Applications of Gold Nanoparticles in Non-Optical Biosensors
Abstract
1. Introduction
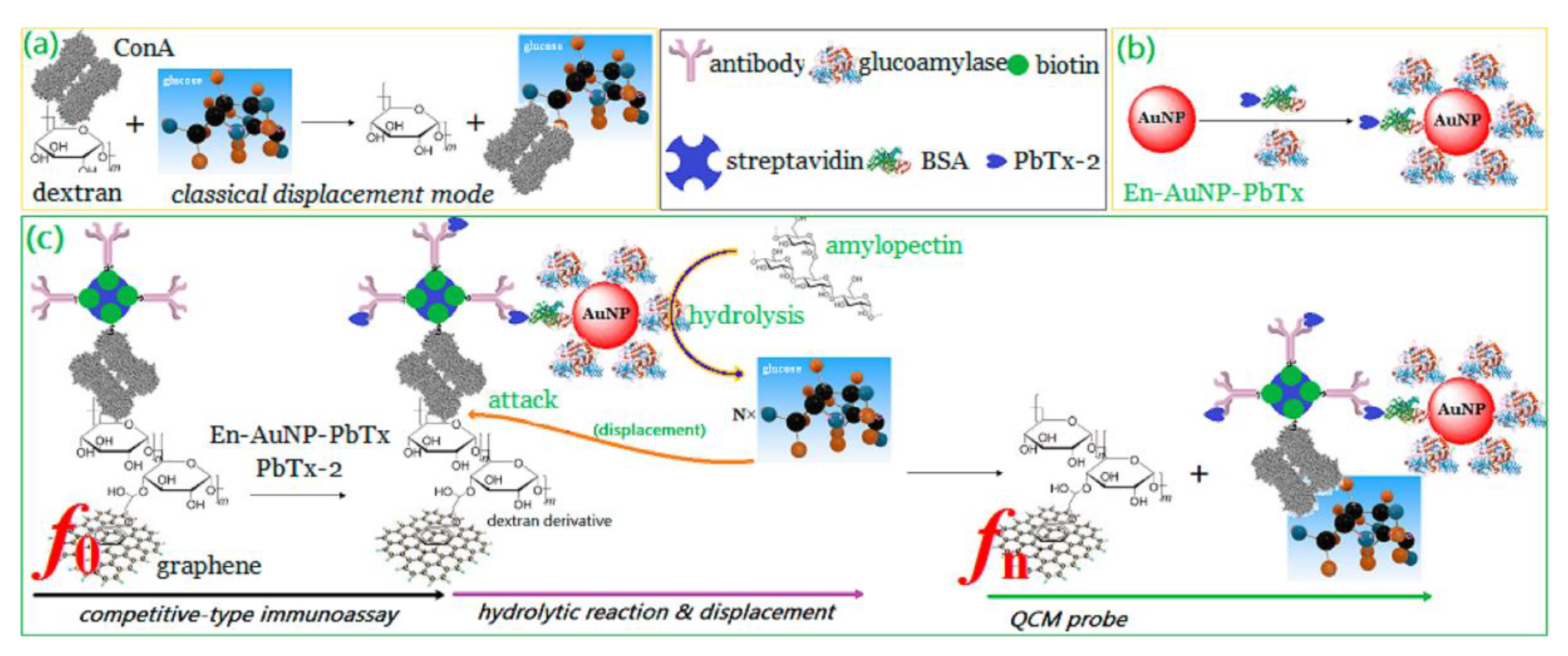
2. Piezoelectric Biosensors
3. Electrochemical Biosensors
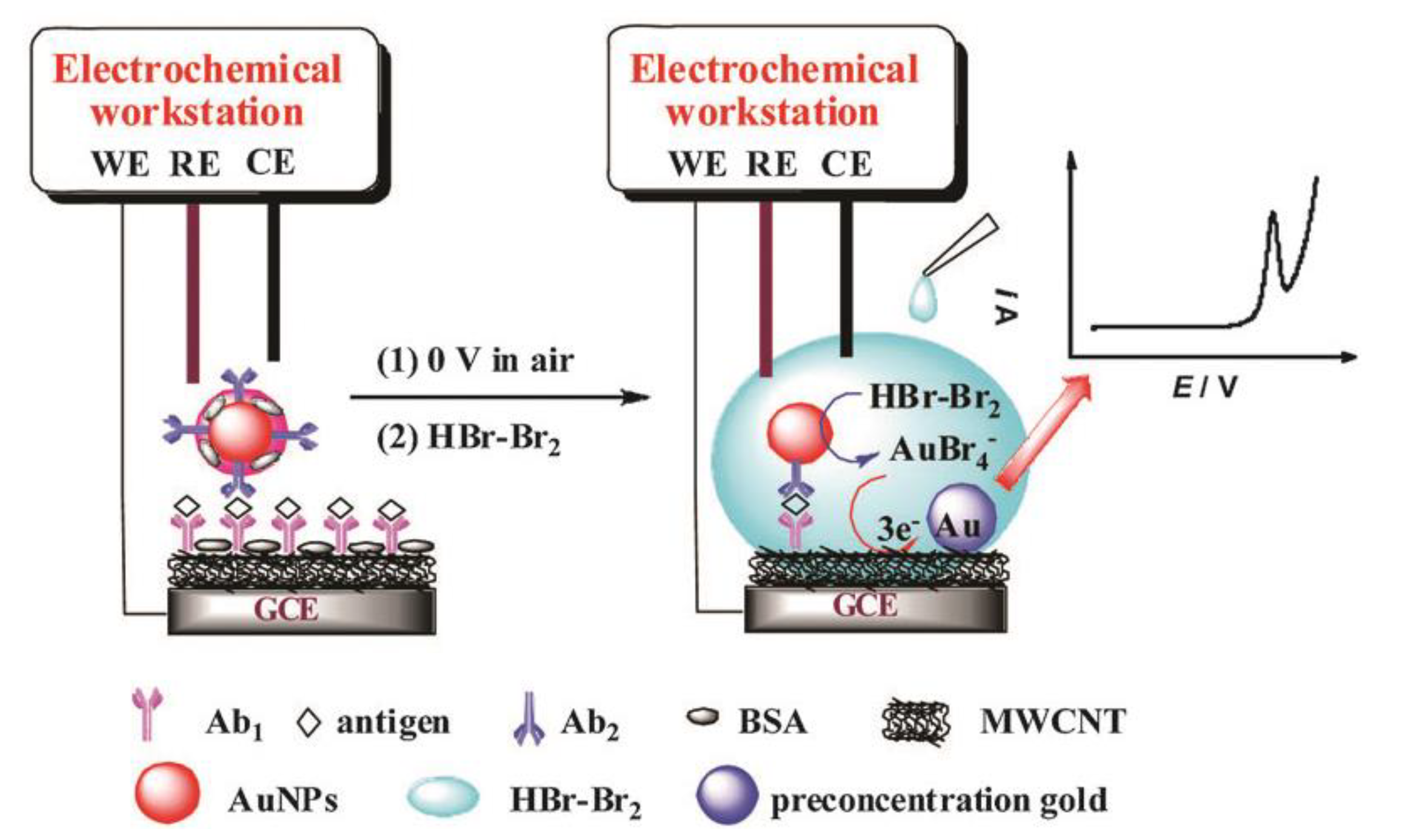
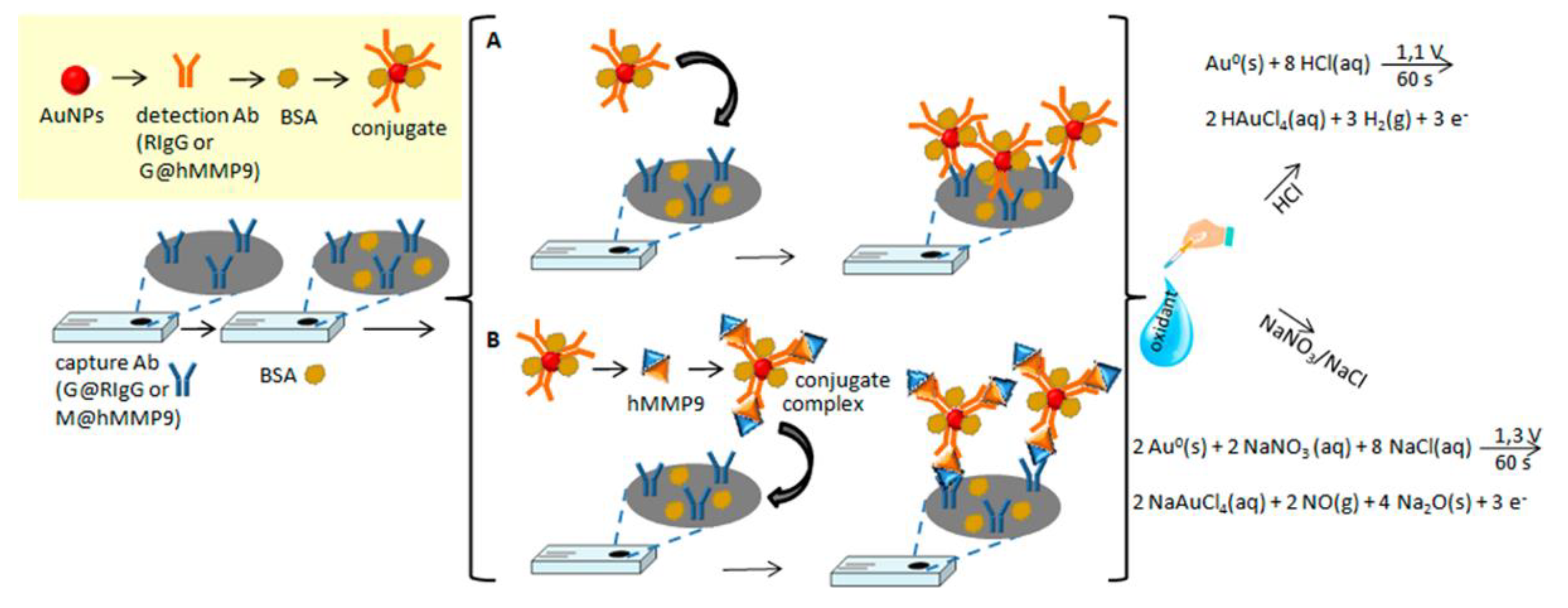
3.1. AuNPs as the Electrochemical Indicators
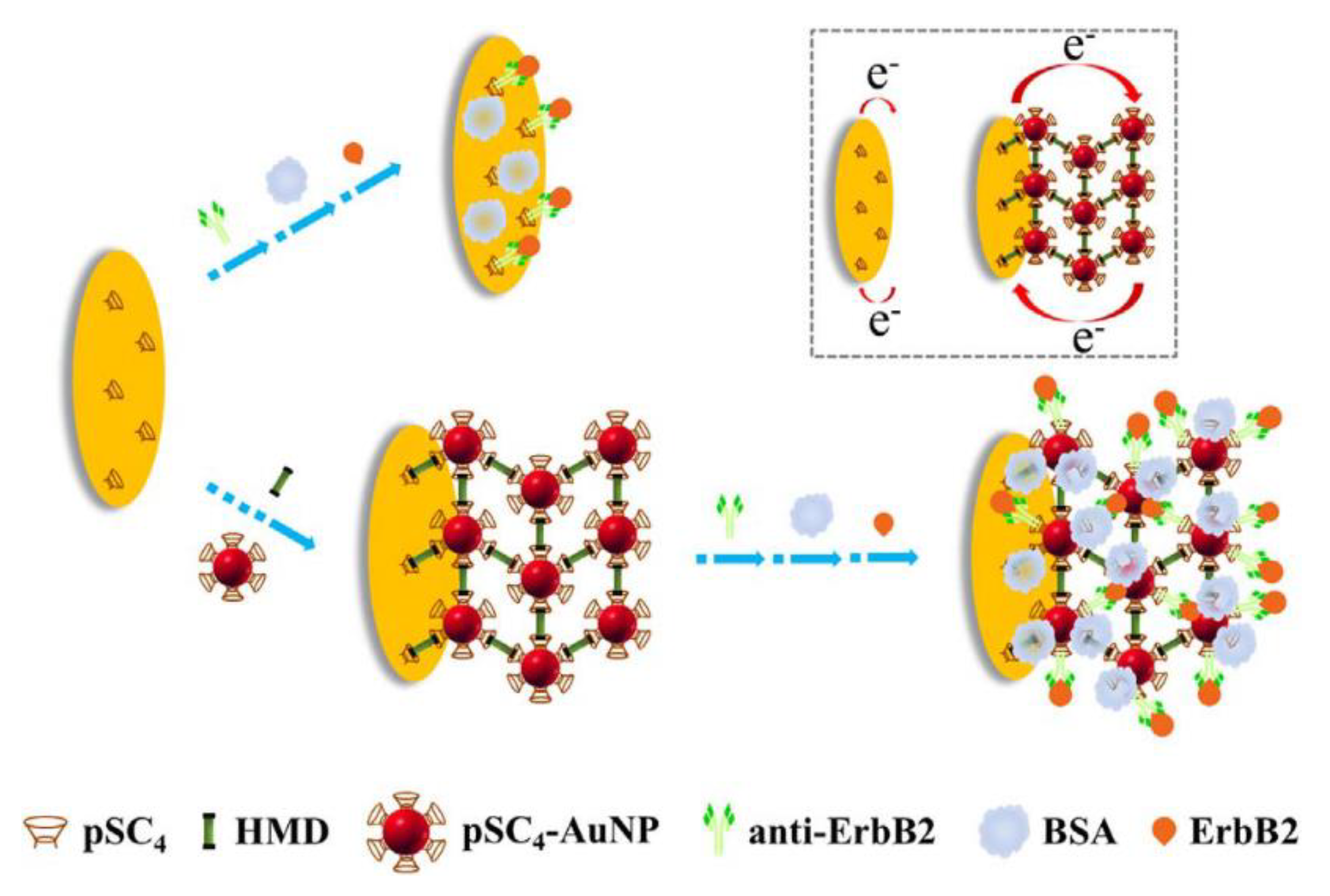
3.2. AuNPs as the Electron Migration Enhancers
3.3. AuNPs as the Immobilization Platform
3.4. AuNPs as the Catalyst
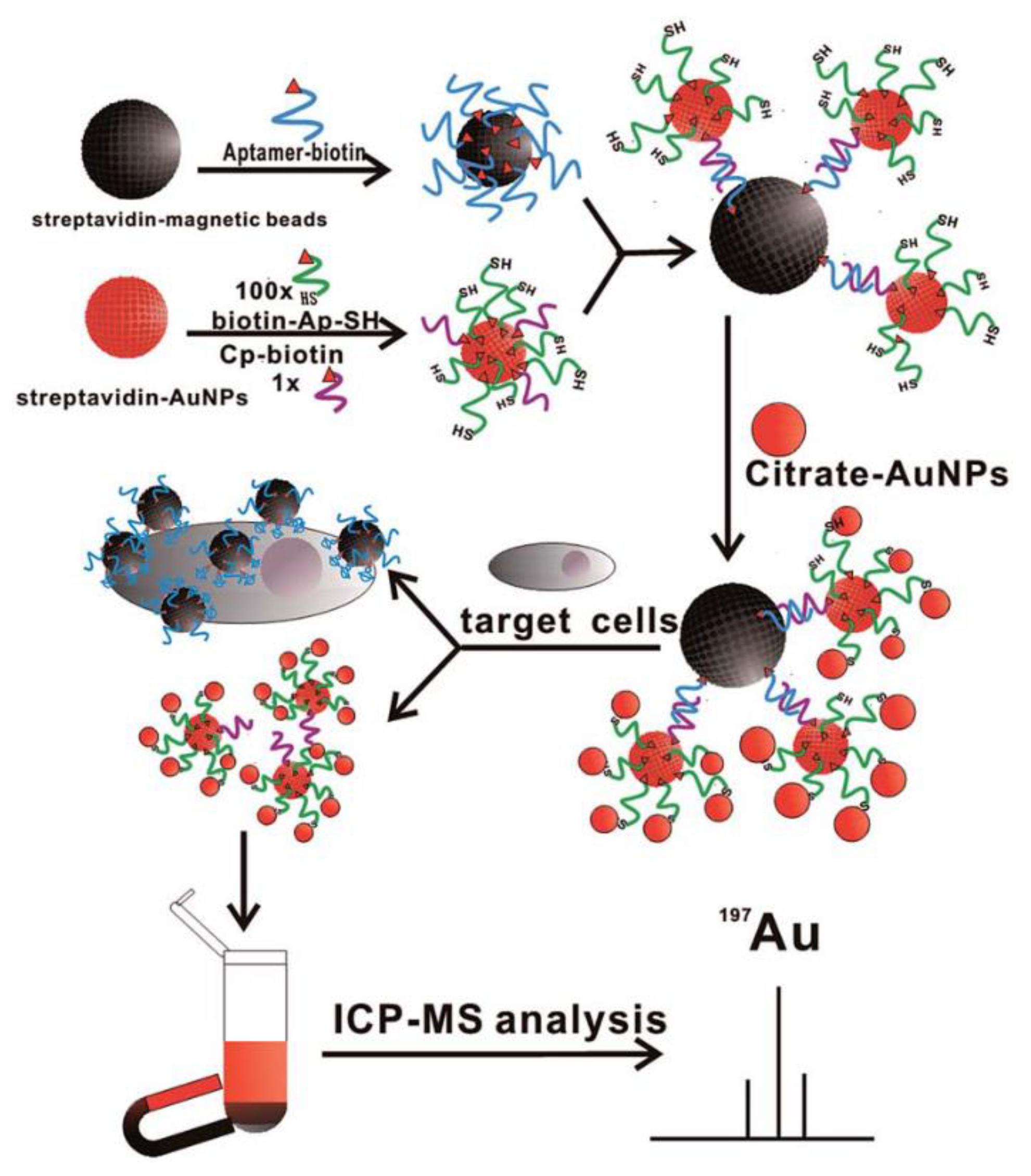
4. ICP-MS Biosensor
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dubertret, B.; Calame, M.; Libchaber, A.J. Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat. Biotechnol. 2001, 19, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Imahori, H.; Fukuzumi, S. Porphyrin monolayer-modified gold clusters as photoactive materials. Adv. Mater. 2001, 13, 1197–1199. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Ramakrishnan, V.; Sainkar, S.R.; Mandale, A.B.; Rao, M.; Sastry, M. Pepsin−gold colloid conjugates: Preparation, characterization, and enzymatic activity. Langmuir 2001, 17, 1674–1679. [Google Scholar] [CrossRef]
- Liu, J.X.; Bao, N.; Luo, X.; Ding, S.N. Nonenzymatic amperometric aptamer cytosensor for ultrasensitive detection of circulating tumor cells and dynamic evaluation of cell surface N-Glycan expression. ACS Omega 2018, 3, 8595–8604. [Google Scholar] [CrossRef]
- Raj, C.R.; Jena, B.K. Efficient electrocatalytic oxidation of NADH at gold nanoparticles self-assembled on three-dimensional sol-gel network. Chem. Commun. 2005, 0, 2005–2007. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Bethell, D.; Kiely, C.J.; Schiffrin, D.J. Self-assembled gold nanoparticle thin films with nonmetallic optical and electronic properties. Langmuir 1998, 14, 5425–5429. [Google Scholar] [CrossRef]
- Halperin, W.P. Quantum size effects in metal particles. Rev. Mod. Phys. 1986, 58, 533–606. [Google Scholar] [CrossRef]
- Ball, P.; Garwin, L. Science at the atomic scale. Nature 1992, 355, 761–766. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Lyon, L.A.; Musick, M.D.; Natan, M.J. Colloidal Au-enhanced surface plasmon resonance immunosensing. Anal. Chem. 1998, 70, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Zengin, A.; Tamer, U.; Caykara, T. Extremely sensitive sandwich assay of kanamycin using surface-enhanced Raman scattering of 2-mercaptobenzothiazole labeled gold@silver nanoparticles. Anal. Chim. Acta 2014, 817, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.F.; Cui, H.; Lai, C.Z.; Liu, L.J. Gold nanoparticle-catalyzed luminol chemiluminescence and its analytical applications. Anal. Chem. 2005, 77, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Mayilo, S.; Kloster, M.A.; Wunderlich, M.; Lutich, A.; Klar, T.A.; Nichtl, A.; Kürzinger, K.; Stefani, F.D.; Feldmann, J. Long-range fluorescence quenching by gold nanoparticles in a sandwich immunoassay for cardiac troponin T. Nano Lett. 2009, 9, 4558–4563. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhao, H.Q.; Li, J.R.; Tang, J.A.; Duan, M.X.; Jiang, L. Study on colloidal Au-enhanced DNA sensing by quartz crystal microbalance. Biochem. Biophys. Res. Commun. 2000, 274, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.C.; O’Shea, S.J.; Li, S.F.Y. Amplified microgravimetric gene sensor using Au nanoparticle modified oligonucleotides. Chem. Commun. 2000, 0, 953–954. [Google Scholar] [CrossRef]
- Andreescu, S.; Luck, L.A. Studies of the binding and signaling of surface-immobilized periplasmic glucose receptors on gold nanoparticles: A glucose biosensor application. Anal. Biochem. 2008, 375, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, S.; Nogami, M. A glucose biosensor based on electrodeposited biocomposites of gold nanoparticles and glucose oxidase enzyme. Analyst 2001, 126, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Mao, C.; Liu, N.N.; Zhu, J.J.; Sheng, J. Direct electrochemistry of horseradish peroxidase based on biocompatible carboxymethyl chitosan–gold nanoparticle nanocomposite. Biosens. Bioelectron. 2006, 22, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Wagner, B.; Karbarz, M.; Nowicka, A.M. A dual DNA biosensor based on two redox couples with a hydrogel sensing platform functionalized with carboxyl groups and gold nanoparticles. Sens. Actuators B Chem. 2015, 208, 220–227. [Google Scholar] [CrossRef]
- Matczuk, M.; Aleksenko, S.S.; Matysik, F.M.; Jarosz, M.; Timerbaev, A.R. Comparison of detection techniques for capillary electrophoresis analysis of gold nanoparticles. Electrophoresis 2015, 36, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, B.; He, M.; Yang, B.; Zhang, J.; Hu, B. Immunomagnetic separation combined with inductively coupled plasma mass spectrometry for the detection of tumor cells using gold nanoparticle labeling. Anal. Chem. 2014, 86, 8082–8089. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, S.; Hu, Z.; Xing, Z.; Zhang, X. Detection of multiple proteins on one spot by laser ablation inductively coupled plasma mass spectrometry and application to immuno-microarray with element-tagged antibodies. Anal. Chem. 2007, 79, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Curie, J.; Curie, P. Development by pressure of polar electricity in hemihedral crystals with inclined faces. Bull. Soc. Min. Fr. 1880, 3, 90. [Google Scholar]
- Wang, L.; Wei, Q.; Wu, C.; Hu, Z.; Ji, J.; Wang, P. The Escherichia coli O157: H7 DNA detection on a gold nanoparticle-enhanced piezoelectric biosensor. Chin. Sci. Bull. 2008, 53, 1175–1184. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Tang, W.; Hu, S.; Li, N.; Liu, F. An in situ assembly of a DNA–streptavidin dendrimer nanostructure: A new amplified quartz crystal microbalance platform for nucleic acid sensing. Chem. Commun. 2015, 51, 10660–10663. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.A.; Ramos-Jesus, J.; Kubota, L.T.; Dutra, R.F. A nanostructured piezoelectric immunosensor for detection of human cardiac troponin T. Sensors 2011, 11, 10785–10797. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Rhee, C.K.; Rahman, M.A. A highly sensitive quartz crystal microbalance immunosensor based on magnetic bead-supported bienzymes catalyzed mass enhancement strategy. Biosens. Bioelectron. 2015, 66, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Chen, M.; Fu, Q.; Smeets, N.M.; Xu, F.; Zhang, Z.; Filipe, C.D.M.; Hoare, T. A highly sensitive immunosorbent assay based on biotinylated graphene oxide and the quartz crystal microbalance. ACS Appl. Mater. Interfaces 2016, 8, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, Q. Hyaluronic acid-coated magnetic nanoparticles-based selective collection and detection of leukemia cells with quartz crystal microbalance. Sens. Actuators B Chem. 2016, 223, 9–14. [Google Scholar] [CrossRef]
