer Graphene Oxide from Humic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. GOH Synthesis
2.2. Characterization
3. Results and Discussion
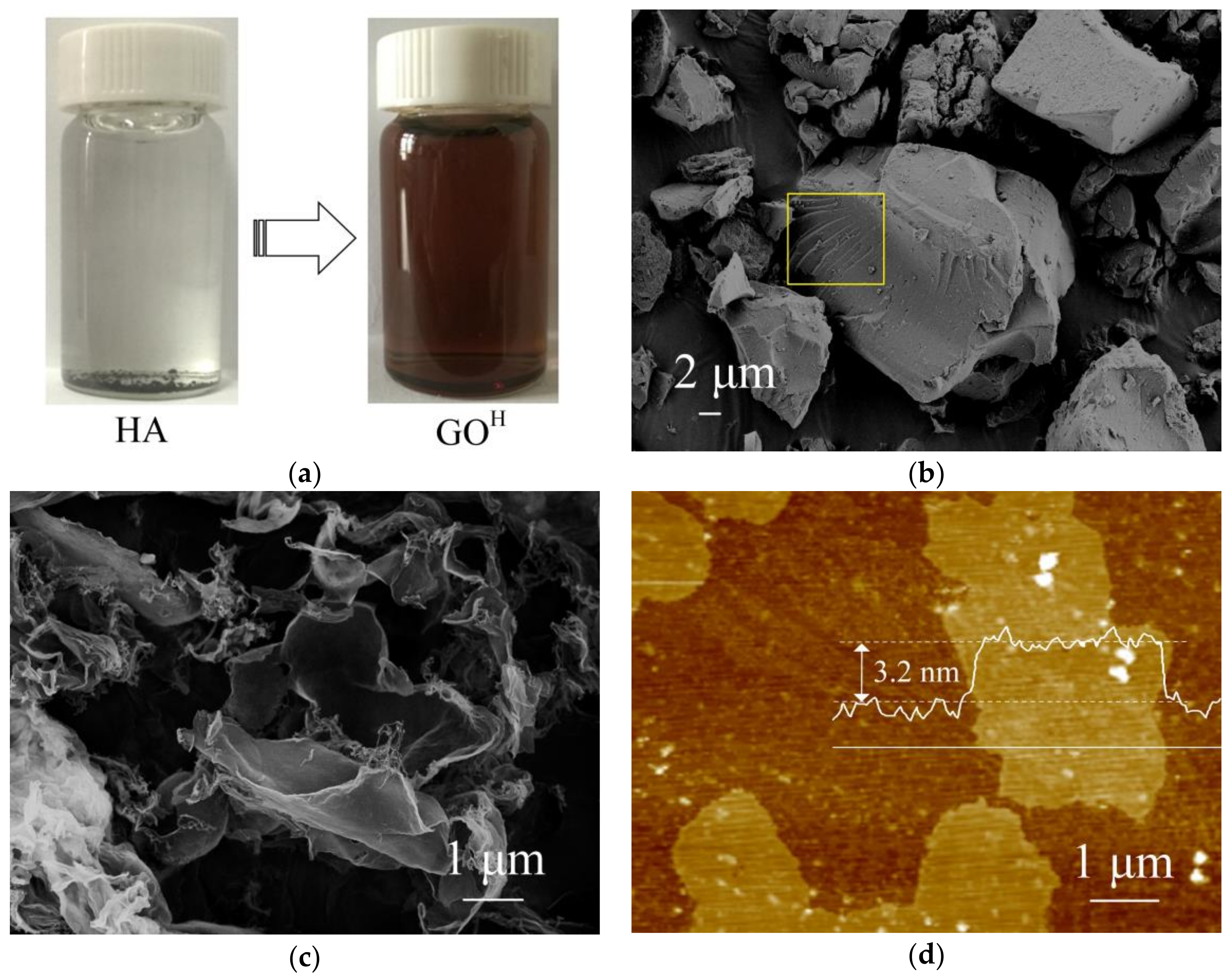
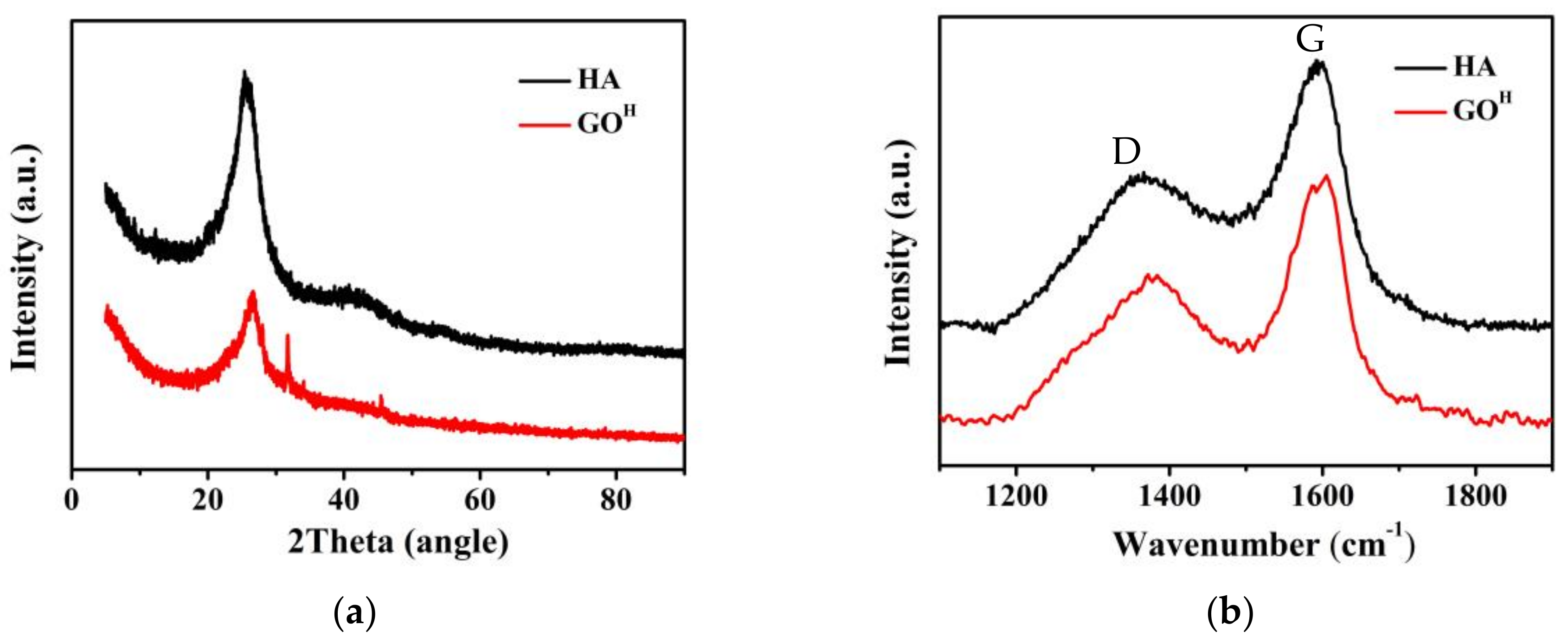
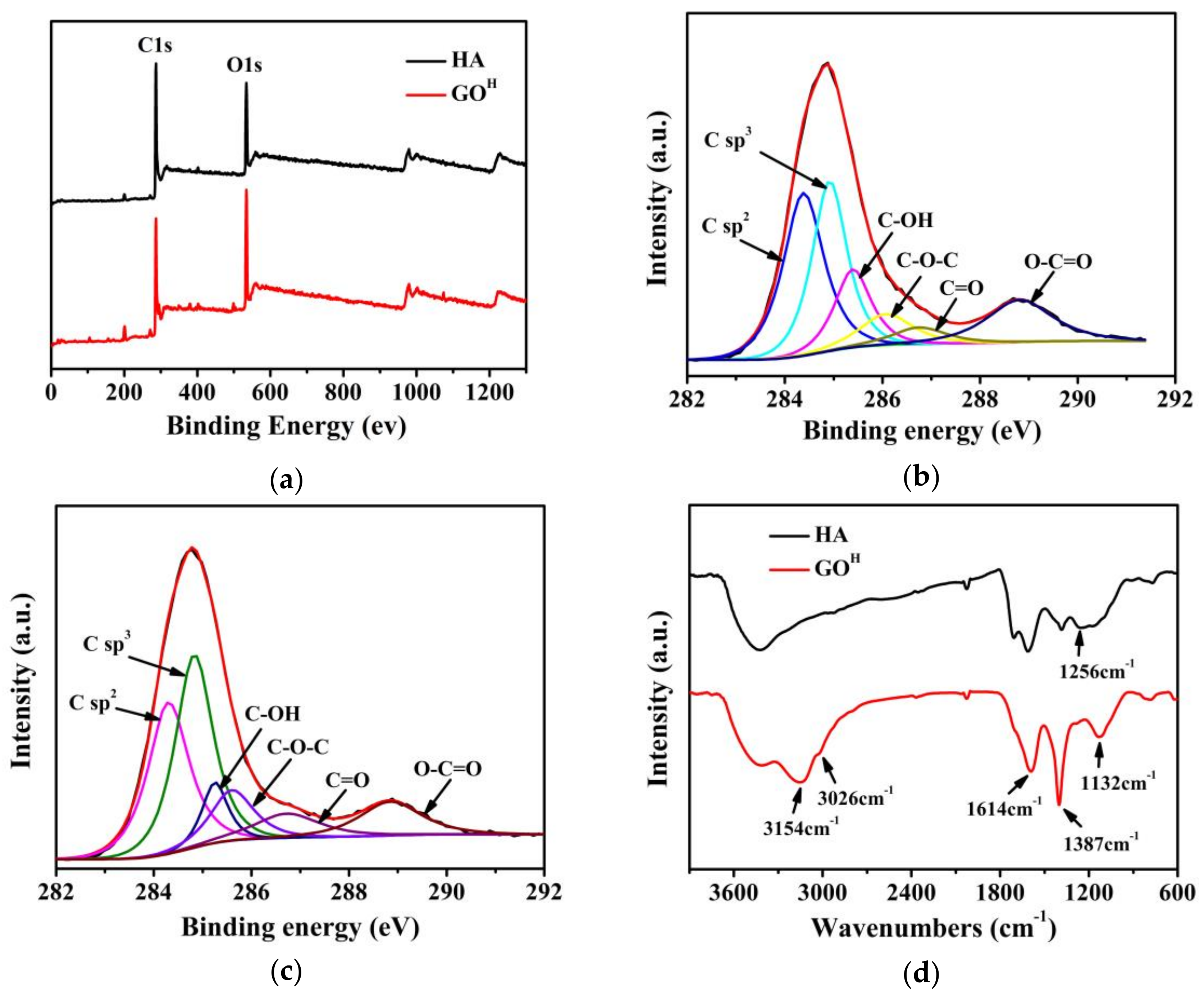
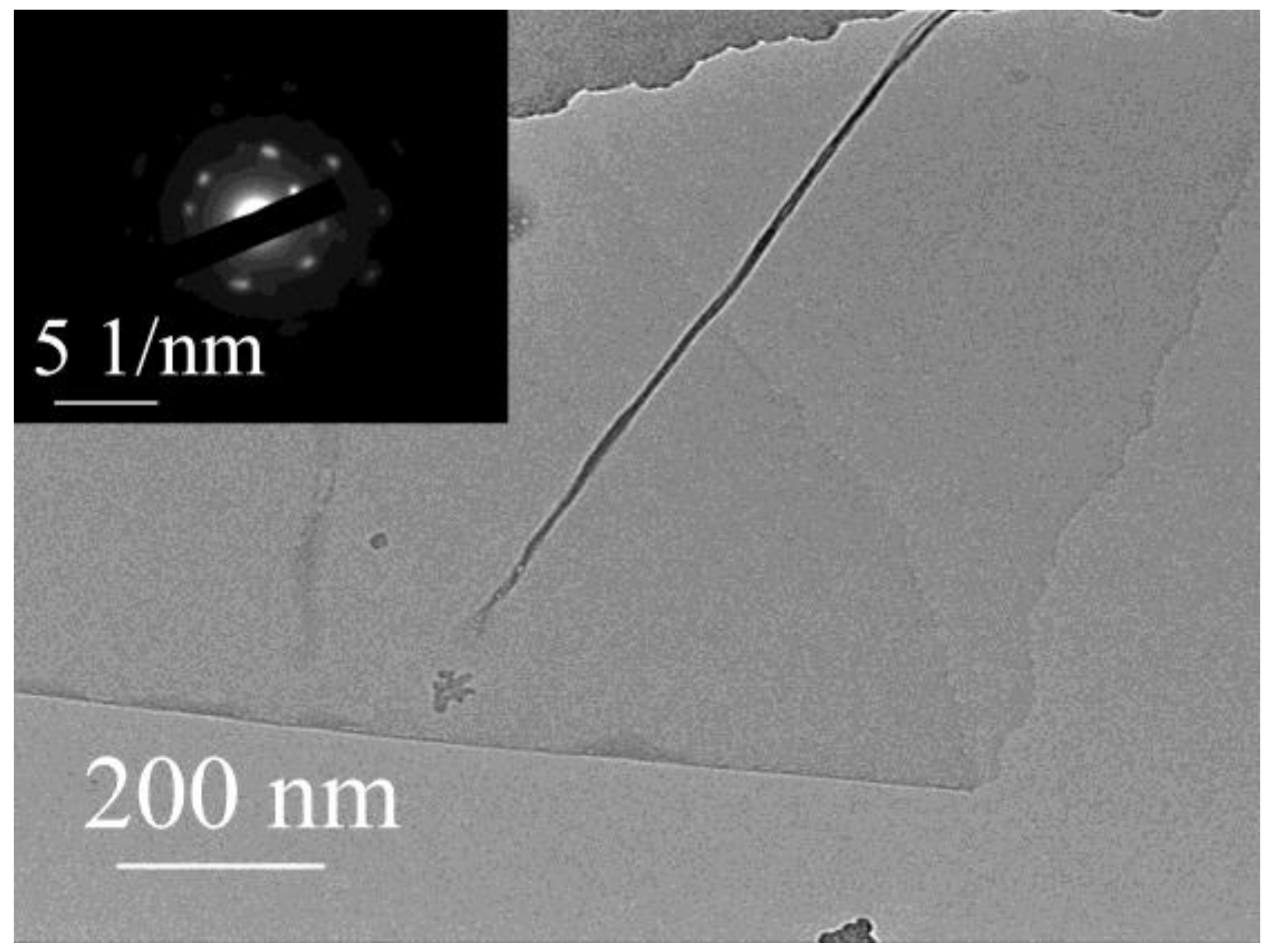
3.1. Characterizations of GOH
3.2. Characterizations of r-GOH
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tai, L.; Zhu, D.; Liu, X.; Yang, T.; Wang, L.; Wang, R.; Jiang, S.; Chen, Z.; Xu, Z.; Li, X. Direct Growth of Graphene on Silicon by Metal-Free Chemical Vapor Deposition. Nano-Micro Lett. 2018, 10, 20. [Google Scholar] [CrossRef]
- Singh, S.P.; Li, Y.; Zhang, J.; Tour, J.M.; Arnusch, C.J. Sulfur-Doped Laser-Induced Porous Graphene Derived from Polysulfone-Class Polymers and Membranes. ACS Nano 2018, 12, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Yap, C.C.; Jumali, M.H.H.; Teridi, M.A.M.; Teh, C.H. A Mini Review: Can Graphene Be a Novel Material for Perovskite Solar Cell Applications? Nano-Micro Lett. 2018, 10, 27. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, H.M. The global growth of graphene. Nat. Nanotechnol. 2014, 9, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, H.; Wang, H.S.; Sun, Q.; Zhang, X.; Cong, C.; Xie, H.; Liu, X.; Zhou, X.; Huang, F.; et al. Silane-catalysed fast growth of large single-crystalline graphene on hexagonal boron nitride. Nat. Nanotechnol. 