Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flax Fabrics Pretreatment
2.3. Functionalization of Flax Fabrics with NPs
2.3.1. Green Synthesis and Deposition of AgNPs onto Flax Fabrics
2.3.2. In-Situ Synthesis of ZnONPs onto AgNPs-Treated Flax Fabrics
2.4. Flax Fabrics Samples Characterization
2.4.1. Ground State Diffuse Reflectance (GSDR)
2.4.2. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive Spectroscopy (EDS)
2.4.3. X-ray Diffraction (XRD)
2.4.4. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.4.5. Thermogravimetric Analysis (TGA)
2.5. Multifunctional Properties Evaluation
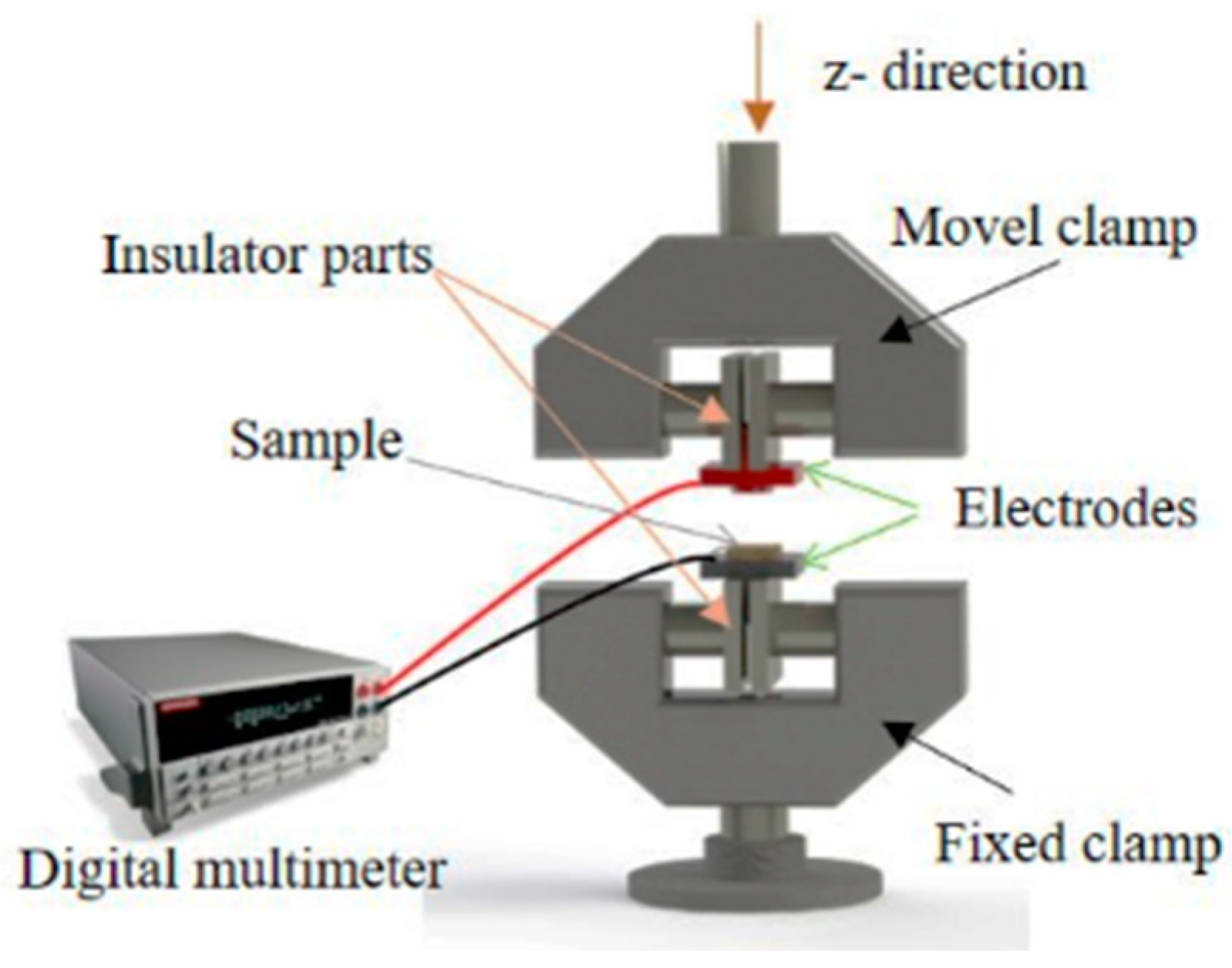
2.5.1. Resistivity Measurements and Electromechanical Characterization
2.5.2. Antibacterial Tests