- Zhang, S.; Bai, H.; Luo, J.; Yang, P.; Cai, J. A recyclable chitosan-based QCM biosensor for sensitive and selective detection of breast cancer cells in real time. Analyst 2014, 139, 6259–6265. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yao, C.; Xia, J.; Wang, J.; Chen, M.; Huang, J.; Chang, K.; Liu, C.; Pan, H.; Fu, W. Rapid parallelized and quantitative analysis of five pathogenic bacteria by ITS hybridization using QCM biosensor. Sens. Actuators B Chem. 2011, 155, 500–504. [Google Scholar] [CrossRef]
- Haddada, M.B.; Salmain, M.; Boujday, S. Gold colloid-nanostructured surfaces for enhanced piezoelectric immunosensing of staphylococcal enterotoxin A. Sens. Actuators B Chem. 2018, 255, 1604–1613. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hung, Y.C.; Chen, K.; Huang, G.S. Detection of gold nanoparticles using an immunoglobulin-coated piezoelectric sensor. Nanotechnology 2008, 19, 495502. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Bloxham, M.; Piletsky, S.A.; Whitcombe, M.J.; Chianella, I. The use of a quartz crystal microbalance as an analytical tool to monitor particle/surface and particle/particle interactions under dry ambient and pressurized conditions: A study using common inhaler components. Analyst 2017, 142, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, D.; Fusella, G.C.; Del Carlo, M.; Pittia, P.; Martinelli, E.; Tortora, L.; Paolesse, R.; Di Natale, C. Gold nanoparticles-peptide based gas sensor arrays for the detection of foodaromas. Biosens. Bioelectron. 2013, 42, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.B.; Ghatak, B.; Debabhuti, N.; Sharma, P.; Ghosh, A.; Tudu, B.; Bhattacharya, N.; Bandyopadhyay, R. Detection of β-caryophyllene in mango using a quartz crystal microbalance sensor. Sens. Actuators B Chem. 2018, 255, 3064–3073. [Google Scholar] [CrossRef]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Haupt, K.; Feller, K.H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.H.; Aik, S.X.L.; Angkasa, J.; Le, Q.; Chooi, K.S.; Li, S.F.Y. Selective and sensitive sensors based on molecularly imprinted poly(vinylidene fluoride) for determination of pesticides and chemical threat agent simulants. Sens. Actuators B Chem. 2018, 258, 228–237. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Y.; Sheng, X.; Peng, Y.; Bai, J.; Lv, Q.; Jia, H.; Jiang, H.; Gao, Z. Rapid high-throughput detection of diethylstilbestrol by using the arrayed langasite crystal microbalance combined with gold nanoparticles through competitive immunoassay. Sens. Actuators B Chem. 2017, 247, 245–253. [Google Scholar] [CrossRef]
- Melani, V.; Haddada, M.B.; Moustaoui, H.; Landoulsi, J.; Djaker, N.; de la Chapelle, M.L.; Spadavecchia, J. Pegylated doxorubicin gold complex: From nanovector to potential intercalant agent for biosensor applications. Front. Lab. Med. 2017, 1, 114–121. [Google Scholar] [CrossRef]
- Yuan, M.; Song, Z.; Fei, J.; Wang, X.; Xu, F.; Cao, H.; Yu, J. Aptasensor for lead (II) based on the use of a quartz crystal microbalance modified with gold nanoparticles. Microchim. Acta 2017, 184, 1397–1403. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lin, Y.M.; Hwang, R.C. Detection of Cu(II) ion in water using a quartz crystal microbalance. J. Electr. Electron. Eng. 2016, 4, 13–17. [Google Scholar] [CrossRef]
- Teh, H.B.; Li, H.; Li, S.F.Y. Highly sensitive and selective detection of Pb2+ ions using a novel and simple DNAzyme-based quartz crystal microbalance with dissipation biosensor. Analyst 2014, 139, 5170–5175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lin, L.; Tang, J.; Duan, M.; Jiang, L. Enhancement of the immobilization and discrimination of DNA probe on a biosensor using gold nanoparticles. Chin. Sci. Bull. 2001, 46, 1074–1077. [Google Scholar]
- Liu, S.F.; Li, J.R.; Jiang, L. Surface modification of platinum quartz crystal microbalance by controlled electroless deposition of gold nanoparticles and its enhancing effect on the HS-DNA immobilization. Colloid Surf. A 2005, 257, 57–62. [Google Scholar] [CrossRef]
- Li, S.; Xia, Y.; Zhang, J.; Han, J.; Jiang, L. Polystyrene spheres coated with gold nanoparticles for detection of DNA. Electrophoresis 2010, 31, 3090–3096. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tang, J.A.; Han, M.; Jiang, L. A novel microgravimetric DNA sensor with high sensitivity. Biochem. Biophys. Res. Commun. 2003, 304, 98–100. [Google Scholar] [CrossRef]
- Kim, N.H.; Baek, T.J.; Park, H.G.; Seong, G.H. Highly sensitive biomolecule detection on a quartz crystal microbalance using gold nanoparticles as signal amplification probes. Anal. Sci. 2007, 23, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, M.; Wang, Z.; Zhang, F.; Xia, J.; Shi, G.; Xia, L.; Li, Y.; Xia, Y.; Xia, L. A label-free immunosensor for detecting common acute lymphoblastic leukemia antigen (CD10) based on gold nanoparticles by quartz crystal microbalance. Sens. Actuators B Chem. 2015, 210, 248–253. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Lin, L.; Li, J.R.; Tang, J.A.; Duan, M.X.; Jiang, L. DNA biosensor with high sensitivity amplified by gold nanoparticles. J. Nanopart. Res. 2001, 3, 321–323. [Google Scholar] [CrossRef]
- Liu, T.; Tang, J.A.; Jiang, L. Sensitivity enhancement of DNA sensors by nanogold surface modification. Biochem. Biophys. Res. Commun. 2002, 295, 14–16. [Google Scholar] [CrossRef]
