Reconstructing Bone with Natural Bone Graft: A Review of In Vivo Studies in Bone Defect Animal Model
Abstract
1. Introduction
2. Natural Bone Graft
2.1. Decellularized Bone Matrix Scaffold
2.2. Demineralized Bone Matrix Scaffold
3. Summary of In Vivo Studies in Bone Defect Animal Model
3.1. Rat Model Studies
3.2. Rabbit Model Studies
3.3. Other Animal Model Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sikavitsas, V.I.; Temenoff, J.S.; Mikos, A.G. Biomaterials and bone mechanotransduction. Biomaterials 2001, 22, 2581–2593. [Google Scholar] [CrossRef]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Jonitz, A.; Lochner, K.; Lindner, T.; Hansmann, D.; Marrot, A.; Bader, R. Oxygen consumption, acidification and migration capacity of human primary osteoblasts within a three-dimensional tantalum scaffold. J. Mater. Sci. Mater. Med. 2011, 22, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lin, X.; Zhang, Q.; Ni, J.; Li, J.; Xiao, J.; Wang, Y.; Ye, Y.; Chen, L.; Jin, K.; et al. Decellularized periosteum as a potential biologic scaffold for bone tissue engineering. Acta Biomater. 2015, 19, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; et al. A multilayer biomaterial for osteochondral regeneration shows superiority vs. microfractures for the treatment of osteochondral lesions in a multicentre randomized trial at 2 years. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Roffi, A.; Di Martino, A.; Marcacci, M. Scaffold-based repair for cartilage healing: A systematic review and technical note. Arthroscopy 2013, 29, 174–186. [Google Scholar] [CrossRef]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Kon, E.; Perdisa, F.; Di Matteo, B.; Di Martino, A.; Iacono, F.; Zaffagnini, S.; Balboni, F.; Vaccari, V.; Marcacci, M. Osteochondral scaffold reconstruction for complex knee lesions: A comparative evaluation. Knee 2013, 20, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Filippeschi, C.; Genchi, G.G.; Mattoli, V.; Mazzolai, B.; Ciofani, G. The Osteoprint: A bioinspired two-photon polymerized 3-D structure for the enhancement of bone-like cell differentiation. Acta Biomater. 2014, 10, 4304–4313. [Google Scholar] [CrossRef]
- Marino, A.; Barsotti, J.; de Vito, G.; Filippeschi, C.; Mazzolai, B.; Piazza, V.; Labardi, M.; Mattoli, V.; Ciofani, G. Two-Photon Lithography of 3D Nanocomposite Piezoelectric Scaffolds for Cell Stimulation. ACS Appl. Mater. Interfaces 2015, 7, 25574–25579. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef] [PubMed]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. 3), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef]
- Bouyer, M.; Guillot, R.; Lavaud, J.; Plettinx, C.; Olivier, C.; Curry, V.; Boutonnat, J.; Coll, J.L.; Peyrin, F.; Josserand, V.; et al. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials 2016, 104, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Levingstone, T.J.; Ramesh, A.; Brady, R.T.; Brama, P.A.J.; Kearney, C.; Gleeson, J.P.; O’Brien, F.J. Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials 2016, 87, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, A.; Hewitt, R.N.; McNamara, L.E.; McCloy, D.; Dominic Meek, R.M.; Dalby, M.J. Biomimetic microtopography to enhance osteogenesis in vitro. Acta Biomater. 2011, 7, 2919–2925. [Google Scholar] [CrossRef] [PubMed]