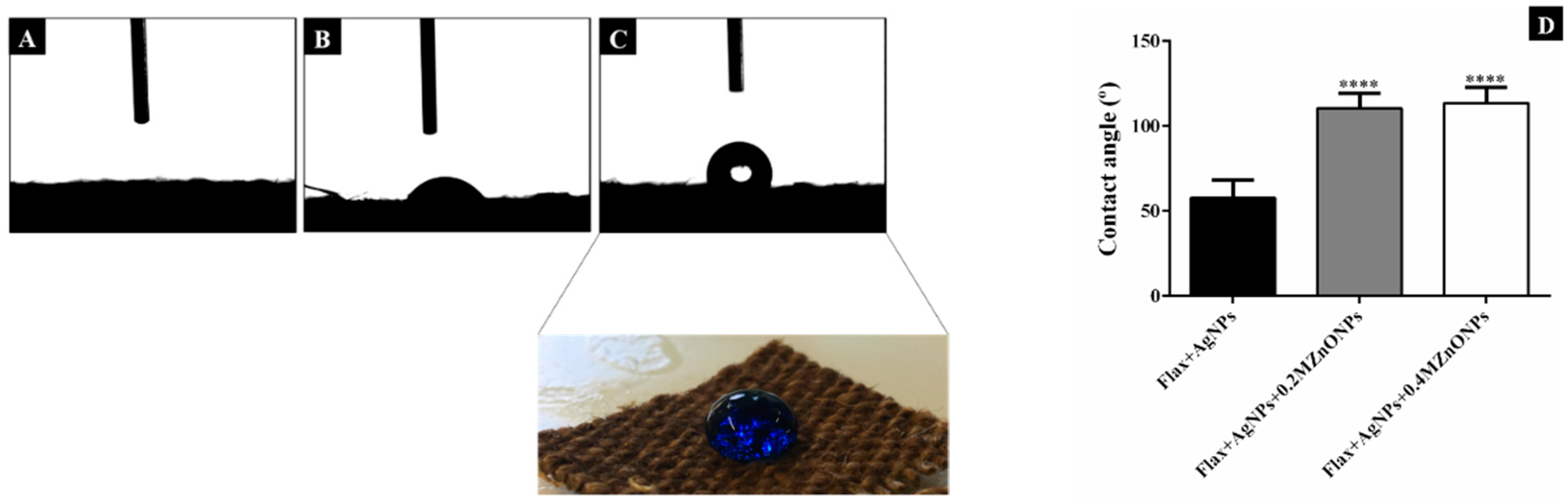
2.5.3. Water Contact Angle Measurement
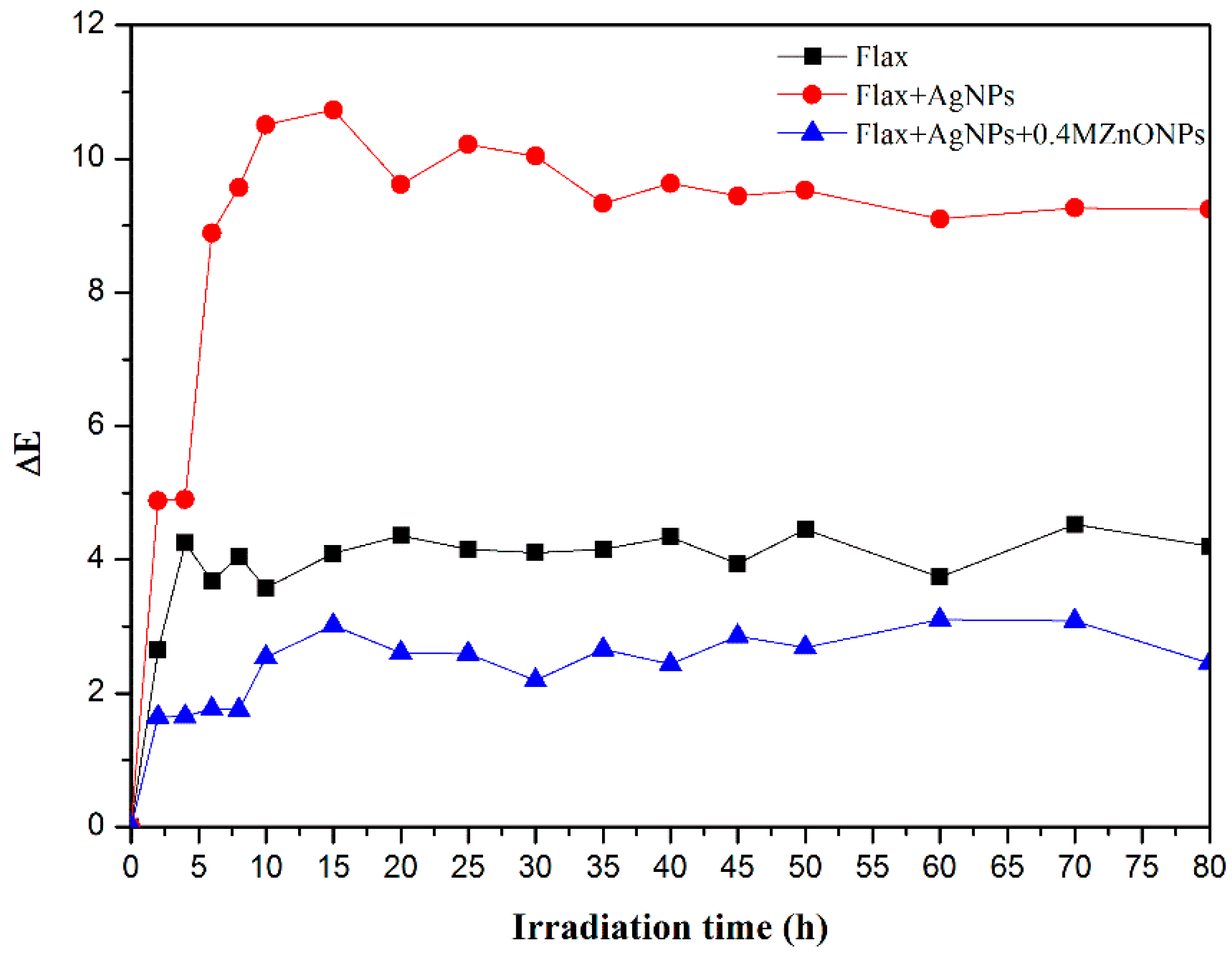
2.5.4. UV Radiation Resistance and Washability
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Flax Fabrics Functionalized with AgNPs and ZnONPs
3.1.1. Ground State Diffuse Reflectance (GSDR)
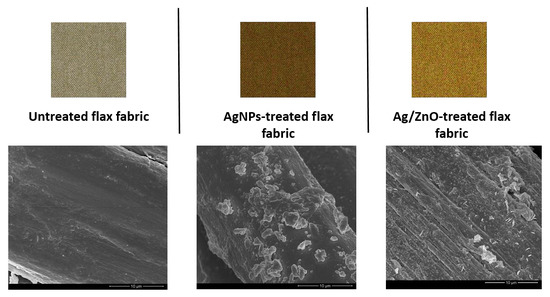
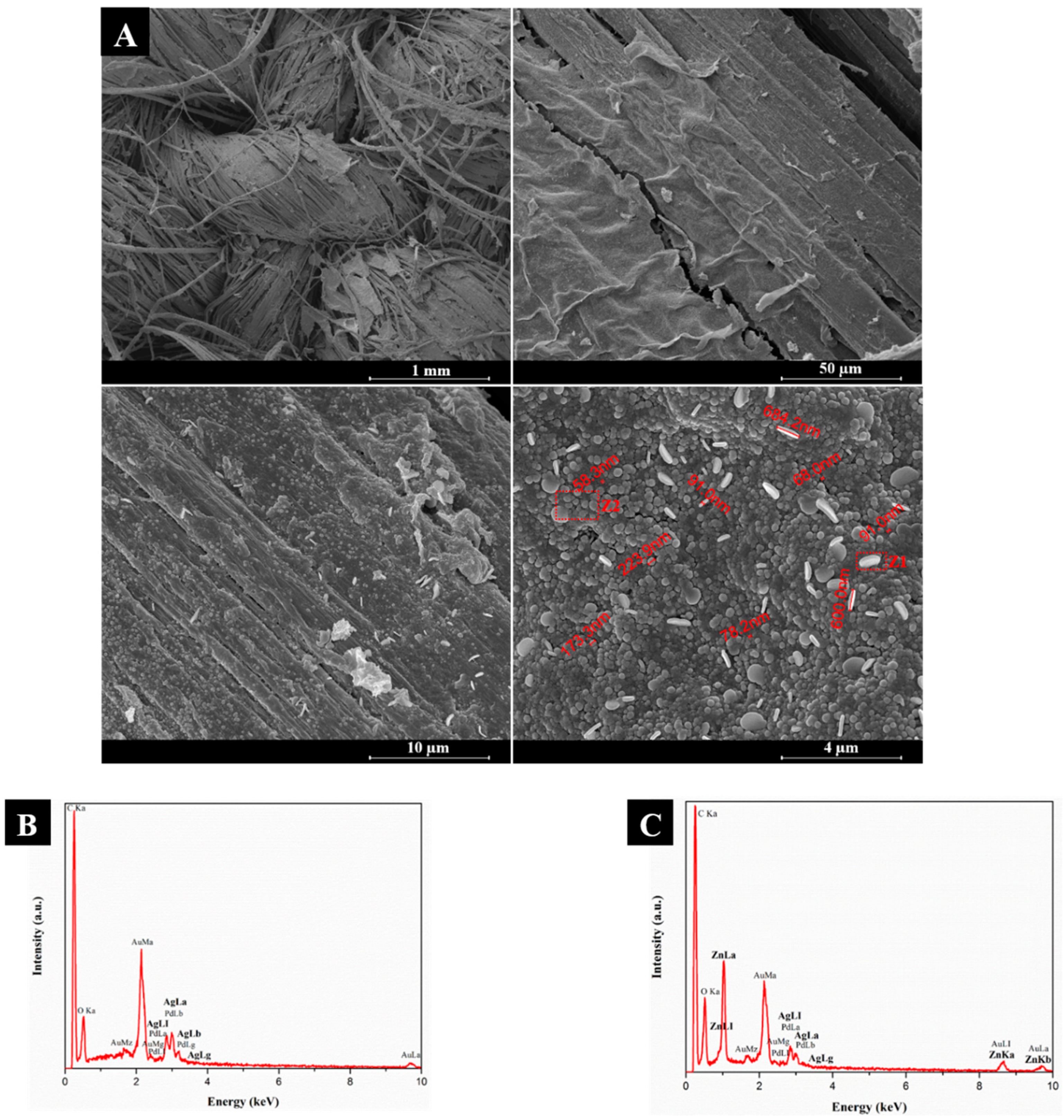
3.1.2. Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive Spectroscopy (EDS)
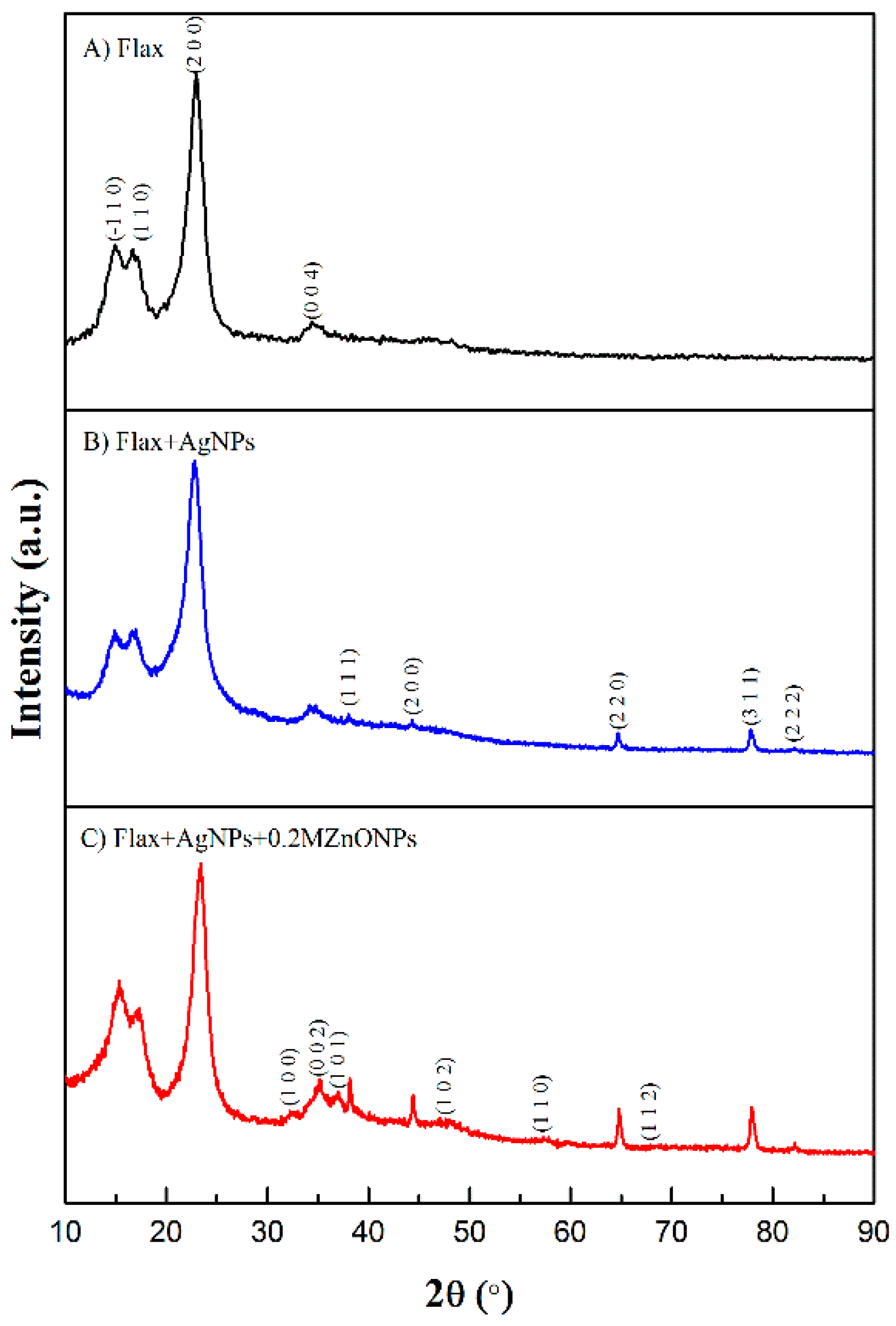
3.1.3. X-ray Diffraction (XRD)
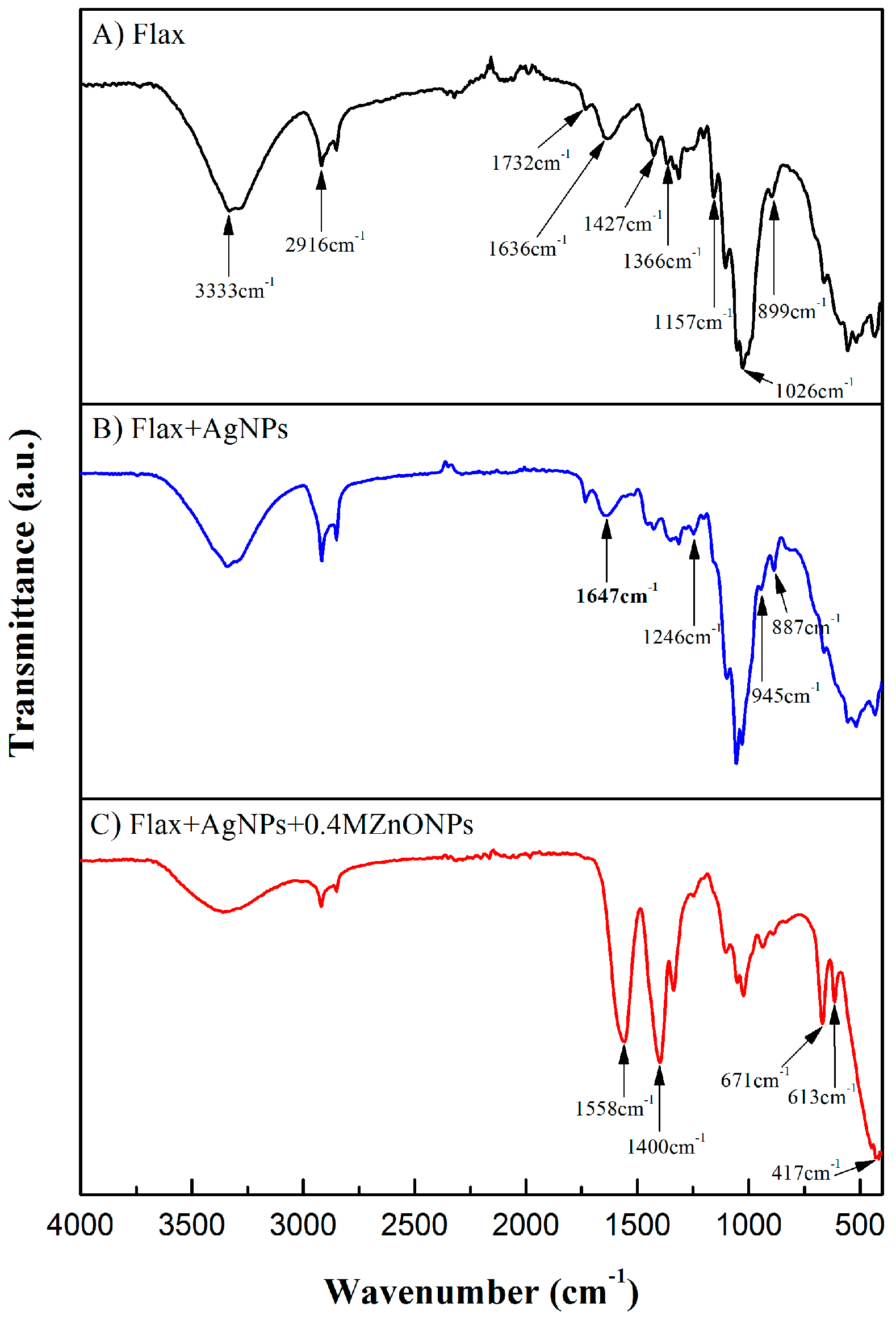
3.1.4. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.1.5. Thermogravimetric Analysis (TGA)
3.2. Multifunctional Flax Fabrics