- Liu, T.; Tang, J.A.; Jiang, L. The enhancement effect of gold nanoparticles as a surface modifier on DNA sensor sensitivity. Biochem. Biophys. Res. Commun. 2004, 313, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Premaratne, G.; Al Mubarak, Z.H.; Senavirathna, L.; Liu, L.; Krishnan, S. Measuring ultra-low levels of nucleotide biomarkers using quartz crystal microbalance and SPR microarray imaging methods: A comparative analysis. Sens. Actuators B Chem. 2017, 253, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chuang, Y.C.; Lu, Y.C.; Lin, H.C.; Yang, Y.L.; Lin, C.S. A method of layer-by-layer gold nanoparticle hybridization in a quartz crystal microbalance DNA sensing system used to detect dengue virus. Nanotechnology 2009, 20, 215501. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, D.K.; Cho, Y.J. Gold nanoparticle-based signal augmentation of quartz crystal microbalance immunosensor measuring C-reactive protein. Curr. Appl. Phys. 2010, 10, 1227–1230. [Google Scholar] [CrossRef]
- Tang, D.; Zhang, B.; Tang, J.; Hou, L.; Chen, G. Displacement-type quartz crystal microbalance immunosensing platform for ultrasensitive monitoring of small molecular toxins. Anal. Chem. 2013, 85, 6958–6966. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Pan, Y.; Fang, H.; Guo, M.; Nie, Z.; Huang, Y.; Yao, S. An aptamer-based quartz crystal microbalance biosensor for sensitive and selective detection of leukemia cells using silver-enhanced gold nanoparticle label. Talanta 2014, 126, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Guo, X.; Sun, W.; Yin, W.; He, P.; Yang, X.; Zhang, X. Target-triggering multiple-cycle signal amplification strategy for ultrasensitive detection of DNA based on QCM and SPR. Anal. Biochem. 2018, 553, 57–61. [Google Scholar] [CrossRef] [PubMed]
- García, M.G.; García, A.C. Adsorptive stripping voltammetric behaviour of colloidal gold and immunogold on carbon paste electrode. Biosens. Bioelectron. 1995, 38, 389–395. [Google Scholar]
- Ozsoz, M.; Erdem, A.; Kerman, K.; Ozkan, D.; Tugrul, B.; Topcuoglu, N.; Ekren, H.; Taylan, M. Electrochemical genosensor based on colloidal gold nanoparticles for the detection of Factor V Leiden mutation using disposable pencil graphite electrodes. Anal. Chem. 2003, 75, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.S.; Pérez-López, B.; Faria, R.C.; Mattoso, L.H.; Hernández-Herrero, M.; Roig-Sagués, A.X.; Costa, M.M.; Merkoçi, A. Electrochemical detection of Salmonella using gold nanoparticles. Biosens. Bioelectron. 2013, 40, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Feng, W.; Lin, L.; Zhang, F.; Cheng, G.; He, P.; Fang, Y. A new amplification strategy for ultrasensitive electrochemical aptasensor with network-like thiocyanuric acid/gold nanoparticles. Biosens. Bioelectron. 2007, 23, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M.; Castaneda, M.T.; Pividori, M.I.; Eritja, R.; Merkoçi, A.; Alegret, S. Magnetically trigged direct electrochemical detection of DNA hybridization using Au67 quantum dot as electrical tracer. Langmuir 2005, 21, 9625–9629. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Castañeda, M.T.; Killard, A.J.; Smyth, M.R.; Alegret, S.; Merkoçi, A. Double-codified gold nanolabels for enhanced immunoanalysis. Anal. Chem. 2007, 79, 5232–5240. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.Y.; Wu, H.; Wee, E.J.; Trau, M.; Wang, Y.; Botella, J.R. Specific and sensitive isothermal electrochemical biosensor for plant pathogen DNA detection with colloidal gold nanoparticles as probes. Sci. Rep. 2017, 7, 38896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Alocilja, E.C. Gold nanoparticle-labeled biosensor for rapid and sensitive detection of bacterial pathogens. J. Biol. Eng. 2015, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Rochelet-Dequaire, M.; Limoges, B.; Brossier, P. Subfemtomolar electrochemical detection of target DNA by catalytic enlargement of the hybridized gold nanoparticle labels. Analyst 2006, 131, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Authier, L.; Grossiord, C.; Brossier, P. Gold nanoparticle-based quantitative electrochemical detection of amplified human cytomegalovirus DNA using disposable microband electrodes. Anal. Chem. 2001, 73, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Kerman, K.; Morita, Y.; Takamura, Y.; Ozsoz, M.; Tamiya, E. Modification of Escherichia coli single-stranded DNA binding protein with gold nanoparticles for electrochemical detection of DNA hybridization. Anal. Chim. Acta 2004, 510, 169–174. [Google Scholar] [CrossRef]
- Ng, B.Y.; Xiao, W.; West, N.P.; Wee, E.J.; Wang, Y.; Trau, M. Rapid, single-cell electrochemical detection of Mycobacterium tuberculosis using colloidal gold nanoparticles. Anal. Chem. 2015, 87, 10613–10618. [Google Scholar] [CrossRef] [PubMed]
- Ilkhani, H.; Sarparast, M.; Noori, A.; Bathaie, S.Z.; Mousavi, M.F. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosens. Bioelectron. 2015, 74, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xu, A.; Liu, L.; Deng, W.; Chen, C.; Tan, Y.; Fu, Y.; Xie, Q.; Yao, S. Ultrasensitive electrochemical immunoassay of proteins based on in situ duple amplification of gold nanoparticle biolabel signals. Chem. Commun. 2015, 51, 8540–8543. [Google Scholar] [CrossRef] [PubMed]
- López-Marzo, A.M.; Hoyos-de-la-Torre, R.; Baldrich, E. NaNO3/NaCl Oxidant and Polyethylene Glycol (PEG) Capped Gold Nanoparticles (AuNPs) as a Novel Green Route for AuNPs Detection in Electrochemical Biosensors. Anal. Chem. 2018, 90, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.R.; Fox, A.P.; Natan, M.J. Morphology-dependent electrochemistry of cytochrome c at Au colloid-modified SnO2 electrodes. J. Am. Chem. Soc. 1996, 118, 1154–1157. [Google Scholar] [CrossRef]