2015, 6, 6499. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Zhang, D.; Yu, P.; Ma, Y. High performance supercapacitors based on reduced graphene oxide in aqueous and ionic liquid electrolytes. Carbon 2011, 49, 573–580. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.; Hu, H.; Li, Y.; Wu, M.; Wang, Z.; Li, G.; Sun, Z.; Chen, C. Facile synthesis of iron oxides/reduced graphene oxide composites: Application for electromagnetic wave absorption at high temperature. Sci. Rep. 2015, 5, 9298. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Sheng, K.; Zhang, P.; Li, C.; Shi, G. Graphene oxide/conducting polymer composite hydrogels. J. Mater. Chem. 2011, 21, 18653. [Google Scholar] [CrossRef]
- Lv, D.; Gordin, M.L.; Yi, R.; Xu, T.; Song, J.; Jiang, Y.B.; Choi, D.; Wang, D. GeOx/Reduced Graphene Oxide Composite as an Anode for Li-Ion Batteries: Enhanced Capacity via Reversible Utilization of Li2O along with Improved Rate Performance. Adv. Funct. Mater. 2014, 24, 1059–1066. [Google Scholar] [CrossRef]
- Xiang, C.; Li, M.; Zhi, M.; Manivannan, A.; Wu, N. A reduced graphene oxide/Co3O4 composite for supercapacitor electrode. J. Power Sources 2013, 226, 65–70. [Google Scholar] [CrossRef]