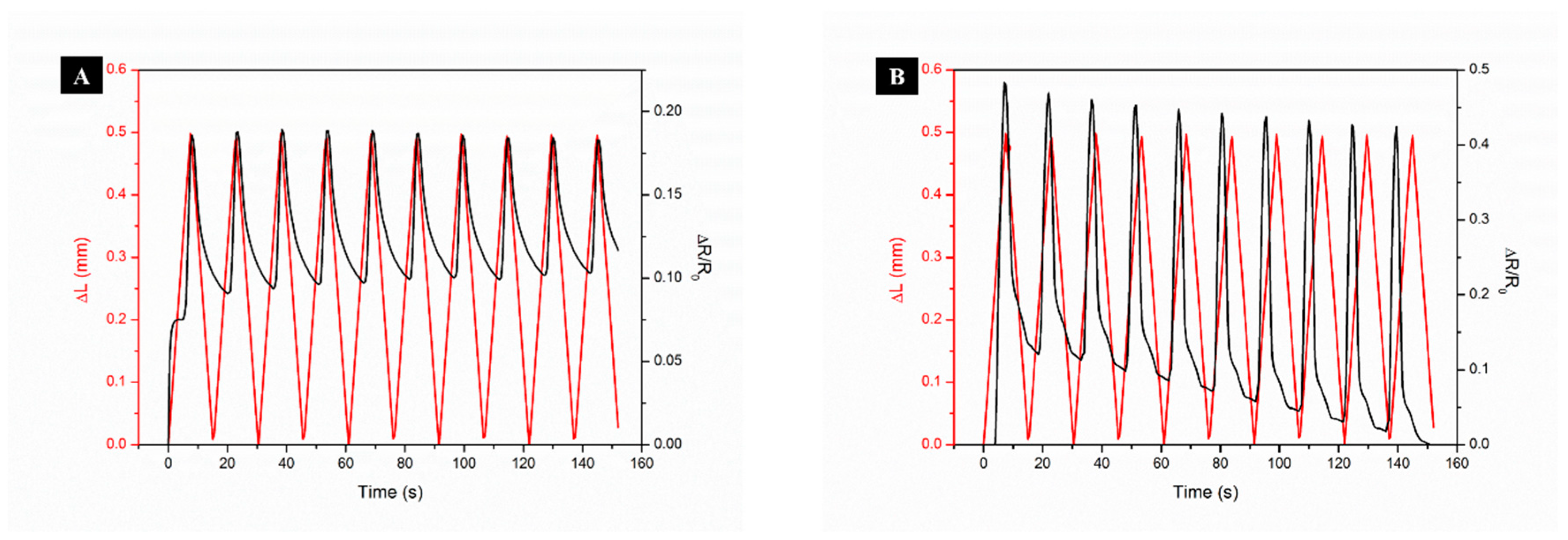
3.2.1. Strain Sensing Mechanism of Flax Fabrics
3.2.2. Antibacterial Activity
3.2.3. Hydrophobicity Properties
3.2.4. UV Radiation Resistance and Wash Durability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koncar, V. 1—Introduction to Smart Textiles and Their Applications. In Woodhead Publishing Series in Textiles; Koncar, V., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 1–8. ISBN 978-0-08-100574-3. [Google Scholar]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X.-M. Fiber-Based Wearable Electronics: A Review of Materials, Fabrication, Devices, and Applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ali, S.; Wijekoon, J.; Gong, R.H.; Fernando, A. A Wearable