- Heydari-Bafrooei, E.; Shamszadeh, N.S. Electrochemical bioassay development for ultrasensitive aptasensing of prostate specific antigen. Biosens. Bioelectron. 2017, 91, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ma, C.; Li, Y.; Liu, B.; Tan, L. One-pot preparation of conducting composite containing abundant amino groups on electrode surface for electrochemical detection of von willebrand factor. Appl. Surf. Sci. 2018, 433, 847–854. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Sun, Y.; Wang, L.; Dong, X.; Ren, J.; He, W.; Xiao, F. Flexible nanohybrid microelectrode based on carbon fiber wrapped by gold nanoparticles decorated nitrogen doped carbon nanotube arrays: In situ electrochemical detection in live cancer cells. Biosens. Bioelectron. 2018, 100, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Vural, T.; Yaman, Y.T.; Ozturk, S.; Abaci, S.; Denkbas, E.B. Electrochemical immunoassay for detection of prostate specific antigen based on peptide nanotube-gold nanoparticle-polyaniline immobilized pencil graphite electrode. J. Colloid Interface Sci. 2018, 510, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Del Caño, R.; Mateus, L.; Sánchez-Obrero, G.; Sevilla, J.M.; Madueno, R.; Blazquez, M.; Pineda, T. Hemoglobin becomes electroactive upon interaction with surface-protected Au nanoparticles. Talanta 2018, 176, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, Z. New immunoprobes based on bovine serum albumin-stabilized copper nanoclusters with triple signal amplification for ultrasensitive electrochemical immunosensing for tumor marker. Sens. Actuators B Chem. 2017, 241, 849–854. [Google Scholar] [CrossRef]
- Bao, J.; Geng, X.; Hou, C.; Zhao, Y.; Huo, D.; Wang, Y.; Wang, Z.; Zeng, Y.; Yang, M.; Fa, H. A simple and universal electrochemical assay for sensitive detection of DNA methylation, methyltransferase activity and screening of inhibitors. J. Electroanal. Chem. 2018, 814, 144–152. [Google Scholar] [CrossRef]
- Jarocka, U.; Sawicka, R.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecki, J.; Radecka, H. An immunosensor based on antibody binding fragments attached to gold nanoparticles for the detection of peptides derived from avian influenza hemagglutinin H5. Sensors 2014, 14, 15714–15728. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, D.; Dong, H.; Song, S.; Koh, K.; Chen, H. para-Sulfonatocalix[4]arene stabilized gold nanoparticles multilayers interfaced to electrodes through host-guest interaction for sensitive ErbB2 detection. Biosens. Bioelectron. 2018, 99, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hua, E.; Liang, M.; Ma, C.; Liu, Z.; Sheng, S.; Liu, M.; Xie, G.; Feng, W. Graphene sheets, polyaniline and AuNPs based DNA sensor for electrochemical determination of BCR/ABL fusion gene with functional hairpin probe. Biosens. Bioelectron. 2014, 51, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.S.; Zhu, X.F.; Xu, J.K.; Lu, L.M.; Wang, W.M.; Yang, T.T.; Xing, H.K.; Yu, Y.F. Label-free electrochemical immunosensor based on Nile blue A-reduced graphene oxide nanocomposites for carcinoembryonic antigen detection. Anal. Biochem. 2016, 500, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.L.; Huang, K.J.; Xing, L.L.; Chen, Y.X. Ultrasensitive electrochemical sensing platform for microRNA based on tungsten oxide-graphene composites coupling with catalyzed hairpin assembly target recycling and enzyme signal amplification. Biosens. Bioelectron. 2016, 86, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yuan, R.; Chai, Y.; Zhuo, Y.; Yuan, Y.; Wang, Y. Simultaneous electrochemical detection of multiple analytes based on dual signal amplification of single-walled carbon nanotubes and multi-labeled graphene sheets. Biomaterials 2012, 33, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, L.; Hua, E.; Jiang, S.; Xie, G. Detection of the human prostate-specific antigen using an aptasensor with gold nanoparticles encapsulated by graphitized mesoporous carbon. Microchim. Acta 2012, 178, 163–170. [Google Scholar] [CrossRef]
- Jeong, B.; Akter, R.; Han, O.H.; Rhee, C.K.; Rahman, M.A. Increased electrocatalyzed performance through dendrimer-encapsulated gold nanoparticles and carbon nanotube-assisted multiple bienzymatic labels: Highly sensitive electrochemical immunosensor for protein detection. Anal. Chem. 2013, 85, 1784–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, J.; Ma, H.; Jiang, Y.; Huang, C.; Han, E.; Yao, B.; He, Y. Optimized dendrimer-encapsulated gold nanoparticles and enhanced carbon nanotube nanoprobes for amplified electrochemical immunoassay of E. coli in dairy product based on enzymatically induced deposition of polyaniline. Biosens. Bioelectron. 2016, 80, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Riskin, M.; Tel-Vered, R.; Bourenko, T.; Granot, E.; Willner, I. Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: Development of an electrochemical TNT sensor based on π-donor−acceptor interactions. J. Am. Chem. Soc. 2008, 130, 9726–9733. [Google Scholar] [CrossRef] [PubMed]
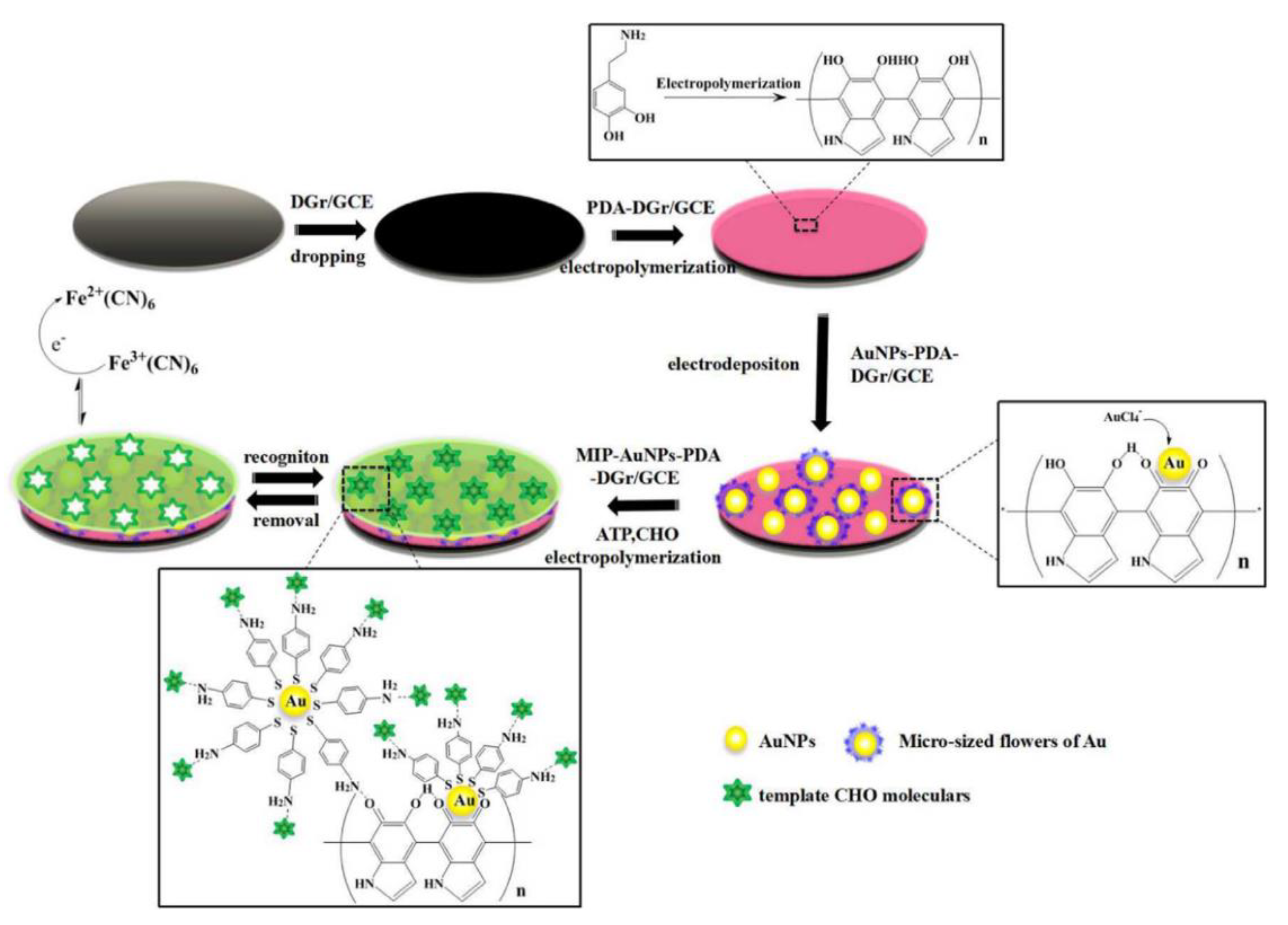
- Yang, H.; Li, L.; Ding, Y.; Ye, D.; Wang, Y.; Cui, S.; Liao, L. Molecularly imprinted electrochemical sensor based on bioinspired Au microflowers for ultra-trace cholesterol assay. Biosens. Bioelectron. 2017, 92, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lu, J.; Zhong, Y.; Yu, Y.; Wang, Y.; Zhang, B.; Chen, Z. Sensitive electrochemical aptamer cytosensor for highly specific detection of cancer cells based on the hybrid nanoelectrocatalysts and enzyme for signal amplification. Biosens. Bioelectron. 2016, 75, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Baca, A.J.; Hu, J.; Zhou, F.; Yan, W.; Pang, D.W. Amplified voltammetric detection of DNA hybridization via oxidation of ferrocene caps on gold nanoparticle/streptavidin conjugates. Anal. Chem. 2003, 75, 3941–3945. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Wang, W.; Kang, T.; Liu, J.; Shiu, K.K.; Leung, C.H.; Ma, D.L. Ultrasensitive electrochemical detection of miRNA-21 by using an iridium(III) complex as catalyst. Biosens. Bioelectron. 2016, 86, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Lan, D.; Li, X.; Zhang, S. Electrochemical DNA biosensor based on nanoporous gold electrode and multifunctional encoded DNA−Au bio bar codes. Anal. Chem. 2008, 80, 9124–9130. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Rong, X.; Wang, Y.; Ding, S.; Tang, W. High-performance and versatile electrochemical aptasensor based on self-supported nanoporous gold microelectrode and enzyme-induced signal amplification. Biosens. Bioelectron. 2018, 102, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Liu, F.; Zhang, Y.; Zhan, T.; He, Y.; Hun, X. Signal amplification technology based on entropy-driven molecular switch for ultrasensitive electrochemical determination of DNA and Salmonella typhimurium. Sens. Actuators B Chem. 2016, 225, 420–427. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Li, H.; Wen, Y.; Fan, X.; Lin, F.; Tan, L.; Yao, S. Ultrasensitive electrochemical aptasensor for thrombin based on the amplification of aptamer–AuNPs–HRP conjugates. Biosens. Bioelectron. 2011, 26, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yin, H.; Li, X.; Li, Z.; Ai, S.; Lin, H. Electrochemical biosensor for protein kinase A activity assay based on gold nanoparticles-carbon nanospheres, phos-tag-biotin and β-galactosidase. Biosens. Bioelectron. 2016, 86, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, N.; Liu, H.; Kang, X.; Liu, X.; Xue, C.; He, X. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Huang, K.J.; Lin, F.; Fang, L.X. Ultrasensitive electrochemical sensing platform based on graphene wrapping SnO2 nanocorals and autonomous cascade DNA duplication strategy. Talanta 2017, 175, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Zhang, H.; Meng, X.; Ai, S. Electrochemical determination of microRNA-21 based on graphene, LNA integrated molecular beacon, AuNPs and biotin multifunctional bio bar codes and enzymatic assay system. Biosens. Bioelectron. 2012, 33, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Zhu, C.; Wu, S.; Mandler, D.; Marks, R.S.; Zhang, H. Amplified detection of femtomolar DNA based on a one-to-few recognition reaction between DNA–Au conjugate and target DNA. Nanoscale 2014, 6, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Wen, W.; Xiong, H.; Zhang, X.; Wang, S. Novel electrochemical aptamer biosensor based on gold nanoparticles signal amplification for the detection of carcinoembryonic antigen. Electrochem. Commun. 2013, 37, 15–19. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Babaie, P.; Mokhtarzadeh, A.; Hajizadeh, N.; Mahboob, S. A novel DNA based bioassay toward ultrasensitive detection of Brucella using gold nanoparticles supported histidine: A new platform for the assay of bacteria in the cultured and human biofluids with and without polymerase chain reactions (PCR). Int. J. Biol. Macromol. 2018, 120, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, K.; Yin, H.; Zhou, Y.; Ai, S. Aptamer based voltammetric determination of ampicillin using a single-stranded DNA binding protein and DNA functionalized gold nanoparticles. Microchim. Acta 2018, 185, 68. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, P.; Su, K.; Li, X.; Qin, Z.; Xu, W.; Chen, J.; Li, C.; Qiu, J. Electrochemical aptasensor for thrombin using co-catalysis of hemin/G-quadruplex DNAzyme and octahedral Cu2O-Au nanocomposites for signal amplification. Biosens. Bioelectron. 2018, 99, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Li, J.J.; Rui, K.; Gai, P.P.; Zhang, J.R.; Zhu, J.J. Sensitive electrochemical detection of telomerase activity using spherical nucleic acids gold nanoparticles triggered mimic-hybridization chain reaction enzyme-free dual signal amplification. Anal. Chem. 2015, 87, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
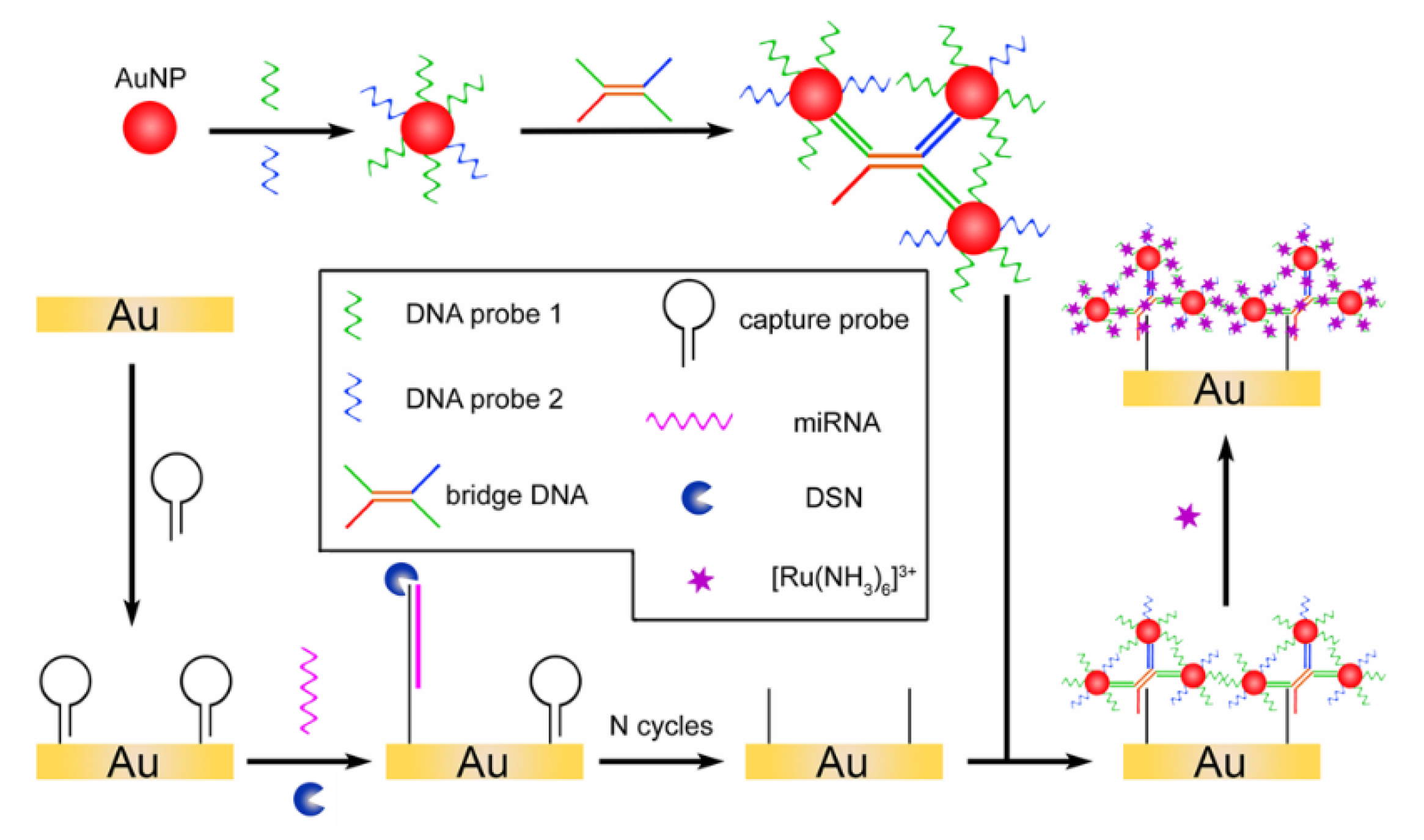
- Bo, B.; Zhang, T.; Jiang, Y.; Cui, H.; Miao, P. Triple Signal Amplification Strategy for Ultrasensitive Determination of miRNA Based on Duplex Specific Nuclease and Bridge DNA–Gold Nanoparticles. Anal. Chem. 2018, 90, 2395–2400. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, Y.; Jiang, L.P.; Bi, S.; Zhu, J.J. Cascade amplification-mediated in situ hot-spot assembly for microRNA detection and molecular logic gate operations. Anal. Chem. 2018, 90, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
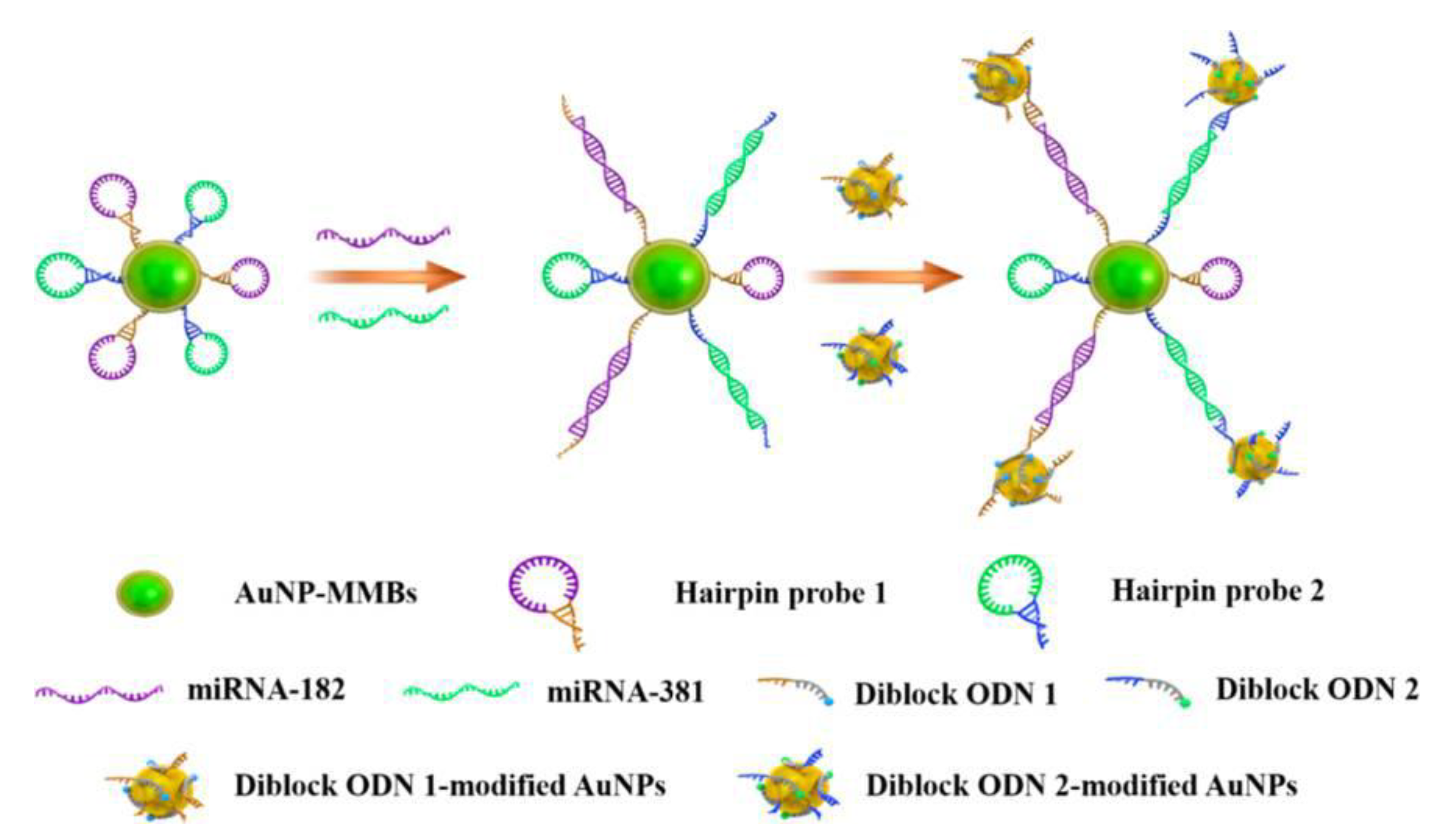
- Wang, J.; Lu, Z.; Tang, H.; Wu, L.; Wang, Z.; Wu, M.; Yi, X.; Wang, J. Multiplexed electrochemical detection of MiRNAs from sera of glioma patients at different stages via the novel conjugates of conducting magnetic microbeads and Diblock oligonucleotide-modified gold nanoparticles. Anal. Chem. 2017, 89, 10834–10840. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Maye, M.M.; Han, L.; Luo, J.; Zhong, C.J. Gold–platinum alloy nanoparticle assembly as catalyst for methanol electrooxidation. Chem. Commun. 2001, 0, 473–474. [Google Scholar] [CrossRef]
- Valden, M.; Lai, X.; Goodman, D.W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 1998, 281, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- El-Deab, M.S.; Okajima, T.; Ohsaka, T. Electrochemical reduction of oxygen on gold nanoparticle-electrodeposited glassy carbon electrodes. J. Electrochem. Soc. 2003, 150, A851–A857. [Google Scholar] [CrossRef]
- De la Escosura-Muñiz, A.; Baptista-Pires, L.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. Magnetic Bead/Gold Nanoparticle Double-Labeled Primers for Electrochemical Detection of Isothermal Amplified Leishmania DNA. Small 2016, 12, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yang, L.; Yu, J.; Wang, L.; Liu, H. Host-guest Interaction Induced Rapid Self-assembled Fe3O4@Au Nanoparticles with High Catalytic Activity. Ind. Eng. Chem. Res. 2018, 57, 9448–9456. [Google Scholar] [CrossRef]
- Zheng, X.; Li, L.; Cui, K.; Zhang, Y.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive Enzyme-free Biosensor by Coupling Cyclodextrin Functionalized Au Nanoparticles and High-Performance Au-Paper Electrode. ACS Appl. Mater. Interfaces 2018, 10, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Liu, Y.; Zhong, J.; Zhang, Z.; Zhao, X.; Liu, X.; Jiang, Y.; Zou, P.; Wang, X.; Wang, Y. Gold nanoparticle/chitosan@N,S co-doped multiwalled carbon nanotubes sensor: Fabrication, characterization, and electrochemical detection of catechol and nitrite. ACS Sustain. Chem. Eng. 2017, 5, 10926–10939. [Google Scholar] [CrossRef]