- Papadimitropoulos, A.; Scotti, C.; Bourgine, P.; Scherberich, A.; Martin, I. Engineered decellularized matrices to instruct bone regeneration processes. Bone 2015, 70, 66–72. [Google Scholar] [CrossRef]
- Liverani, C.; Mercatali, L.; Cristofolini, L.; Giordano, E.; Minardi, S.; Porta, G.D.; De Vita, A.; Miserocchi, G.; Spadazzi, C.; Tasciotti, E.; et al. Investigating the Mechanobiology of Cancer Cell-ECM Interaction Through Collagen-Based 3D Scaffolds. Cell. Mol. Bioeng. 2017, 10, 223–234. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Peric, M.; Dumic-Cule, I.; Grcevic, D.; Matijasic, M.; Verbanac, D.; Paul, R.; Grgurevic, L.; Trkulja, V.; Bagi, C.M.; Vukicevic, S. The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 2015, 70, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.S.; Bemenderfer, T.B.; Wessel, A.R.; Kacena, M.A. A review of mouse critical size defect models in weight bearing bones. Bone 2013, 55, 241–247. [Google Scholar] [CrossRef]
- Freytes, D.O.; Badylak, S.F.; Webster, T.J.; Geddes, L.A.; Rundell, A.E. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials 2004, 25, 2353–2361. [Google Scholar] [CrossRef]
- Dahl, S.L.; Koh, J.; Prabhakar, V.; Niklason, L.E. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003, 12, 659–666. [Google Scholar] [CrossRef]
- Woods, T.; Gratzer, P.F. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials 2005, 26, 7339–7349. [Google Scholar] [CrossRef]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Lee, D.J.; Padilla, R.; Zhang, H.; Hu, W.S.; Ko, C.C. Biological assessment of a calcium silicate incorporated hydroxyapatite-gelatin nanocomposite: A comparison to decellularized bone matrix. Biomed. Res. Int. 2014, 2014, 837524. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Guo, Q.; Huang, J.; Zhang, L.; Yao, J.; Yang, F.; Wang, S.; Xu, W.; Wang, A.; et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials 2008, 29, 2378–2387. [Google Scholar] [CrossRef]
- Chen, G.; Dong, C.; Yang, L.; Lv, Y. 3D Scaffolds with Different Stiffness but the Same Microstructure for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2015, 7, 15790–15802. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Z.; Sun, S.; Huang, H.; Sun, X.; Wang, Z.; Zhang, Y.; Zhang, B. Adipose-derived stem cells modified genetically in vivo promote reconstruction of bone defects. Cytotherapy 2010, 12, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Wu, L.A.; Zhang, M.; Zhang, R.; Sun, H.H. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: Promises, strategies, and translational perspectives. Biomaterials 2011, 32, 3189–3209. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, C.; Iop, L.; Sciro, M.; Harder, M.; Mavrilas, D.; Korossis, S.; Bagno, A.; Palu, G.; Aguiari, P.; Gerosa, G. A sterilization method for decellularized xenogeneic cardiovascular scaffolds. Acta Biomater. 