Adsorption of Sb (III) on Oxidized Exfoliated Graphite Nanoplatelets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ox-xGnP and Characterization Methods
2.3. Description Of the Adsorption Experiments
3. Results
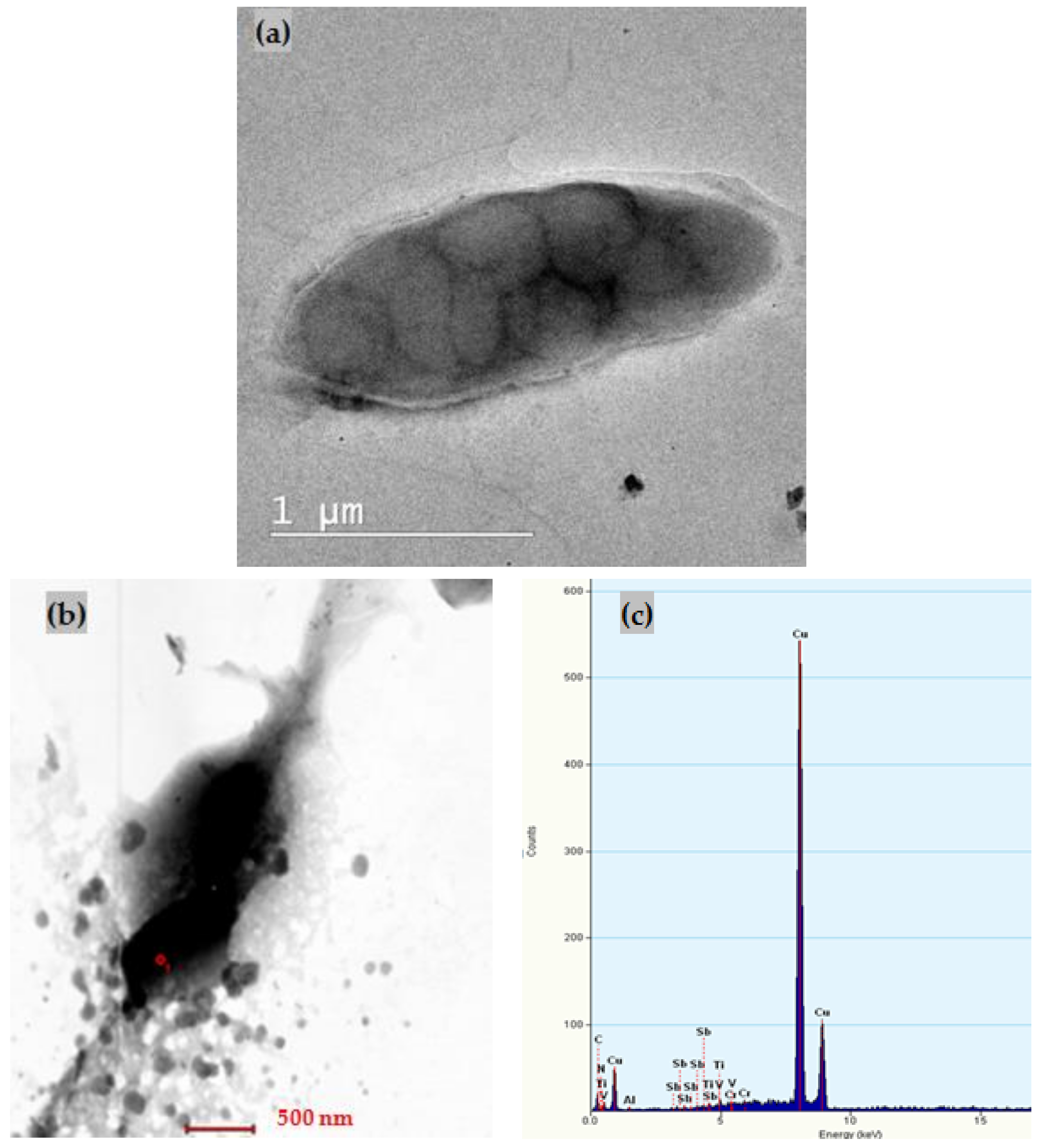
3.1. Characterization of ox-xGnP
3.2. Adsorption Study. Effects of the Adsorption Parameters
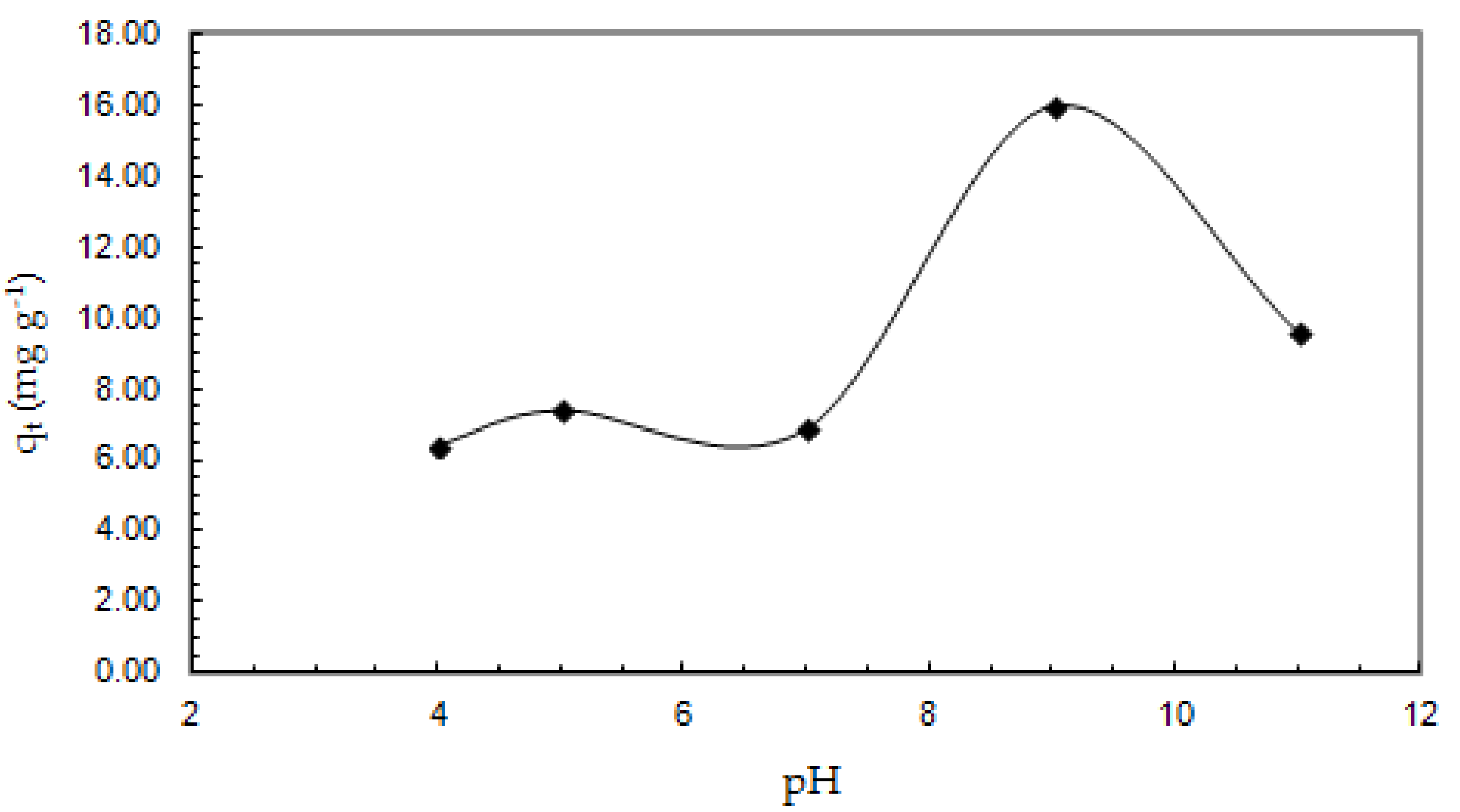
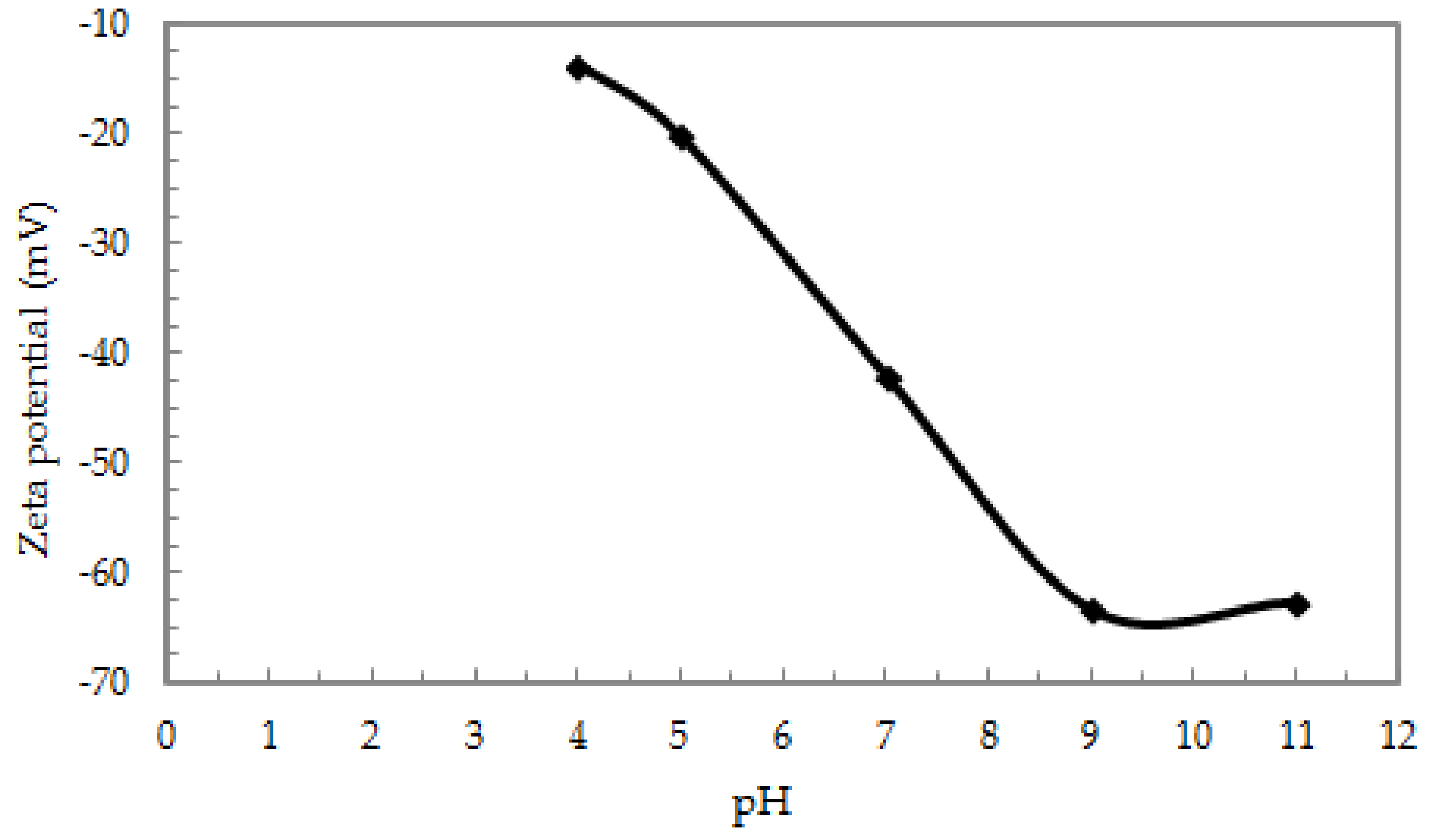
3.2.1. The Effect of pH on the Adsorption Capacity of Sb (III) from Aqueous Solutions Studied in the pH Range of 4.0–11.0
3.2.2. Effect of the Amount of ox-xGnP on the Adsorption Process
3.2.3. Effect of the Initial Concentration of Sb (III) on the Adsorption Process
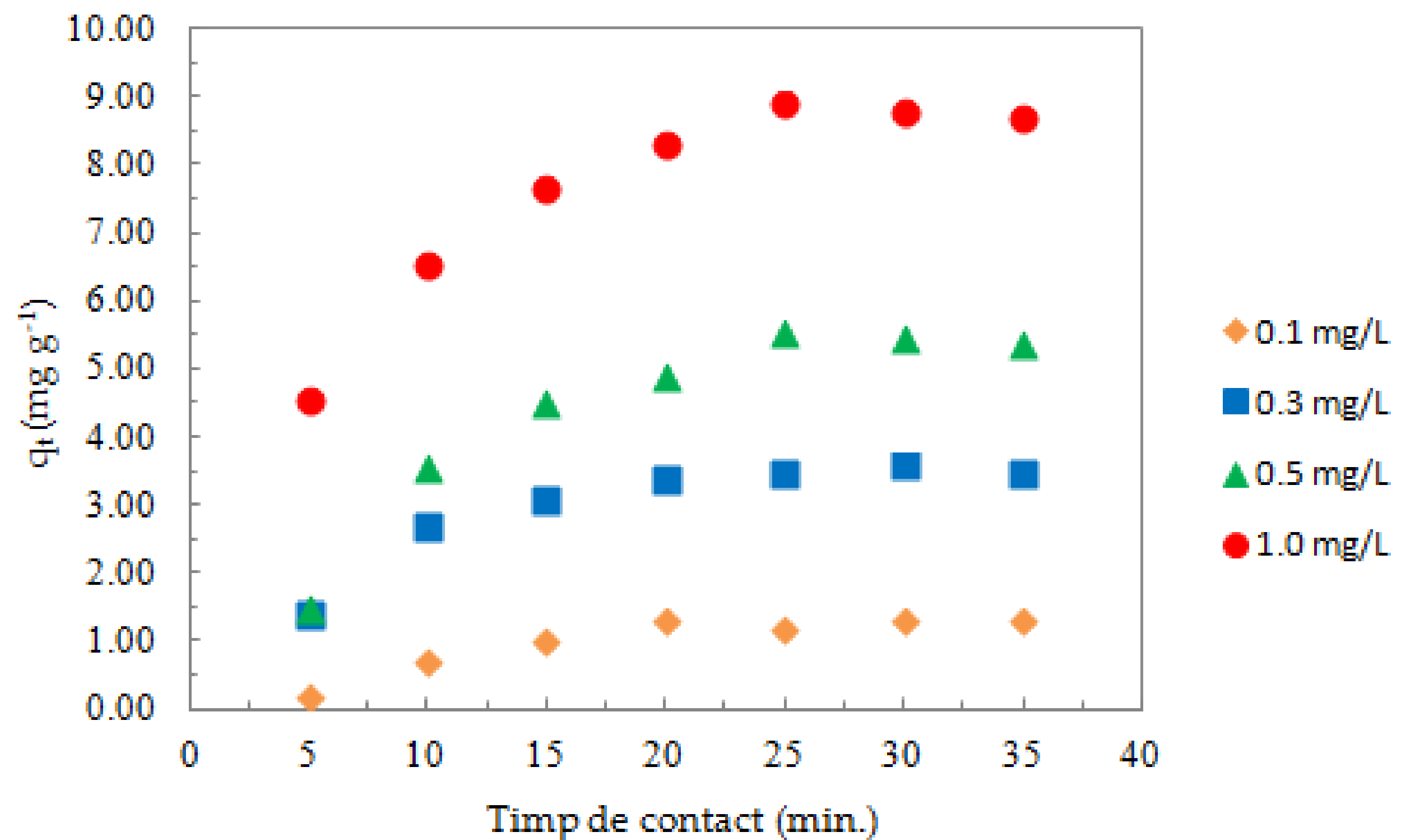
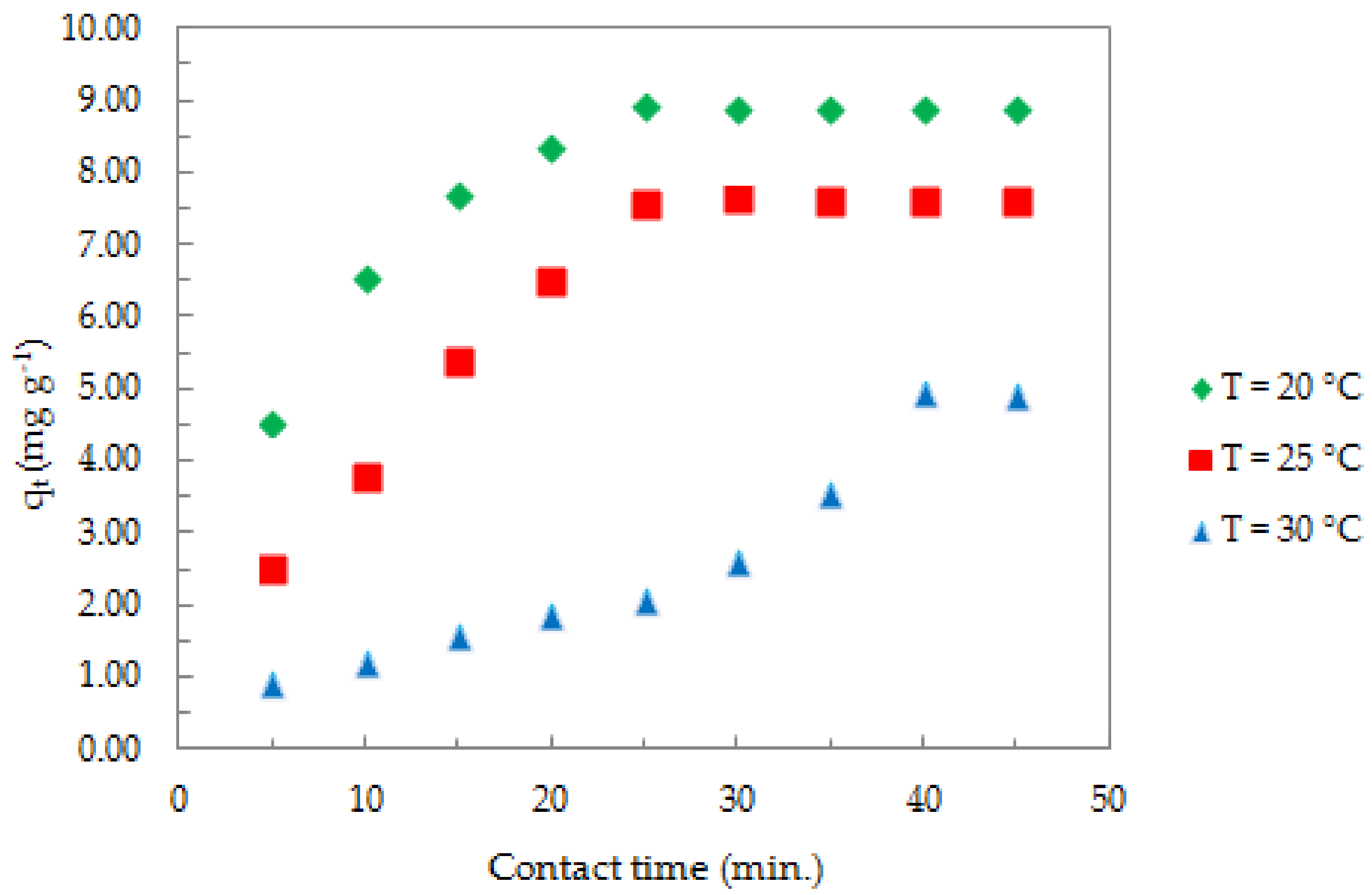
3.2.4. Effect of Contact Time and Temperature on the Adsorption Process
3.3. Adsorption Kinetics Study
3.4. Study of Adsorption Isotherms
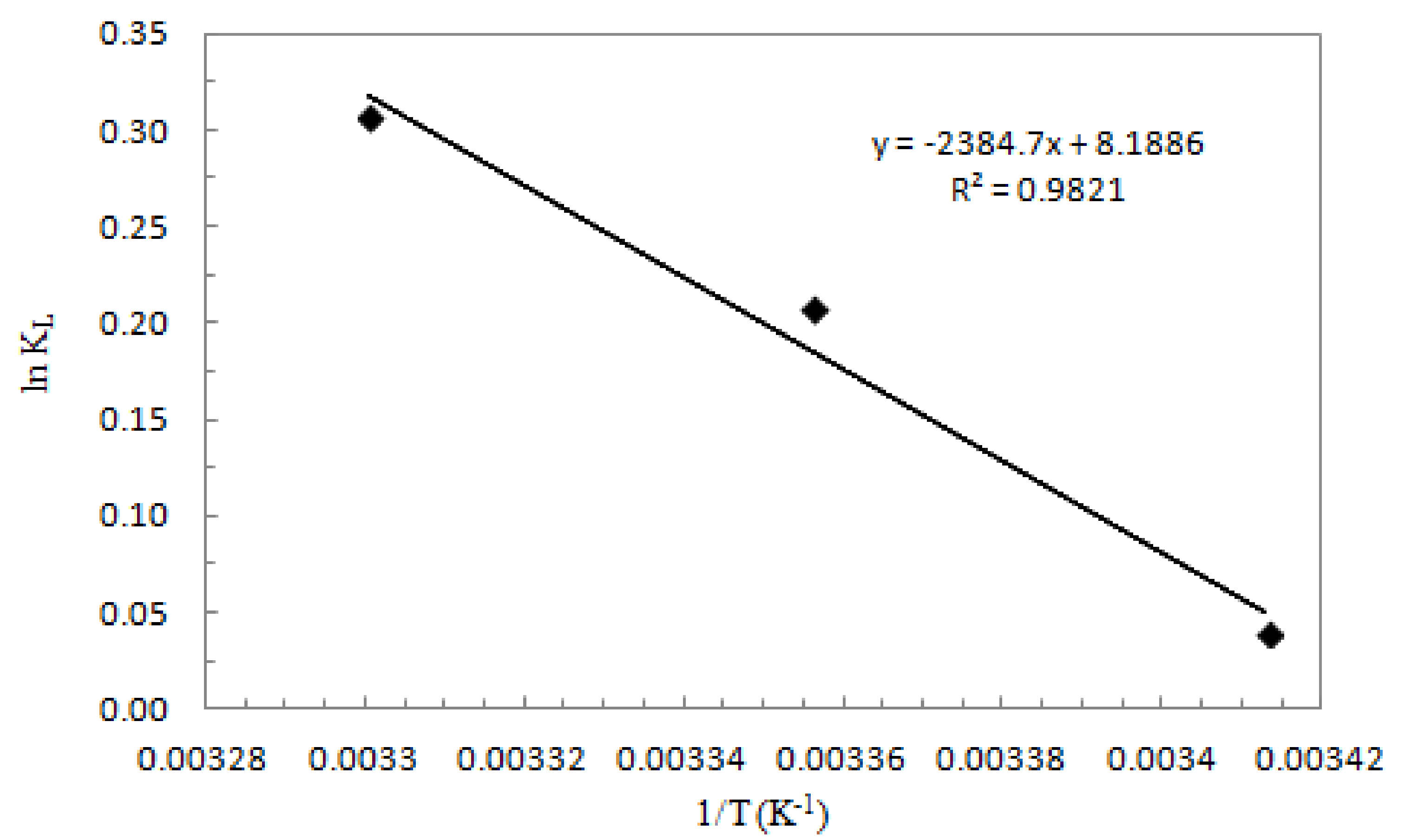
3.5. Thermodynamic Study
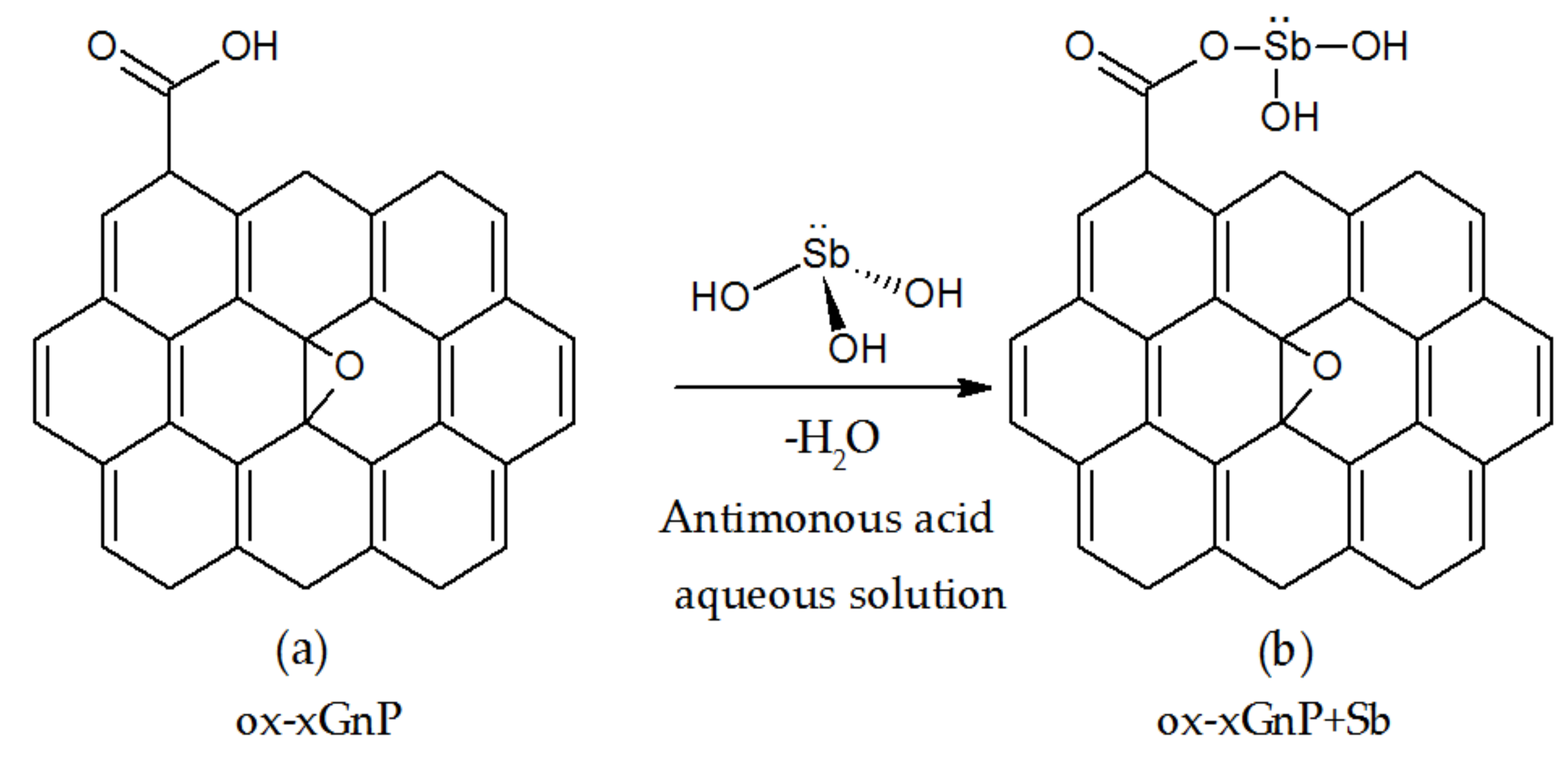
3.6. Proposed Mechanism for Adorption of Sb (III) onto ox-xGnP
- (1)
- The adsorption model was Langmuir-type, which means that the active centers on the ox-xGnP surface were the same, evenly distributed and did not influence each other;