Piezo-Resistive Sensor for Capturing Cardiorespiratory Signals. Sens. Actuators A Phys. 2018, 282, 215–229. [Google Scholar] [CrossRef]
- Ferreira, D.; Ferreira, A.; Fangueiro, R. Searching for Natural Conductive Fibrous Structures via a Green Sustainable Approach Based on Jute Fibers and Silver Nanoparticles. Polymers 2018, 10, 63. [Google Scholar] [CrossRef]
- Shaban, M.; Mohamed, F.; Abdallah, S. Production and Characterization of Superhydrophobic and Antibacterial Coated Fabrics Utilizing ZnO Nanocatalyst. Sci. Rep. 2018, 8, 3925. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Sultana, M.; Pervez, M.; Habib, M.; Liu, H.-H. Surface Functionalization of “Rajshahi Silk” Using Green Silver Nanoparticles. Fibers 2017, 5, 35. [Google Scholar] [CrossRef]
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards Multifunctional Cellulosic Fabric: UV Photo-Reduction and in-Situ Synthesis of Silver Nanoparticles into Cellulose Fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886. [Google Scholar] [CrossRef]
- Pandimurugan, R.; Thambidurai, S. UV Protection and Antibacterial Properties of Seaweed Capped ZnO Nanoparticles Coated Cotton Fabrics. Int. J. Biol. Macromol. 2017, 105, 788–795. [Google Scholar] [CrossRef]
- Seki, Y. Conductive Cotton Fabrics Coated with Myristic Acid/Zinc Oxide Nanoparticles. Polym. Plast. Technol. Eng. 2018, 57, 766–774. [Google Scholar] [CrossRef]