2018, 67, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, R.S.; Jana, S.; Tefft, B.J.; Helder, M.R.; Young, M.D.; Hennessy, R.R.; Stoyles, N.J.; Lerman, A. Supercritical carbon dioxide-based sterilization of decellularized heart valves. JACC Basic Transl. Sci. 2017, 2, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.M.; Pandit, A.; Zeugolis, D.I. Influence of sterilisation methods on collagen-based devices stability and properties. Expert Rev. Med. Devices 2014, 11, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Somers, P.; Cuvelier, C.A.; Somer, F.D.; Cornelissen, M.; Cox, E.; Verloo, M.; Chiers, K.; van Nooten, G. Gamma radiation alters the ultrastructure in tissue-engineered heart valve scaffolds. Tissue Eng. Part A 2009, 15, 3597–3604. [Google Scholar] [CrossRef]
- Gouk, S.S.; Lim, T.M.; Teoh, S.H.; Sun, W.Q. Alterations of human acellular tissue matrix by gamma irradiation: Histology, biomechanical property, stability, in vitro cell repopulation, and remodeling. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 205–217. [Google Scholar] [CrossRef]
- Matuska, A.M.; McFetridge, P.S. The effect of terminal sterilization on structural and biophysical properties of a decellularized collagen-based scaffold; implications for stem cell adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 397–406. [Google Scholar] [CrossRef]
- Kasimir, M.T.; Rieder, E.; Seebacher, G.; Nigisch, A.; Dekan, B.; Wolner, E.; Weigel, G.; Simon, P. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J. Heart Valve Dis. 2006, 15, 278–286. [Google Scholar]
- Wang, X.; Li, Y.; Han, R.; He, C.; Wang, G.; Wang, J.; Zheng, J.; Pei, M.; Wei, L. Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-beta3 gene promoted pig cartilage defect repair. PLoS ONE 2014, 9, e116061. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lin, H.; Wang, J.; Zhao, Y.; Wang, B.; Zhao, W.; Sun, W.; Dai, J. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials 2007, 28, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Aghdasi, B.; Montgomery, S.R.; Daubs, M.D.; Wang, J.C. A review of demineralized bone matrices for spinal fusion: The evidence for efficacy. Surgeon 2013, 11, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Mikulski, A.; Lietze, A. Solubilized and insolubilized bone morphogenetic protein. Proc. Natl. Acad. Sci. USA 1979, 76, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Gruskin, E.; Doll, B.A.; Futrell, F.W.; Schmitz, J.P.; Hollinger, J.O. Demineralized bone matrix in bone repair: History and use. Adv. Drug Deliv. Rev. 2012, 64, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Chan, H.L.; Chong, L.Y.; Jheng, Y.H.; Chang, P.C. Evaluation of the osteogenic potential of growth factor-rich demineralized bone matrix in vivo. J. Periodontol. 2015, 86, 36–43. [Google Scholar] [CrossRef]
- Berven, S.; Tay, B.K.; Kleinstueck, F.S.; Bradford, D.S. Clinical applications of bone graft substitutes in spine surgery: Consideration of mineralized and demineralized preparations and growth factor supplementation. Eur. Spine J. 2001, 10 (Suppl. 2), S169–S177. [Google Scholar]
- Hu, Q.; Liu, M.; Chen, G.; Xu, Z.; Lv, Y. Demineralized Bone Scaffolds with Tunable Matrix Stiffness for Efficient Bone Integration. ACS Appl. Mater. Interfaces 2018, 10, 27669–27680. [Google Scholar] [CrossRef]