- Liang, K.; Li, X.; Kang, S.Z.; Qin, L.; Li, G.; Mu, J. Catalytic performance of ferroferric oxide/reduced graphene oxide/silver nanoparticle composite microflowers. Carbon 2014, 80, 716–724. [Google Scholar] [CrossRef]
- Dimiev, A.; Kosynkin, D.V.; Alemany, L.B.; Chaguine, P.; Tour, J.M. Pristine graphite oxide. J. Am. Chem. Soc. 2012, 134, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.; Beall, G.W. Graphene oxide and graphene from low grade coal: Synthesis, characterization and applications. Curr. Opin. Colloid Interface Sci. 2015, 20, 362–366. [Google Scholar] [CrossRef]
- Wandruszka, R.V. Humic acids: Their detergent qualities and potential uses in pollution remediation. Geochem. Trans. 2000, 2, 10. [Google Scholar] [CrossRef]
- Liu, P.; Wang, L.; Zhou, Y.; Pan, T.; Lu, X.; Zhang, D. Effect of hydrothermal treatment on the structure and pyrolysis product distribution of Xiaolongtan lignite. Fuel 2016, 164, 110–118. [Google Scholar] [CrossRef]
- Shi, W.; Fan, H.; Ai, S.; Zhu, L. Preparation of fluorescent graphene quantum dots from humic acid for bioimaging application. New J. Chem. 2015, 39, 7054–7059. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.G. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Rakhi, R.B.; Chen, W.; Alshareef, H.N. Effect of pH-induced chemical modification of hydrothermally reduced graphene oxide on supercapacitor performance. J. Power Sources 2013, 233, 313–319. [Google Scholar] [CrossRef]
- Buglione, L.; Chng, E.L.K.; Ambrosi, A.; Sofer, Z.; Pumera, M. Graphene materials preparation methods have dramatic influence upon their capacitance. Electrochem. Commun. 2012, 14, 5–8. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Cheng, H.; Hu, Y.; Shi, G.; Dai, L.; Qu, L. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J. Am. Chem. Soc. 2012, 134, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, A.R.; Sidhureddy, B.; Keeler, W.; Chen, A. Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem. Commun. 2017, 76, 42–46. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Yu, B.J.; Sun, S.; Guo, Y.; Chang, Z.Z.; Li, Q.; Wang, C.Y. High-Performance Anode of Sodium Ion Battery from Polyacrylonitrile/Humic Acid Composite Electrospun Carbon Fibers. Electrochim. Acta 2017, 232, 348–356. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Zeng, C.; Favas, G.; Wu, H.; Chaffee, A.L.; Hayashi, J.I.; Li, C.Z. Effects of Pretreatment in Steam on the Pyrolysis Behavior of Loy Yang Brown Coal. Energy Fuels 2006, 20, 281–286. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, Z.; Neff, D.; Zhamu, A.; Jang, B.Z. Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett. 2010, 10, 4863–4868. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, Z.; Wang, H.; Ding, J.; Zahiri, B.; Holt, C.M.; Tan, X.; Mitlin, D. Colossal pseudocapacitance in a high functionality–high surface area carbon anode doubles the energy of an asymmetric supercapacitor. Energy Environ. Sci. 2014, 7, 1708–1718. [Google Scholar] [CrossRef]
- Li, X.; Xing, W.; Zhuo, S.; Zhou, J.; Li, F.; Qiao, S.Z.; Lu, G.Q. Preparation of capacitor’s electrode from sunflower seed shell. Bioresour. Technol. 2011, 102, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiang, Y.; Fan, H.; Liu, M.; Zhuo, O.; Wang, X.; Wu, Q.; Yang, L.; Ma, Y.; Hu, Z. Porous 3D Few-Layer Graphene-like Carbon for Ultrahigh-Power Supercapacitors with Well-Defined Structure-Performance Relationship. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Yuan, R.; Zhang, C.; Huang, G.; Guo, H.; Chen, Z.; Chen, L.; Yi, G.; Zhang, Y.; Yu, J. Facile synthesis of graphene nanosheets from humic acid for supercapacitors. Fuel Process. Technol. 2017, 165, 112–122. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Liu, Z.; Li, D.; Wang, H.; Li, Q. One-pot construction of 3-D nitrogen-doped activated graphene-like nanosheets for high-performance supercapacitors. Electrochim. Acta 2016, 190, 378–387. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Hou, S.; Zhang, W.; Zhou, J.; Zhao, Z. Preparation of edge-nitrogenated graphene nanoplatelets as an efficient electrode material for supercapacitors. Electrochim. Acta 2016, 208, 47–54. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Kang, W.; Geng, Q.; Xing, B.; Liu, Q.; Jia, J.; Zhang, C. One-Step Green Hydrothermal Synthesis of Few-Layer Graphene Oxide from Humic Acid. Nanomaterials 2018, 8, 215. https://doi.org/10.3390/nano8040215
Huang G, Kang W, Geng Q, Xing B, Liu Q, Jia J, Zhang C. One-Step Green Hydrothermal Synthesis of Few-Layer Graphene Oxide from Humic Acid. Nanomaterials. 2018; 8(4):215. https://doi.org/10.3390/nano8040215
Chicago/Turabian StyleHuang, Guangxu, Weiwei Kang, Qianhao Geng, Baolin Xing, Quanrun Liu, Jianbo Jia, and Chuanxiang Zhang. 2018. "One-Step Green Hydrothermal Synthesis of Few-Layer Graphene Oxide from Humic Acid" Nanomaterials 8, no. 4: 215. https://doi.org/10.3390/nano8040215
APA StyleHuang, G., Kang, W., Geng, Q., Xing, B., Liu, Q., Jia, J., & Zhang, C. (2018). One-Step Green Hydrothermal Synthesis of Few-Layer Graphene Oxide from Humic Acid. Nanomaterials, 8(4), 215. https://doi.org/10.3390/nano8040215