- (2)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Smichowski, P. Antimony in the environment as a global pollutant: A review on analytical methodologies for its determination in atmospheric aerosols. Talanta 2008, 75, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.L.C.; dos Santos, W.N.L.; dos Santos, I.F.; Junior, M.M.S.; Silva, L.O.B.; Barbosa, U.A.; de Santana, F.A.; Queiroz, A.F.d.S. Strategies of sample preparation for speciation analysis of inorganic antimony using hydride generation atomic spectrometry. Microchem. J. 2014, 114, 22–31. [Google Scholar] [CrossRef]
- Kang, M.; Kawasaki, M.; Tamada, S.; Kamei, T.; Magara, Y. Effect of pH on the removal of arsenic and antimony using reverse osmosis membranes. Desalination 2000, 131, 293–298. [Google Scholar] [CrossRef]
- Navarro, P.; Simpson, J.; Alguacil, F.J. Removal of antimony (III) from copper in sulphuric acid solutions by solvent extraction with LIX 1104SM. Hydrometallurgy 1999, 53, 121–131. [Google Scholar] [CrossRef]
- Gannon, K.; Wilson, D.J. Removal of antimony from aqueous systems. Sep. Sci. Technol. 1986, 21, 475–493. [Google Scholar] [CrossRef]
- Kang, M.; Kamei, T.; Magara, Y. Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res. 2003, 37, 4171–4179. [Google Scholar] [CrossRef]