- Lin, F.H.; Liao, C.J.; Chen, K.S.; Sun, J.S. Preparation of a biphasic porous bioceramic by heating bovine cancellous bone with Na4P2O7·10H2O addition. Biomaterials 1999, 20, 475–484. [Google Scholar] [CrossRef]
- Kim, H.P.; Ji, Y.H.; Rhee, S.C.; Dhong, E.S.; Park, S.H.; Yoon, E.S. Enhancement of bone regeneration using osteogenic-induced adipose-derived stem cells combined with demineralized bone matrix in a rat critically-sized calvarial defect model. Curr. Stem Cell Res. Ther. 2012, 7, 165–172. [Google Scholar] [PubMed]
- Dallari, D.; Savarino, L.; Albisinni, U.; Fornasari, P.; Ferruzzi, A.; Baldini, N.; Giannini, S. A prospective, randomised, controlled trial using a Mg-hydroxyapatite–demineralized bone matrix nanocomposite in tibial osteotomy. Biomaterials 2012, 33, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zotz, T.G.; Paula, J.B.; Moser, A.D. Experimental model of heterotopic ossification in Wistar rats. Braz. J. Med. Biol. Res. 2012, 45, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J. A review of osteoinductive testing methods and sterilization processes for demineralized bone. Cell Tissue Bank. 2005, 6, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Schouten, C.C.; Hartman, E.H.; Spauwen, P.H.; Jansen, J.A. DBM induced ectopic bone formation in the rat: The importance of surface area. J. Mater. Sci. Mater. Med. 2005, 16, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Mauney, J.R.; Jaquiery, C.; Volloch, V.; Heberer, M.; Martin, I.; Kaplan, D.L. In vitro and in vivo evaluation of differentially demineralized cancellous bone scaffolds combined with human bone marrow stromal cells for tissue engineering. Biomaterials 2005, 26, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Powers, R.M., Jr.; Wolfinbarger, L., Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J. Periodontol. 1997, 68, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Diachina, S.; Lee, Y.T.; Zhao, L.; Zou, R.; Tang, N.; Han, H.; Chen, X.; Ko, C.C. Decellularized bone matrix grafts for calvaria regeneration. J. Tissue Eng. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Hwang, M.P.; Du, P.; Ko, J.; Ha, C.W.; Do, S.H.; Park, K. Bioactive cell-derived matrices combined with polymer mesh scaffold for osteogenesis and bone healing. Biomaterials 2015, 50, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.C.; Ji, Y.H.; Gharibjanian, N.A.; Dhong, E.S.; Park, S.H.; Yoon, E.S. In vivo evaluation of mixtures of uncultured freshly isolated adipose-derived stem cells and demineralized bone matrix for bone regeneration in a rat critically sized calvarial defect model. Stem Cells Dev. 2011, 20, 233–242. [Google Scholar] [CrossRef]
- Stancoven, B.W.; Lee, J.; Dixon, D.R.; McPherson, J.C., 3rd; Bisch, F.C.; Wikesjo, U.M.; Susin, C. Effect of bone morphogenetic protein-2, demineralized bone matrix and systemic parathyroid hormone (1-34) on local bone formation in a rat calvaria critical-size defect model. J. Periodontal Res. 2013, 48, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Sun, J.; Zhang, W.; Liang, H.; Shi, Q.; Li, X.; Chen, Y.; Zhuang, Y.; Dai, J. Demineralized Bone Matrix Scaffolds Modified by CBD-SDF-1α Promote Bone Regeneration via