- Jena, B.K.; Raj, C.R. Electrochemical biosensor based on integrated assembly of dehydrogenase enzymes and gold nanoparticles. Anal. Chem. 2006, 78, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Han, J.; Jiang, L.; Wang, Y.; Li, Y.; Dong, Y.; Wei, Q. An ultrasensitive sandwich-type electrochemical immunosensor based on signal amplification strategy of gold nanoparticles functionalized magnetic multi-walled carbon nanotubes loaded with lead ions. Biosens. Bioelectron. 2015, 68, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Xu, J.; Xia, J.; Zhang, F.; Wang, Z. An electrochemical aptasensor based on the conversion of liquid-phase colorimetric assay into electrochemical analysis for sensitive detection of lysozyme. Sens. Actuators B Chem. 2018, 255, 2136–2142. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Tang, L.; Li, J. Electrochemical DNA sensor by the assembly of graphene and DNA-conjugated gold nanoparticles with silver enhancement strategy. Analyst 2011, 136, 4732–4737. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, J.; Wang, M.; Yan, F.; Ju, H. Triple signal amplification of graphene film, polybead carried gold nanoparticles as tracing tag and silver deposition for ultrasensitive electrochemical immunosensing. Anal. Chem. 2012, 84, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Min, I.H.; Choi, L.; Ahn, K.S.; Kim, B.K.; Lee, B.Y.; Kim, K.S.; Choi, H.N.; Lee, W.Y. Electrochemical determination of carbohydrate-binding proteins using carbohydrate-stabilized gold nanoparticles and silver enhancement. Biosens. Bioelectron. 2010, 26, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, Y.; He, P.; Fang, Y. Electrochemical detection of DNA hybridization based on silver-enhanced gold nanoparticle label. Anal. Chim. Acta 2002, 469, 165–172. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Polsky, R. Magnetically-induced solid-state electrochemical detection of DNA hybridization. J. Am. Chem. Soc. 2002, 124, 4208–4209. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Xu, Y.; Zhu, N.; He, P.; Fang, Y. An electrochemical DNA hybridization detection assay based on a silver nanoparticle label. Analyst 2002, 127, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Polsky, R.; Xu, D. Silver-enhanced colloidal gold electrochemical stripping detection of DNA hybridization. Langmuir 2001, 17, 5739–5741. [Google Scholar] [CrossRef]
- Pan, Y.; Shan, W.; Fang, H.; Guo, M.; Nie, Z.; Huang, Y.; Yao, S. Sensitive and visible detection of apoptotic cells on Annexin-V modified substrate using aminophenylboronic acid modified gold nanoparticles (APBA-GNPs) labeling. Biosens. Bioelectron. 2014, 52, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Yan, F.; Wu, J.; Leng, C.; Ju, H. Ultrasensitive multiplexed immunoassay with electrochemical stripping analysis of silver nanoparticles catalytically deposited by gold nanoparticles and enzymatic reaction. Anal. Chem. 2011, 83, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiong, Z.; Chen, Z. Ultrasensitive electrochemical microcystin-LR immunosensor using gold nanoparticle functional polypyrrole microsphere catalyzed silver deposition for signal amplification. Sens. Actuators B Chem. 2017, 246, 623–630. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, Q.; Liu, Q.; Li, Y.; Liu, H.; Wang, P.; Chen, L.; Zhang, D.; Li, Y.; Dong, Y. An ultrasensitive sandwich-type electrochemical immunosensor based on the signal amplification strategy of echinoidea-shaped Au@Ag-Cu2O nanoparticles for prostate specific antigen detection. Biosens. Bioelectron. 2018, 99, 450–457. [Google Scholar] [CrossRef] [PubMed]
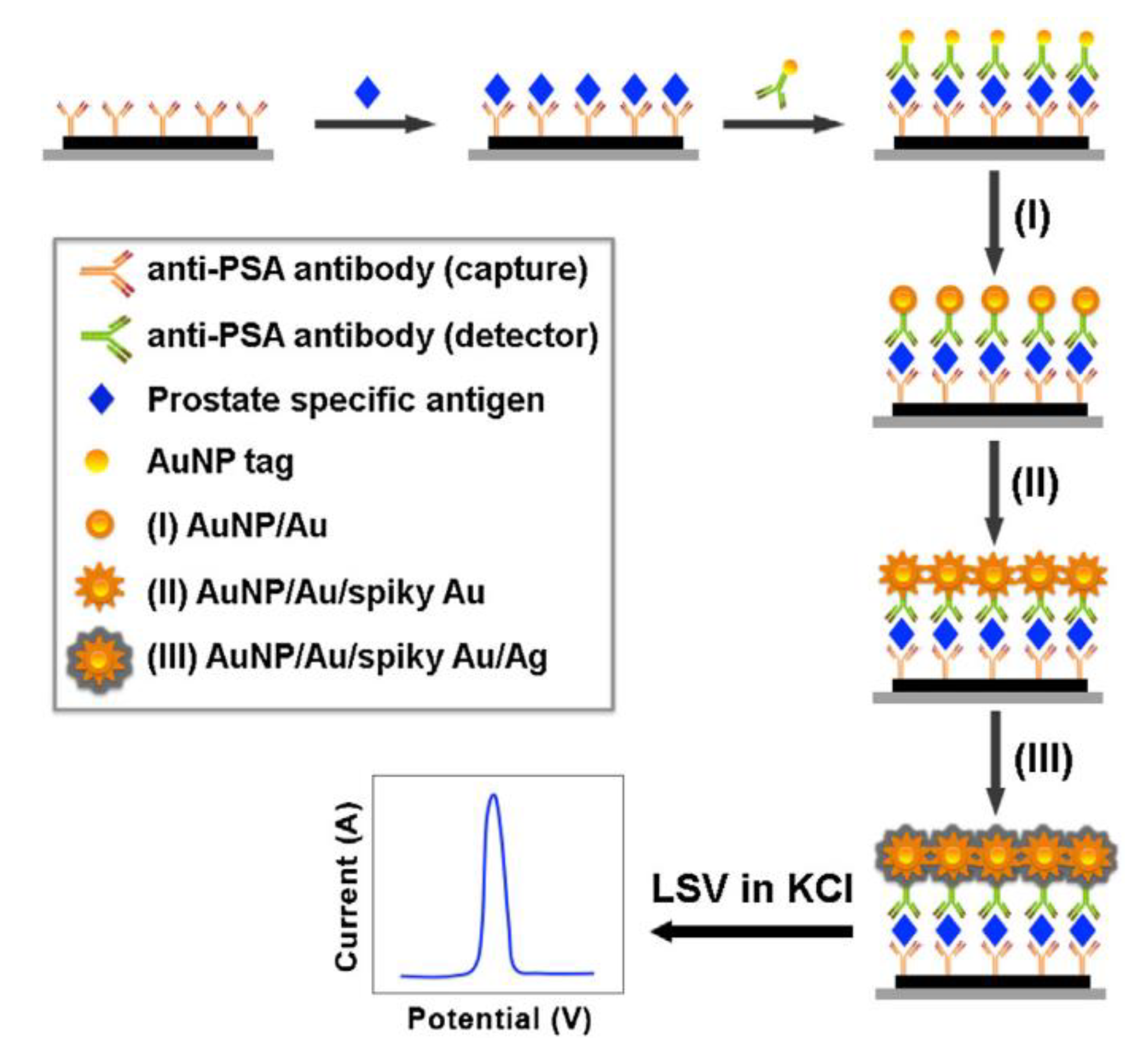
- Duangkaew, P.; Wutikhun, T.; Laocharoensuk, R. Triple signal amplification strategy based on size and shape transformation of ultrasmall sub-10 nm gold nanoparticles tag towards sensitivity improvement of electrochemical immunosensors. Sens. Actuators B Chem. 2017, 239, 430–437. [Google Scholar] [CrossRef]
- Houk, R.S.; Fassel, V.A.; Flesch, G.D.; Svec, H.J.; Gray, A.L.; Taylor, C.E. Inductively coupled argon plasma as an ion source for mass spectrometric determination of trace elements. Anal. Chem. 1980, 52, 2283–2289. [Google Scholar] [CrossRef]
- Yuan, H.; Gao, S.; Liu, X.; Li, H.; Günther, D.; Wu, F. Accurate U-Pb age and trace element determinations of zircon by laser ablation-inductively coupled plasma-mass spectrometry. Geostand. Geoanal. Res. 2004, 28, 353–370. [Google Scholar] [CrossRef]
- Jenner, G.A.; Longerich, H.P.; Jackson, S.E.; Fryer, B.J. ICP-MS—A powerful tool for high-precision trace-element analysis in earth sciences: Evidence from analysis of selected USGS reference samples. Chem. Geol. 1990, 83, 133–148. [Google Scholar] [CrossRef]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U-Pb zircon geochronology Fryer. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Becker, J.S.; Zoriy, M.; Matusch, A.; Wu, B.; Salber, D.; Palm, C.; Becker, J.S. Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom. Rev. 2010, 29, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Jarujamrus, P.; Chawengkirttikul, R.; Shiowatana, J.; Siripinyanond, A. Towards chloramphenicol detection by inductively coupled plasma mass spectrometry (ICP-MS) linked immunoassay using gold nanoparticles (AuNPs) as element tags. Anal. At. Spectrom. 2012, 27, 884–890. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Yang, L.; Du, G.; Pai-Panandiker, A.S.; Huang, X.; Yan, B. Enhancement of cell recognition in vitro by dual-ligand cancer targeting gold nanoparticles. Biomaterials 2011, 32, 2540–2545. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hamme II, A.T. Gold nanoparticle labeling based ICP-MS detection/measurement of bacteria, and their quantitative photothermal destruction. Mater. Chem. B 2015, 3, 3573–3582. [Google Scholar] [CrossRef] [PubMed]
- Dersch, J.M.; Nguyen, T.T.; Østergaard, J.; Stürup, S.; Gammelgaard, B. Selective analysis of human serum albumin based on SEC-ICP-MS after labelling with iophenoxic acid. Anal. Bioanal. Chem. 2015, 407, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Jeong, A.; Lim, H.B. Magnetophoretic separation ICP-MS immunoassay using Cs-doped multicore magnetic nanoparticles for the determination of salmonella typhimurium. Talanta 2018, 178, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortes, M.; Encinar, J.R.; Costa-Fernandez, J.M.; Sanz-Medel, A. Highly sensitive nanoparticle-based immunoassays with elemental detection: Application to Prostate-Specific Antigen quantification. Biosens. Bioelectron. 2016, 85, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yan, X.; Huang, Y.; Wen, R.; Li, Z.; Yang, L.; Yang, C.J.; Wang, Q. ICP-MS-based multiplex and ultrasensitive assay of viruses with lanthanide-coded biospecific tagging and amplification strategies. Anal. Chem. 2013, 85, 9428–9432. [Google Scholar] [CrossRef] [PubMed]