- Koparal, A.S.; Özgür, R.; Öğütveren, Ü.B.; Bergmann, H. Antimony removal from model acid solutions by electrodeposition. Sep. Purif. Technol. 2004, 37, 107–116. [Google Scholar] [CrossRef]
- Guin, R.; Das, S.; Saha, S. The anion exchange behavior of Te and Sb. J. Radioanal. Nuclear Chem. 1998, 230, 269–272. [Google Scholar] [CrossRef]
- Saito, T.; Tsuneda, S.; Hirata, A.; Nishiyama, S.Y.; Saito, K.; Saito, K.; Sugita, K.; Uezu, K.; Tamada, M.; Sugo, T. Removal of Antimony (III) Using Polyol-Ligand-Containing Porous Hollow-Fiber Membranes. Sep. Sci. Technol. 2004, 39, 3011–3022. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Sarı, A.; Tuzen, M. Biosorption of antimony from aqueous solution by lichen (Physcia tribacia) biomass. Chem. Eng. J. 2010, 163, 382–388. [Google Scholar] [CrossRef]
- Wu, F.; Sun, F.; Wu, S.; Yan, Y.; Xing, B. Removal of antimony (III) from aqueous solution by freshwater cyanobacteria Microcystis biomass. Chem. Eng. J. 2012, 183, 172–179. [Google Scholar] [CrossRef]
- Sarı, A.; Şahinogğlu, G.N.R.; Tuzen, M. Antimony (III) adsorption from aqueous solution using raw perlite and Mn-modified perlite: Equilibrium, thermodynamic, and kinetic studies. Ind. Eng. Chem. Res. 2012, 51, 6877–6886. [Google Scholar] [CrossRef]