Recruiting Endogenous Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 27511–27522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.C.; Gertzman, A.A.; Xie, L.; Nizkorodov, A.; Hyzy, S.L.; Truncale, K.; Guldberg, R.E.; Schwartz, Z.; Boyan, B.D. Endogenous regeneration of critical-size chondral defects in immunocompromised rat xiphoid cartilage using decellularized human bone matrix scaffolds. Tissue Eng. Part A 2012, 18, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.U.; Kemper, N.; Breathwaite, E.; Dutta, S.M.; Hsu, E.L.; Hsu, W.K.; Francis, M.P. Demineralized bone matrix fibers formable as general and custom 3D printed mold-based implants for promoting bone regeneration. Biofabrication 2016, 8, 035007. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wei, Y.; Villasante, A.; Ng, J.J.D.; Arkonac, D.E.; Chao, P.G.; Vunjak-Novakovic, G. Stem cell delivery in tissue-specific hydrogel enabled meniscal repair in an orthotopic rat model. Biomaterials 2017, 132, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Acarturk, T.O.; Hollinger, J.O. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast. Reconstr. Surg. 2006, 118, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Blanquaert, F.; Saffar, J.L.; Colombier, M.L.; Carpentier, G.; Barritault, D.; Caruelle, J.P. Heparan-like molecules induce the repair of skull defects. Bone 1995, 17, 499–506. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 299–308. [Google Scholar] [CrossRef]
- Chim, H.; Schantz, J.T. Human circulating peripheral blood mononuclear cells for calvarial bone tissue engineering. Plast. Reconstr. Surg. 2006, 117, 468–478. [Google Scholar] [CrossRef]
- Sakata, Y.; Ueno, T.; Kagawa, T.; Kanou, M.; Fujii, T.; Yamachika, E.; Sugahara, T. Osteogenic potential of cultured human periosteum-derived cells-a pilot study of human cell transplantation into a rat calvarial defect model. J. Craniomaxillofac. Surg. 2006, 34, 461–465. [Google Scholar] [CrossRef]
- Cunniffe, G.M.; Vinardell, T.; Murphy, J.M.; Thompson, E.M.; Matsiko, A.; O’Brien, F.J.; Kelly, D.J. Porous decellularized tissue engineered hypertrophic cartilage as a scaffold for large bone defect healing. Acta Biomater. 2015, 23, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Lane, J.M.; Burstein, A.H.; Kopman, C.R.; Vigorita, V.J. The healing of segmental bone defects induced by demineralized bone matrix. A radiographic and biomechanical study. J. Bone Jt. Surg. Am. 1984, 66, 274–279. [Google Scholar] [CrossRef]
- Chen, G.; Yang, L.; Lv, Y. Cell-free scaffolds with different stiffness but same microstructure promote bone regeneration in rabbit large bone defect model. J. Biomed. Mater. Res. A 2016, 104, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lv, Y. Matrix elasticity-modified scaffold loaded with SDF-1α improves the in situ regeneration of segmental bone defect in rabbit radius. Sci. Rep. 2017, 7, 1672. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; McBride, S.; Dean, D.D.; Sylvia, V.L.; Doll, B.A.; Hollinger, J.O. In vivo performance of combinations of autograft, demineralized bone matrix, and tricalcium phosphate in a rabbit femoral defect model. Biomed. Mater. 2014, 9, 035010. [Google Scholar] [CrossRef] [PubMed]