- Merkoçi, A.; Aldavert, M.; Tarrasón, G.; Eritja, R.; Alegret, S. Toward an ICPMS-linked DNA assay based on gold nanoparticles immunoconnected through peptide sequences. Anal. Chem. 2005, 77, 6500–6503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Z.; Yu, B.; Shi, J.; Zhang, X. Application of the biological conjugate between antibody and colloid Au nanoparticles as analyte to inductively coupled plasma mass spectrometry. Anal. Chem. 2002, 74, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Quinn, Z.A.; Baranov, V.I.; Tanner, S.D.; Wrana, J.L. Simultaneous determination of proteins using an element-tagged immunoassay coupled with ICP-MS detection. J. Anal. At. Spectrom. 2002, 17, 892–896. [Google Scholar] [CrossRef]
- Hsu, I.H.; Chen, W.H.; Wu, T.K.; Sun, Y.C. Gold nanoparticle-based inductively coupled plasma mass spectrometry amplification and magnetic separation for the sensitive detection of a virus-specific RNA sequence. J. Chromatogr. A 2011, 1218, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, B.; He, M.; Zhang, Y.; Xiao, G.; Hu, B. Magnetic immunoassay coupled with inductively coupled plasma mass spectrometry for simultaneous quantification of alpha-fetoprotein and carcinoembryonic antigen in human serum. Spectrochim. Acta B 2015, 106, 20–27. [Google Scholar] [CrossRef]
- He, Q.; Zhu, Z.; Jin, L.; Peng, L.; Guo, W.; Hu, S. Detection of HIV-1 p24 antigen using streptavidin–biotin and gold nanoparticles based immunoassay by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2014, 29, 1477–1482. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; He, M.; Zhang, Y.; Peng, L.; Hu, B. Boronic acid recognition based-gold nanoparticle-labeling strategy for the assay of sialic acid expression on cancer cell surface by inductively coupled plasma mass spectrometry. Analyst 2016, 141, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xi, Z.; Zeng, X.; Fang, L.; Jiang, W.; Wu, Y.; Xu, L.; Fu, F. Magnetic bead-based AuNP labelling combined with inductively coupled plasma mass spectrometry for sensitively and specifically counting cancer cells. J. Anal. At. Spectrom. 2016, 31, 679–685. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; He, M.; Xiao, G.; Hu, B. Gold nanoparticle labeling with tyramide signal amplification for highly sensitive detection of alpha fetoprotein in human serum by ICP-MS. Talanta 2018, 176, 40–46. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, D.; Li, M.; Fang, L.; Yang, W.; Xu, L.; Fu, F. Rolling circle amplification combined with gold nanoparticles-tag for ultra sensitive and specific quantification of DNA by inductively coupled plasma mass spectrometry. Biosens. Bioelectron. 2014, 58, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, B.; He, M.; Wang, H.; Hu, B. Gold nanoparticles labeling with hybridization chain reaction amplification strategy for the sensitive detection of HepG2 cells by inductively coupled plasma mass spectrometry. Biosens. Bioelectron. 2016, 86, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Luo, J.; Zhang, N.B.; Wei, Q.L. Nucleic acid quantification using nicking–displacement, rolling circle amplification and bio-bar-code mediated triple-amplification. Anal. Chim. Acta 2015, 881, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Li, Y.; Jiang, Y.; Yan, X.P. Gold nanoparticles amplified ultrasensitive quantification of human urinary protein by capillary electrophoresis with on-line inductively coupled plasma mass spectroscopic detection. J. Proteome Res. 2010, 9, 3545–3550. [Google Scholar] [CrossRef] [PubMed]
- Degueldre, C.; Favarger, P.Y.; Wold, S. Gold colloid analysis by inductively coupled plasma-mass spectrometry in a single particle mode. Anal. Chim. Acta 2006, 555, 263–268. [Google Scholar] [CrossRef]
- Degueldre, C.; Favarger, P.Y. Thorium colloid analysis by single particle inductively coupled plasma-mass spectrometry. Talanta 2004, 62, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Beermann, B.; Carrillo-Nava, E.; Scheffer, A.; Buscher, W.; Jawalekar, A.M.; Seela, F.; Hinz, H.J. Association temperature governs structure and apparent thermodynamics of DNA–gold nanoparticles. Biophys. Chem. 2007, 126, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, A.; Brüchert, W.; Bettmer, J. Size characterisation of Au nanoparticles by ICP-MS coupling techniques. J. Anal. At. Spectrom. 2006, 21, 431–434. [Google Scholar] [CrossRef]
- Bao, D.; Oh, Z.G.; Chen, Z. Characterization of silver nanoparticles internalized by Arabidopsis plants using single particle ICP-MS analysis. Front. Plant. Sci. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, N.; Ceylan, H.; Guler, M.O.; Tekinay, A.B. Intracellular accumulation of gold nanoparticles leads to inhibition of macropinocytosis to reduce the endoplasmic reticulum stress. Sci. Rep. 2017, 7, 40493. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Sikora, K.N.; Tang, R.; Liu, Y.; Lee, Y.W.; Kim, S.T.; Jiang, Z.; Vachet, R.W.; Rotello, V.M.; Rotello, V.M. Quantitative differentiation of cell surface-bound and internalized cationic gold nanoparticles using mass spectrometry. ACS Nano 2016, 10, 6731–6736. [Google Scholar] [CrossRef] [PubMed]
- Tavares, A.J.; Poon, W.; Zhang, Y.N.; Dai, Q.; Besla, R.; Ding, D.; Ouyang, B.; Li, A.; Chen, J.; Zheng, J.; et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl. Acad. Sci. USA 2017, 114, E10871–E10880. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Min, K.H.; Wang, Z.; Kim, J.; Jacobson, O.; Huang, P.; Zhu, G.; Liu, Y.; Yung, B.; Niu, G.; et al. Development of Sialic Acid-coated Nanoparticles for Targeting Cancer and Efficient Evasion of the Immune System. Theranostics 2017, 7, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Zazo, H.; Colino, C.I.; Warzecha, K.T.; Hoss, M.; Gbureck, U.; Trautwein, C.; Tacke, F.; Lanao, J.M.; Bartneck, M. Gold Nanocarriers for Macrophage-Targeted Therapy of Human Immunodeficiency Virus. Macromol. Biosci. 2017, 17, 1600359. [Google Scholar] [CrossRef] [PubMed]
- Allabashi, R.; Stach, W.; de la Escosura-Muñiz, A.; Liste-Calleja, L.; Merkoçi, A. ICP-MS: A powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J. Nanopart. Res. 2009, 11, 2003. [Google Scholar] [CrossRef]