- Fan, H.-T.; Tang, Q.; Sun, Y.; Zhang, Z.-G.; Li, W.-X. Selective removal of antimony (III) from aqueous solution using antimony (III)-imprinted organic–inorganic hybrid sorbents by combination of surface imprinting technique with sol–gel process. Chem. Eng. J. 2014, 258, 146–156. [Google Scholar] [CrossRef]
- Gupta, V.K.; Tyagi, I.; Sadegh, H.; Ghoshekand, R.S.; Makhlouf, A.S.H.; Maazinejad, B. Nanoparticles as Adsorbent; A Positive Approach for Removal of Noxious Metal Ions: A Review. Sci. Technol. Dev. 2015, 34, 195–214. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv. Colloid Interface Sci. 2014, 204, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, B.; Lu, Q.; Qu, Q. Graphene-based materials: Fabrication and application for adsorption in analytical chemistry. J. Chromatogr. A 2014, 1362, 1–15. [Google Scholar] [CrossRef] [PubMed]
- AlAqad, K.M.; Abulkibash, A.M.; Al Hamouz, O.C.S.; Saleh, T.A. Silver nanoparticles decorated graphene modified Carbon paste electrode for molecular methimazole determination. Chem. Data Collect. 2017, 11, 168–182. [Google Scholar] [CrossRef]
- Ion, A.C.; Alpatova, A.; Ion, I.; Culetu, A. Study on phenol adsorption from aqueous solutions on exfoliated graphitic nanoplatelets. Mater. Sci. Eng. B 2011, 176, 588–595. [Google Scholar] [CrossRef]