- Zhukauskas, R.; Dodds, R.A.; Hartill, C.; Arola, T.; Cobb, R.R.; Fox, C. Histological and radiographic evaluations of demineralized bone matrix and coralline hydroxyapatite in the rabbit tibia. J. Biomater. Appl. 2010, 24, 639–656. [Google Scholar] [CrossRef]
- Gu, H.; Xiong, Z.; Yin, X.; Li, B.; Mei, N.; Li, G.; Wang, C. Bone regeneration in a rabbit ulna defect model: Use of allogeneic adipose-derivedstem cells with low immunogenicity. Cell Tissue Res. 2014, 358, 453–464. [Google Scholar] [CrossRef]
- Lin, X.; Elliot, J.J.; Carnes, D.L.; Fox, W.C.; Pena, L.A.; Campion, S.L.; Takahashi, K.; Atkinson, B.L.; Zamora, P.O. Augmentation of osseous phenotypes in vivo with a synthetic peptide. J. Orthop. Res. 2007, 25, 531–539. [Google Scholar] [CrossRef]
- Moxham, J.P.; Wong, K.K.; Kibblewhite, D.J. Transforming growth factor-beta1 shows an incremental osteoinductive dose-response relationship. Laryngoscope 2009, 119, 126–130. [Google Scholar] [CrossRef]
- Moxham, J.P. Oncostatin-M enhances osteoinduction in a rabbit critical calvarial defect model. Laryngoscope 2007, 117, 1790–1797. [Google Scholar] [CrossRef]
- Follmar, K.E.; Prichard, H.L.; DeCroos, F.C.; Wang, H.T.; Levin, L.S.; Klitzman, B.; Olbrich, K.C.; Erdmann, D. Combined bone allograft and adipose-derived stem cell autograft in a rabbit model. Ann. Plast. Surg. 2007, 58, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Kaempfen, A.; Todorov, A.; Guven, S.; Largo, R.D.; Jaquiery, C.; Scherberich, A.; Martin, I.; Schaefer, D.J. Engraftment of Prevascularized, Tissue Engineered Constructs in a Novel Rabbit Segmental Bone Defect Model. Int. J. Mol. Sci. 2015, 16, 12616–12630. [Google Scholar] [CrossRef] [PubMed]
- Kiely, P.D.; Brecevich, A.T.; Taher, F.; Nguyen, J.T.; Cammisa, F.P.; Abjornson, C. Evaluation of a new formulation of demineralized bone matrix putty in a rabbit posterolateral spinal fusion model. Spine J. 2014, 14, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- ten Koppel, P.G.; van Osch, G.J.; Verwoerd, C.D.; Verwoerd-Verhoef, H.L. A new in vivo model for testing cartilage grafts and biomaterials: The ‘rabbit pinna punch-hole’ model. Biomaterials 2001, 22, 1407–1414. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Qiu, P.; Zhang, Q.; Wang, S.; Su, M.; Chen, Y.; Jin, K.; Qin, A.; Fan, S.; et al. Biphasic hierarchical extracellular matrix scaffold for osteochondral defect regeneration. Osteoarthr. Cartil. 2018, 26, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Luo, K.; Rong, Z.; Wang, Z.; Luo, F.; Zhang, Z.; Sun, D.; Dong, S.; Xu, J.; Dai, F. Periostin Upregulates Wnt/beta-Catenin Signaling to Promote the Osteogenesis of CTLA4-Modified Human Bone Marrow-Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 41634. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.L.; Tremp, M.; Ho, C.K.; Sun, Y.; Liu, K.; Li, Q. Prefabrication of a functional bone graft with a pedicled periosteal flap as an in vivo bioreactor. Sci. Rep. 2017, 7, 18038. [Google Scholar] [CrossRef]