- Liu, J.; Murphy, K.E.; MacCuspie, R.I.; Winchester, M.R. Capabilities of single particle inductively coupled plasma mass spectrometry for the size measurement of nanoparticles: A case study on gold nanoparticles. Anal. Chem. 2014, 86, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Xing, Z.; Dong, Y.; Zhang, S.; Zhang, X. One-step homogeneous DNA assay with single-nanoparticle detection. Angew. Chem. 2011, 123, 3524–3527. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, W.; Zhao, D.; Hao, Q.; Li, J.; Huang, J.; Shi, J.; Chao, J.; Su, S.; Wang, L. Label-Free Electrochemical Sensing Platform for MicroRNA-21 Detection Using Thionine and Gold Nanoparticles Co-Functionalized MoS2 Nanosheet. ACS. Appl. Mater. Interfaces 2017, 9, 35597–35603. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jiang, P.; Chen, Z.; Nie, L. Magnetic Fe3O4@Mesoporous Silica Composite Microspheres: Synthesis and Biomedical Applications. Nanosci. Nanotechnol. Lett. 2017, 9, 1849–1860. [Google Scholar] [CrossRef]
- Yang, B.; Chen, B.; He, M.; Yin, X.; Xu, C.; Hu, B. Aptamer-Based Dual-Functional Probe for Rapid and Specific Counting and Imaging of MCF-7 Cells. Anal. Chem. 2018, 90, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Liu, F.; Ma, P.; Xiao, X. Applications of gold nanoparticles in optical biosensors. J. Biomed. Nanotechnol. 2014, 10, 2700–2721. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Jia, H.; Liu, Z.; Qiu, X.; Gao, Y.; Xu, J.; Lu, L.; Yu, Y. Label-free electrochemical immunoassay for α-fetoprotein based on a redox matrix of Prussian blue-reduced graphene oxide/gold nanoparticles-poly(3,4-ethylenedioxythiophene) composite. J. Electroanal. Chem. 2017, 799, 625–633. [Google Scholar] [CrossRef]
- Nie, L.; Chen, Z.; Zou, H.; Chang, H. Development of flowing automatic quartz crystal microbalance system for DNA detection. J. Nanosci. Nanotechnol. 2013, 13, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, P.H.; Xu, W.H.; Wei, Y.; Li, L.N.; Huang, Y.Y.; Sun, Y.F.; Chen, X.; Liu, J.H.; Huang, X.J. Reliable electrochemical sensing arsenic(III) in nearly groundwater pH based on efficient adsorption and excellent electrocatalytic ability of AuNPs/CeO2-ZrO2 nanocomposite. Sens. Actuators B Chem. 2018, 255, 226–234. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z. A cascade reaction signal-amplified amperometric immunosensor platform for ultrasensitive detection of tumour marker. Sens. Actuators B Chem. 2018, 254, 642–647. [Google Scholar] [CrossRef]
- Tian, L.; Qian, K.; Qi, J.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. ACS. Appl. Mater. Interfaces 2018, 99, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Liu, F.; Yang, H. Preparation of Quantum Dot Fluorescence Encoded Polystyrene Microbeads. Nanosci. Nanotechnol. Lett. 2017, 9, 941–944. [Google Scholar] [CrossRef]
- Su, S.; Sun, H.; Cao, W.; Chao, J.; Peng, H.; Zuo, X.; Yuwen, L.H.; Wang, L. Dual-target electrochemical biosensing based on DNA structural switching on gold nanoparticle-decorated MoS2 nanosheets. ACS. Appl. Mater. Interfaces 2016, 8, 6826–6833. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Xiao, X.; Yang, H. Preparation and Biomedical Applications of Gold Nanocluster. J. Nanosci. Nanotechnol. 2016, 16, 8164–8175. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Yang, H.C.; Jiang, P.F.; Chen, Z.; Ji, C.Y.; Nie, L.B. Multi-Functional Fe3O4@mSiO2-AuNCs Composite Nanoparticles Used as Drug Delivery System. J. Biomed. Nanotechnol. 2017, 13, 1292–1299. [Google Scholar] [CrossRef]
- Maciejewska-Prończuk, J.; Morga, M.; Adamczyk, Z.; Oćwieja, M.; Zimowska, M. Homogeneous gold nanoparticle monolayers-QCM and electrokinetic characteristics. Colloid Surf. A 2017, 514, 226–235. [Google Scholar] [CrossRef]

| Analytes a | Electrode Modification b | Functions of AuNPs | Detection Limits | Ref. |
|---|---|---|---|---|
| Mtb DNA | SPCE/SA | Electrochemical indicators | 1 CFU | [71] |
| EGFR | GCE | Electrochemical indicators | 50 pg/mL | [72] |
| hIgG, hPSA | GCE/MWCNT/AB | Electrochemical indicators | 0.3 fg/mL, 0.1 fg/mL | [73] |
| hMMP9 | SPCE/AB | Electrochemical indicators | 0.06 ng/mL | [74] |
| PSA | GCE/AuNPs/AB | Electron migration enhancers | 145.69 fg/mL | [81] |
| M.SssI MTase | GCE/AuNPs/CP | Electron migration enhancers | 0.04 U/mL | [82] |
| ErbB2 | GE/pSC4/HMD/AuNPs | Electron migration enhancers | 0.5 ng/mL | [84] |
| ssDNA | GCE/CS-GS/PANI/AuNPs/CP | Electron migration enhancers | 2.11 pM | [85] |
| CEA | GCE/NB-ERGO/AuNPs/AB | Electron migration enhancers | 1 pg/mL | [86] |
| MicroRNA | GCE/WO3-Gr/AuNPs/CP | Electron migration enhancers | 0.05 fM | [87] |
| PDGF, TB | GCE/SWCNTs@AuNPs/AB | Electron migration enhancers | 8 pM, 11 pM | [88] |
| PSA | PGE/GMCs@AuNPs/AB | Electron migration enhancers | 0.25 ng/mL | [89] |
| CEA | GE/Cys/AuNPs@PAMAM/Th/AB | Electron migration enhancers | 4.4 pg/mL | [90] |
| ssDNA | GE/CP | Immobilization platform | 50 fM | [105] |
| Ampicillin | GCE/AuNPs/Aptamer | Immobilization platform | 0.3 pM | [108] |
| TB | GCE/AuNPs/Aptamer | Immobilization platform | 23 fM | [109] |
| MiRNA | GE/CP | Immobilization platform | 6.8 aM | [111] |
| MiRNA-141 | GE/CP | Immobilization platform | 25.1 aM | [112] |
| MiRNA-182, MiRNA-381 | MGE | Immobilization platform | 0.2 fM, 0.12 fM | [113] |
| AFP | GCE/AuNPs/AB | Catalyst | 3.33 fg/mL | [122] |
| Lysozyme | GE/CP | Catalyst | 0.32 pM | [123] |
| Microcystin-LR | GCE/CNT/PEG | Catalyst | 0.1 ng/L | [133] |
| PSA | GCE/Au@N-GQDs/AB | Catalyst | 0.003 pg/mL | [134] |
| PSA | SPCE/CNT/AB | Catalyst | 1.2 pg/mL | [135] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of Gold Nanoparticles in Non-Optical Biosensors. Nanomaterials 2018, 8, 977. https://doi.org/10.3390/nano8120977
Jiang P, Wang Y, Zhao L, Ji C, Chen D, Nie L. Applications of Gold Nanoparticles in Non-Optical Biosensors. Nanomaterials. 2018; 8(12):977. https://doi.org/10.3390/nano8120977
Chicago/Turabian StyleJiang, Pengfei, Yulin Wang, Lan Zhao, Chenyang Ji, Dongchu Chen, and Libo Nie. 2018. "Applications of Gold Nanoparticles in Non-Optical Biosensors" Nanomaterials 8, no. 12: 977. https://doi.org/10.3390/nano8120977
APA StyleJiang, P., Wang, Y., Zhao, L., Ji, C., Chen, D., & Nie, L. (2018). Applications of Gold Nanoparticles in Non-Optical Biosensors. Nanomaterials, 8(12), 977. https://doi.org/10.3390/nano8120977