- Lu, J.; Drzal, L.T.; Worden, R.M.; Lee, I. Simple fabrication of a highly sensitive glucose biosensor using enzymes immobilized in exfoliated graphite nanoplatelets nafion membrane. Chem. Mater. 2007, 19, 6240–6246. [Google Scholar] [CrossRef]
- Cho, H.-H.; Wepasnick, K.; Smith, B.A.; Bangash, F.K.; Fairbrother, D.H.; Ball, W.P. Sorption of aqueous Zn [II] and Cd [II] by multiwall carbon nanotubes: The relative roles of oxygen-containing functional groups and graphenic carbon. Langmuir 2009, 26, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Ion, A.C.; Ion, I.; Culetu, A. Lead adsorption onto exfoliated graphitic nanoplatelets in aqueous solutions. Mater. Sci. Eng. B 2011, 176, 504–509. [Google Scholar] [CrossRef]
- Agnihotri, S.; Rostam-Abadi, M.; Rood, M.J. Temporal changes in nitrogen adsorption properties of single-walled carbon nanotubes. Carbon 2004, 42, 2699–2710. [Google Scholar] [CrossRef]
- Capra, L.; Manolache, M.; Ion, I.; Ion, A.C. Validation of a method for determination of antimony in drinking water by ICP-OES. Univ. Politeh. Buchar. Sci. Bull. Ser. B-Chem. Mater. Sci. 2016, 78, 103–112. Available online: https://www.scientificbulletin.upb.ro/SeriaB (accessed on 12 November 2018).