- Bhumiratana, S.; Bernhard, J.C.; Alfi, D.M.; Yeager, K.; Eton, R.E.; Bova, J.; Shah, F.; Gimble, J.M.; Lopez, M.J.; Eisig, S.B.; et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci. Transl. Med. 2016, 8, 343ra83. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Lu, S.B.; Guo, Q.Y.; Zhao, B.; Zhang, L.; Wang, A.Y.; Xu, W.J.; Xia, Q.; Ma, X.L.; et al. Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin. Med. J. 2011, 124, 3930–3938. [Google Scholar]
- Bae, J.C.; Lee, J.J.; Shim, J.H.; Park, K.H.; Lee, J.S.; Bae, E.B.; Choi, J.W.; Huh, J.B. Development and Assessment of a 3D-Printed Scaffold with rhBMP-2 for an Implant Surgical Guide Stent and Bone Graft Material: A Pilot Animal Study. Materials 2017, 10, 1434. [Google Scholar] [CrossRef]
- Huber, E.; Pobloth, A.M.; Bormann, N.; Kolarczik, N.; Schmidt-Bleek, K.; Schell, H.; Schwabe, P.; Duda, G.N.; Wildemann, B. Demineralized Bone Matrix as a Carrier for Bone Morphogenetic Protein-2: Burst Release Combined with Long-Term Binding and Osteoinductive Activity Evaluated In Vitro and In Vivo. Tissue Eng. Part A 2017, 23, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Vindas Bolanos, R.A.; Cokelaere, S.M.; Estrada McDermott, J.M.; Benders, K.E.; Gbureck, U.; Plomp, S.G.; Weinans, H.; Groll, J.; van Weeren, P.R.; Malda, J. The use of a cartilage decellularized matrix scaffold for the repair of osteochondral defects: The importance of long-term studies in a large animal model. Osteoarthr. Cartil. 2017, 25, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, Z.; Qin, X.; Wu, B.; Wu, L.; Zhao, X.; Li, Y. Chest wall reconstruction in a canine model using polydioxanone mesh, demineralized bone matrix and bone marrow stromal cells. Biomaterials 2009, 30, 3224–3233. [Google Scholar] [CrossRef]
- Fujishiro, T.; Bauer, T.W.; Kobayashi, N.; Kobayashi, H.; Sunwoo, M.H.; Seim, H.B., 3rd; Turner, A.S. Histological evaluation of an impacted bone graft substitute composed of a combination of mineralized and demineralized allograft in a sheep vertebral bone defect. J. Biomed. Mater. Res. A 2007, 82, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Hesse, E.; Kluge, G.; Atfi, A.; Correa, D.; Haasper, C.; Berding, G.; Shin, H.O.; Viering, J.; Langer, F.; Vogt, P.M.; et al. Repair of a segmental long bone defect in human by implantation of a novel multiple disc graft. Bone 2010, 46, 1457–1463. [Google Scholar] [CrossRef]
- Kakabadze, A.; Mardaleishvili, K.; Loladze, G.; Karalashvili, L.; Chutkerashvili, G.; Chakhunashvili, D.; Kakabadze, Z. Reconstruction of mandibular defects with autogenous bone and decellularized bovine bone grafts with freeze-dried bone marrow stem cell paracrine factors. Oncol. Lett. 2017, 13, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the matrisome-an inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Z.; Chen, B.; Yang, M.; Zhao, Y.; Sun, W.; Xiao, Z.; Zhang, J.; Dai, J. Loading of VEGF to the heparin cross-linked demineralized bone matrix improves vascularization of the scaffold. J. Mater. Sci. Mater. Med. 2010, 21, 309–317. [Google Scholar] [CrossRef] [PubMed]
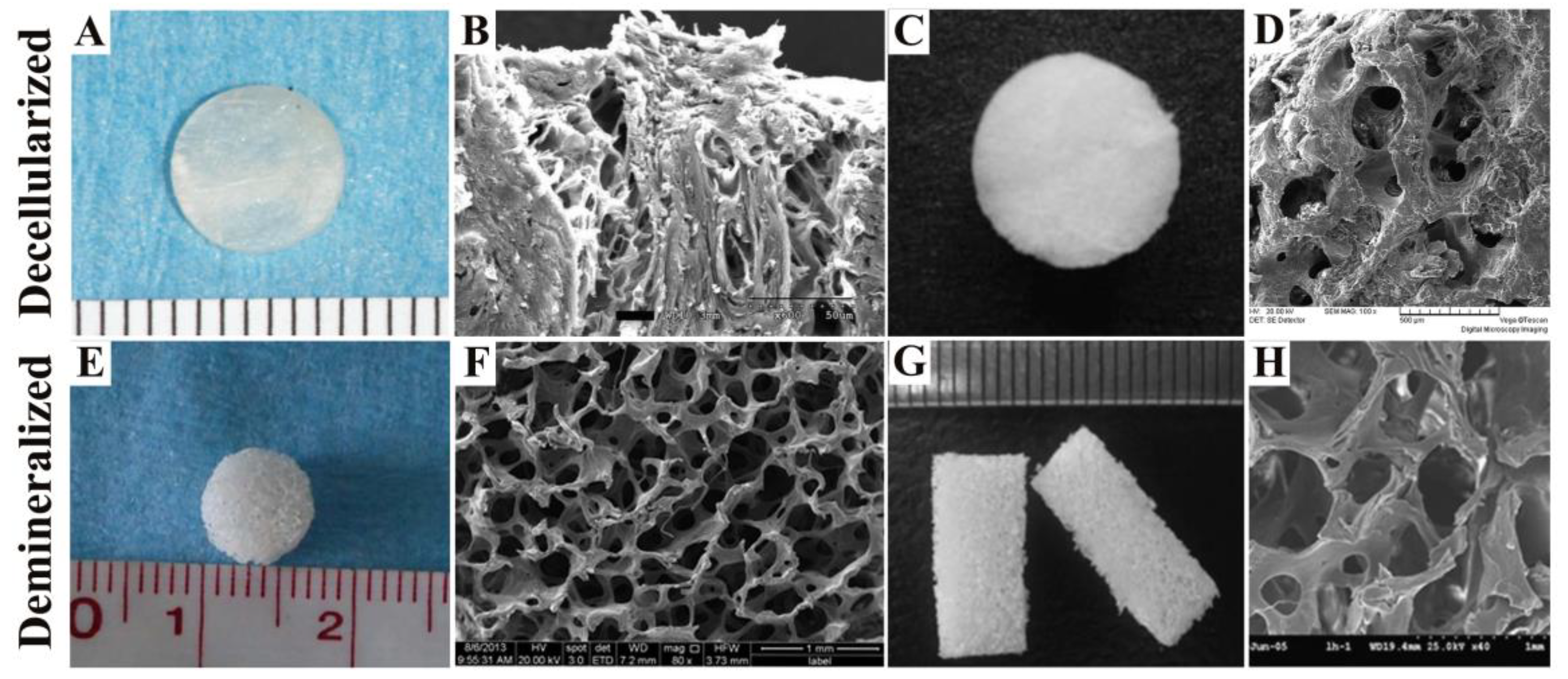
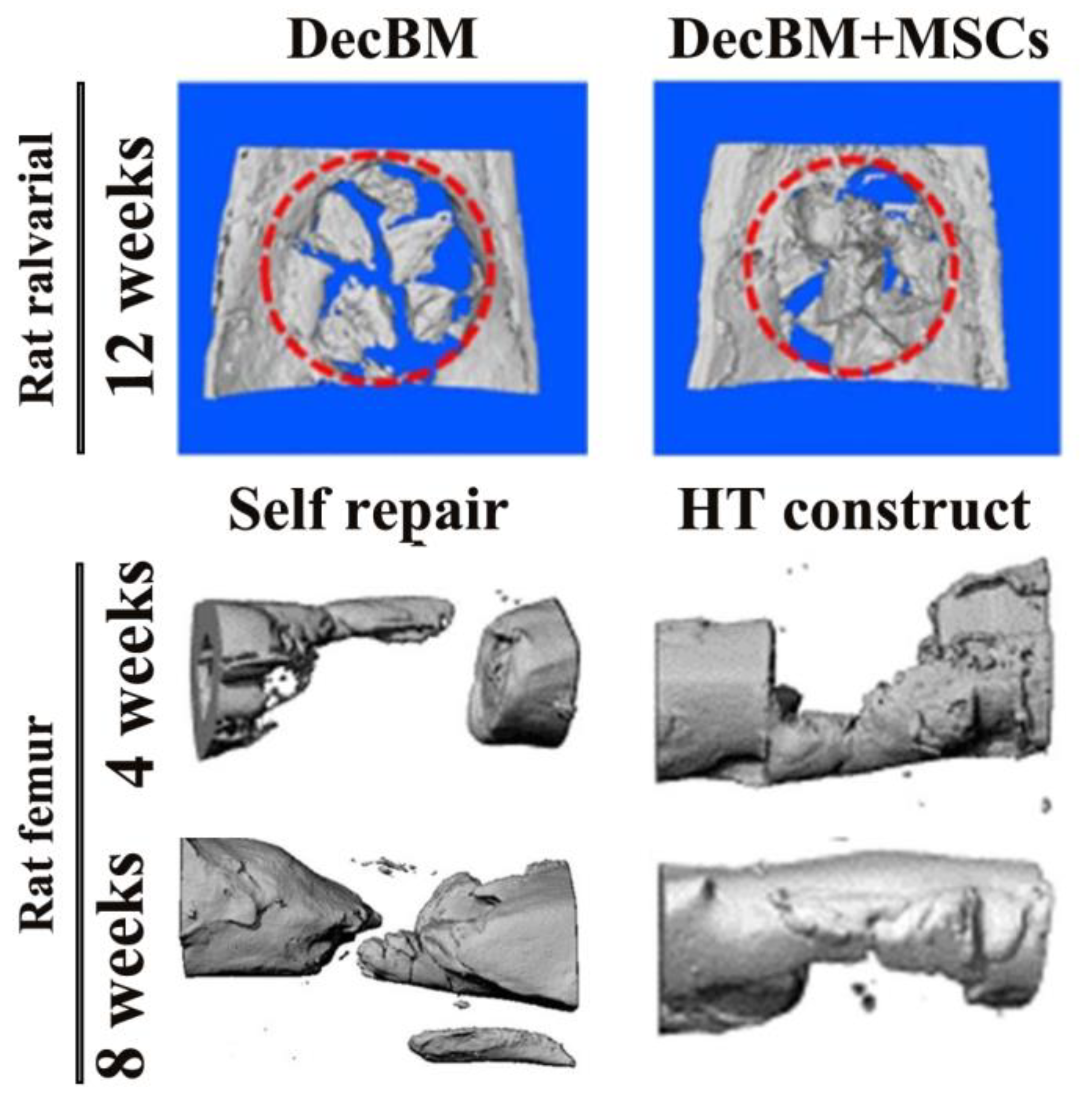
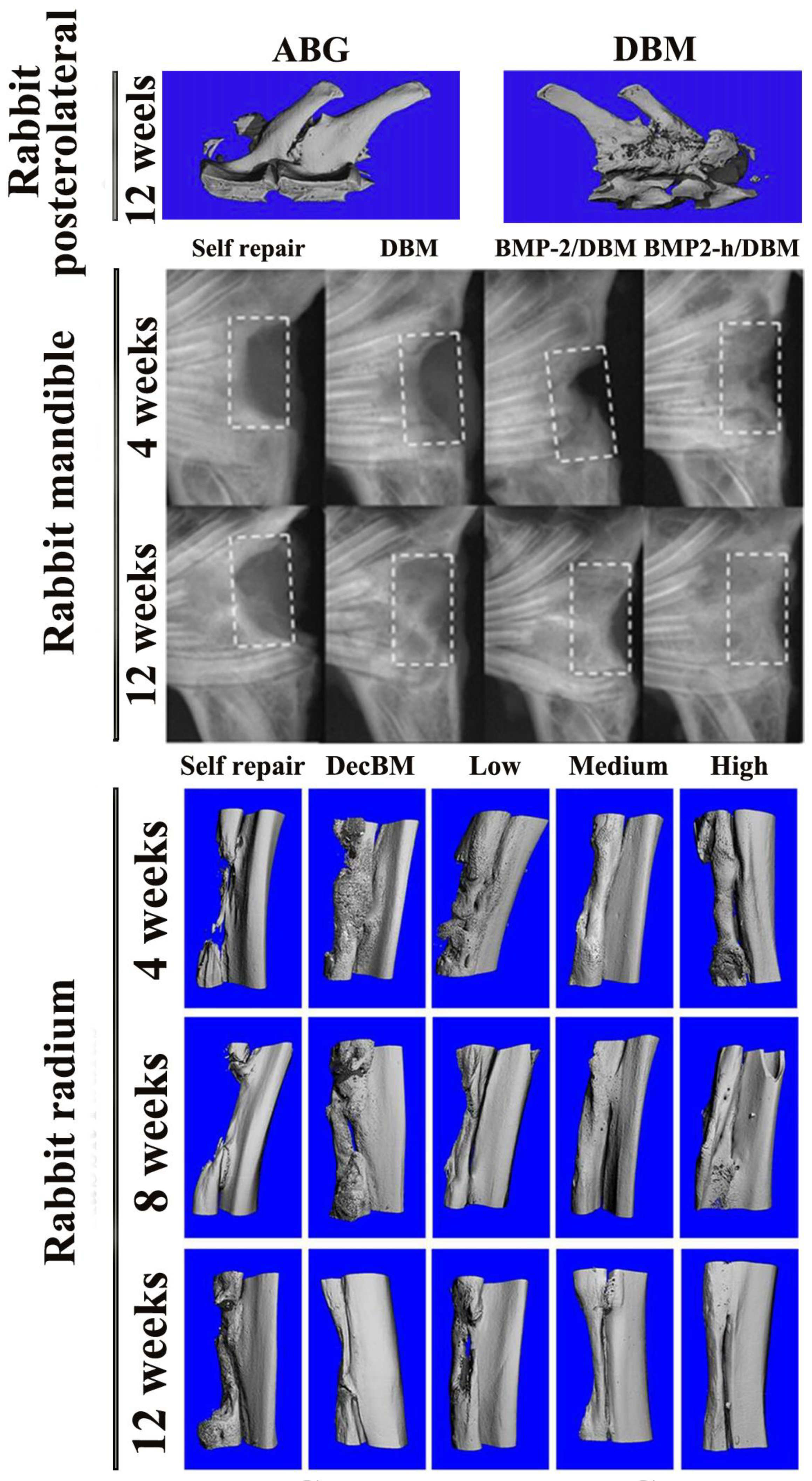
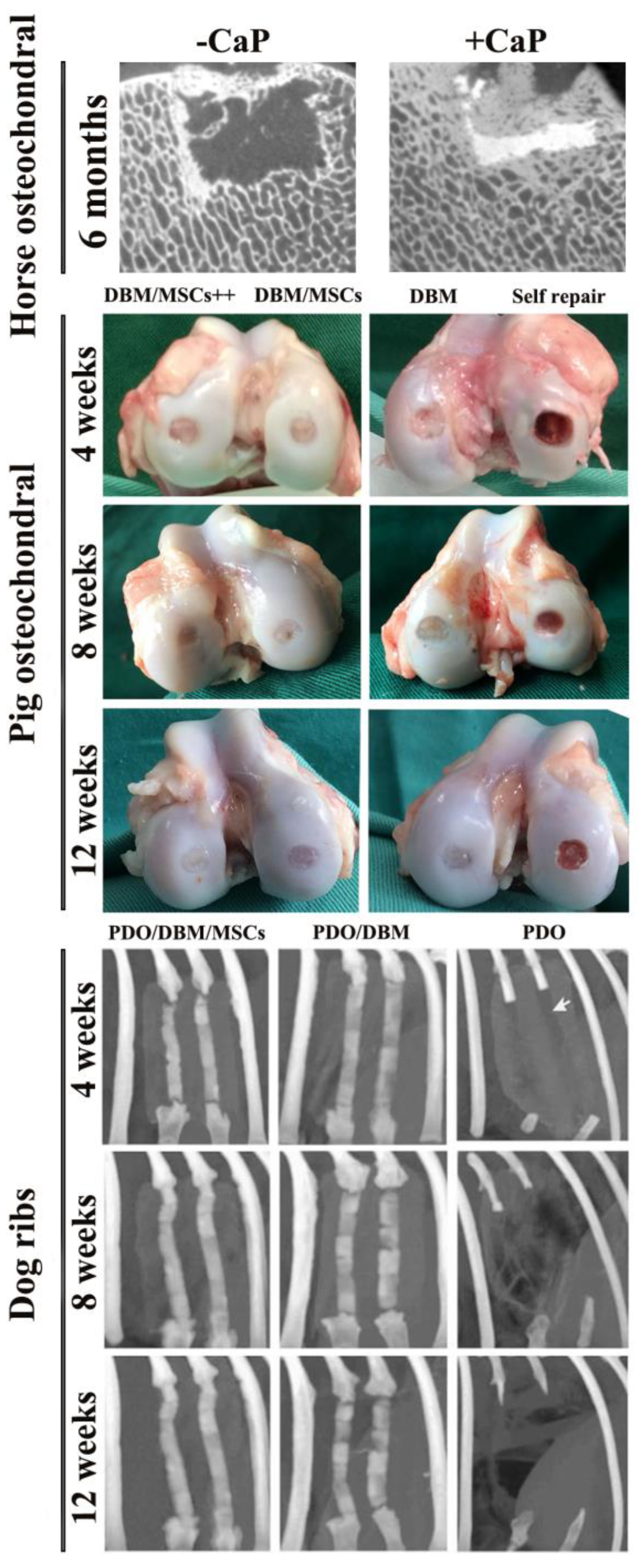
| Comparison Category | Decellularized Bone Matrix Scaffold | DBM Scaffold |
|---|---|---|
| Preparation method | Physical methods (snap freezing, mechanical force and mechanical agitation, etc.) [25] | Treated with decalcification reagents (hydrochloric acid and EDTA-2Na, etc.) |
| Chemistry methods (alkaline solution, acid, nonionic detergents and Tritonn X-100, etc.) [26] | ||
| Enzymatic methods (exonucleases, endonucleases and trypsin, etc.) [27] | ||
| Characteristics | Effectively remove cells from host bone tissue | A complex consisting of collagen, non-collagen, and lower concentrations of growth factors |
| Advantages | Reduce or eliminate the antigenicity of bone | Decalcification exposes osteogenic factors |
| Suitable mechanical strength; better biocompatibility | Good biological properties, osteoinduction and bone conduction activity | |
| The same structure and composition as natural bone | Biodegradable | |
| Weak immunogenicity | ||
| Maintain natural bone-like pore structure and 3D structure | ||
| Disadvantages | Decellularization still causes damage to natural ECM components and microstructures | Low biomechanical strength |
| The difference in ECM from different donor sources is difficult to exclude | Not suitable for repairing bone defects in load-bearing defect models | |
| It is difficult to completely avoid inflammation and immune response |
| Defect Sites | Grafts | Descriptions | References |
|---|---|---|---|
| Calvarial | Decellularized bone | Well new bone fusion around the particles | [58] |
| Calvarial | Decellularized bone matrix as the coating layer of the poly PLGA/PLA scaffold | Increased formation of new bone | [59] |
| Calvarial | DBM combined with PLA and SVF | Significantly promote the regenerative repair | [60] |
| Calvarial | DBM combined with ADSCs | Have more new bone formation | [51] |
| Calvarial | DBM | Significantly limits bone formation | [61] |
| Femur | CBD-SDF-1α modified DBM scaffold | Effectively mobilize CD34+ and c-kit+ endogenous stem cells to the injure site | [62] |
| Xiphoid cartilage | Decellularized human bone matrix scaffold | Achieve endogenous cartilage formation | [63] |
| Spine | DBM fibers | Promoted spine fusion repair | [64] |
| Meniscal | Decellularized meniscus ECM hydrogel | Contribute to tissue regeneration and protection from joint space narrowing | [65] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Lv, Y. Reconstructing Bone with Natural Bone Graft: A Review of In Vivo Studies in Bone Defect Animal Model. Nanomaterials 2018, 8, 999. https://doi.org/10.3390/nano8120999
Liu M, Lv Y. Reconstructing Bone with Natural Bone Graft: A Review of In Vivo Studies in Bone Defect Animal Model. Nanomaterials. 2018; 8(12):999. https://doi.org/10.3390/nano8120999
Chicago/Turabian StyleLiu, Mengying, and Yonggang Lv. 2018. "Reconstructing Bone with Natural Bone Graft: A Review of In Vivo Studies in Bone Defect Animal Model" Nanomaterials 8, no. 12: 999. https://doi.org/10.3390/nano8120999
APA StyleLiu, M., & Lv, Y. (2018). Reconstructing Bone with Natural Bone Graft: A Review of In Vivo Studies in Bone Defect Animal Model. Nanomaterials, 8(12), 999. https://doi.org/10.3390/nano8120999