- Choi, E.-Y.; Han, T.H.; Hong, J.; Kim, J.E.; Lee, S.H.; Kim, H.W.; Kim, S.O. Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Pan, M.; Wu, G.; Liu, C.; Lin, X.; Huang, X. Enhanced Adsorption of Zn (II) onto Graphene Oxides Investigated Using Batch and Modeling Techniques. Nanomaterials 2018, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, V.V.; Fleck, M.; Petrosyan, A.M. Structure and vibrational spectra of L-alanine L-alaninium picrate monohydrate. J. Mol. Struct. 2012, 1015, 51–55. [Google Scholar] [CrossRef]
- Vijayan, N.; Rajasekaran, S.; Bhagavannarayana, G.; Ramesh Babu, R.; Gopalakrishnan, R.; Palanichamy, M.; Ramasamy, P. Growth and characterization of nonlinear optical amino acid single crystal: L-alanine. Cryst. Growth Design 2006, 6, 2441–2445. [Google Scholar] [CrossRef]
- Merline, G.K.P.; Chitra, M. Investigation on optical, thermal, dielectric and mechanical properties of antimony potassium tartrate on l-alanine single crystals. J. Mater. Sci. Mater. Electron. 2018, 29, 5509–5517. [Google Scholar] [CrossRef]
- Reddy, J.R.; Ravi, G.; Suresh, P.; Veldurthi, N.K.; Velchuri, R.; Vithal, M. Antimony potassium tartrate. J. Therm. Anal. Calorim. 2014, 115, 1321–1327. [Google Scholar] [CrossRef]
- Das, A.; Kasaliwal, G.R.; Jurk, R.; Boldt, R.; Fischer, D.; Stöckelhuber, K.W.; Heinrich, G. Rubber composites based on graphene nanoplatelets, expanded graphite, carbon nanotubes and their combination: A comparative study. Compos. Sci. Technol. 2012, 72, 1961–1967. [Google Scholar] [CrossRef]
- Constantino, L.V.; Quirino, J.N.; Abrão, T.; Parreira, P.S.; Urbano, A.; Santos, M.J. Sorption–desorption of antimony species onto calcined hydrotalcite: Surface structure and control of competitive anions. J. Hazard. Mater. 2018, 344, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Çıtak, D.; Tuzen, M. Equilibrium, thermodynamic and kinetic studies on adsorption of Sb (III) from aqueous solution using low-cost natural diatomite. Chem. Eng. J. 2010, 162, 521–527. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Rudzinski, W.; Panczyk, T. Remarks on the current state of adsorption kinetic theories for heterogeneous solid surfaces: A comparison of the ART and the SRT approaches. Langmuir 2002, 18, 439–449. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. On the applicability of the pseudo-second order equation to represent the kinetics of adsorption at solid/solution interfaces: A theoretical analysis based on the statistical rate theory. Adsorption 2009, 15, 181. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. Available online: http://cedb.asce.org/CEDBsearch/record.jsp?dockey=0013042 (accessed on 12 November 2018).
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Mahvi, A.H.; Rastkari, N.; Saeedi, R.; Nazmara, S.; Iravani, E. Adsorption of bisphenol A (BPA) from aqueous solutions by carbon nanotubes: Kinetic and equilibrium studies. Desalin. Water Treat. 2015, 54, 84–92. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Zeitschrift für Physikalische Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Bergmann, C.P.; Machado, F.M. Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Springer: Berlin, Germany, 2015. [Google Scholar]
- Balarak, D. Kinetics, isotherm and thermodynamics studies on bisphenol A adsorption using barley husk. Int. J. ChemTech Res. 2016, 9, 681–690. Available online: http://www.sphinxsai.com/2016/ch_vol9_no5/3/(681-690)V9N5CT.pdf (accessed on 12 November 2018).
- Watkins, R.; Weiss, D.; Dubbin, W.; Peel, K.; Coles, B.; Arnold, T. Investigations into the kinetics and thermodynamics of Sb (III) adsorption on goethite (α-FeOOH). J. Colloid Interface Sci. 2006, 303, 639–646. [Google Scholar] [CrossRef] [PubMed]




| Amount of ox-xGnP (mg) | Concentration of Sb (III) (mg L−1) | Qe (mg g−1) |
|---|---|---|
| 1.0 | 0.3 | 3.60 |
| 1.0 | 0.7 | 6.86 |
| 2.0 | 0.3 | 2.85 |
| 2.0 | 0.7 | 5.10 |
| qeexp. (mg g−1) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Intra-Particle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qecal. (mg g−1) | k1 (min−1) | R2 | qecal. (mg g−1) | k2 (g mg−1 min−1) | R2 | kid (mg g−1 min−1/2) | C (mg g−1) | R2 | |
| 8.91 | 8.10 | 0.129 | 0.999 | 11.3 | 199 | 0.998 | 1.64 | 1.17 | 0.978 |
| T (K) | Freundlich Isotherm | Langmuir Isotherm | ||||||
|---|---|---|---|---|---|---|---|---|
| KF | n | 1/n | R2 | KL (L mg−1) | qm (mg g−1) | R2 | RL | |
| T = 293 | 9.66 | 1.45 | 0.69 | 0.965 | 1.04 | 18.2 | 0.994 | 0.49 |
| T = 298 | 8.88 | 1.51 | 0.66 | 0.965 | 1.23 | 15.3 | 0.989 | 0.45 |
| T = 303 | 4.66 | 1.50 | 0.66 | 0.849 | 1.36 | 7.65 | 0.923 | 0.42 |
| ΔH0 (kJ mol−1) | ΔS0 (kJ mol−1 K−1) | ΔG0 (kJ mol−1) | ||
|---|---|---|---|---|
| T = 293 (K) | T = 298 (K) | T = 303 (K) | ||
| −19.8 | 0.068 | −39.8 | −40.1 | −40.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capra, L.; Manolache, M.; Ion, I.; Stoica, R.; Stinga, G.; Doncea, S.M.; Alexandrescu, E.; Somoghi, R.; Calin, M.R.; Radulescu, I.; et al. Adsorption of Sb (III) on Oxidized Exfoliated Graphite Nanoplatelets. Nanomaterials 2018, 8, 992. https://doi.org/10.3390/nano8120992
Capra L, Manolache M, Ion I, Stoica R, Stinga G, Doncea SM, Alexandrescu E, Somoghi R, Calin MR, Radulescu I, et al. Adsorption of Sb (III) on Oxidized Exfoliated Graphite Nanoplatelets. Nanomaterials. 2018; 8(12):992. https://doi.org/10.3390/nano8120992
Chicago/Turabian StyleCapra, Luiza, Mihaela Manolache, Ion Ion, Rusandica Stoica, Gabriela Stinga, Sanda Maria Doncea, Elvira Alexandrescu, Raluca Somoghi, Marian Romeo Calin, Ileana Radulescu, and et al. 2018. "Adsorption of Sb (III) on Oxidized Exfoliated Graphite Nanoplatelets" Nanomaterials 8, no. 12: 992. https://doi.org/10.3390/nano8120992
APA StyleCapra, L., Manolache, M., Ion, I., Stoica, R., Stinga, G., Doncea, S. M., Alexandrescu, E., Somoghi, R., Calin, M. R., Radulescu, I., Ivan, G. R., Deaconu, M., & Ion, A. C. (2018). Adsorption of Sb (III) on Oxidized Exfoliated Graphite Nanoplatelets. Nanomaterials, 8(12), 992. https://doi.org/10.3390/nano8120992