- Lim, Z.H.; Chia, Z.X.; Kevin, M.; Wong, A.S.W.; Ho, G.W. A Facile Approach towards ZnO Nanorods Conductive Textile for Room Temperature Multifunctional Sensors. Sens. Actuators B Chem. 2010, 151, 121–126. [Google Scholar] [CrossRef]
- Fangueiro, R.; Rana, S. Natural Fibres: Advances in Science and Technology Towards Industrial Applications, 1st ed.; Springer: Dordrecht, The Netherlands, 2016; p. 456. ISBN 978-94-017-7513-7. [Google Scholar]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and Properties of Natural Fiber Polymer Composites: A Comprehensive Review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Fangueiro, R.; Rana, S. Advances in Natural Fibre Composites: Raw Materials, Processing and Analysis, 1st ed.; Springer International Publishing: New York, NY, USA, 2017; p. 280. ISBN 978-3-319-64640-4. [Google Scholar]
- Shaker, K.; Ashraf, M.; Jabbar, M.; Shahid, S.; Nawab, Y.; Zia, J.; Rehman, A. Bioactive Woven Flax-Based Composites: Development and Characterisation. J. Ind. Text. 2015, 46, 549–561. [Google Scholar] [CrossRef]
- Borda d’ Agua, R.; Branquinho, R.; Duarte, M.P.; Mauricio, E.; Fernando, A.L.; Martins, R.; Fortunato, E. Efficient Coverage of ZnO Nanoparticles on Cotton Fibres for Antibacterial Finishing Using a Rapid and Low Cost in Situ Synthesis. New J. Chem. 2018, 42, 1052–1060. [Google Scholar] [CrossRef]
- Beeby, S.; Ensel, G.; Kraft, M.; White, N. MEMS Mechanical Sensors; Artech House: Boston, MA, USA, 2004; p. 282. ISBN 9781580535366. [Google Scholar]
- Roy, A.; Gauri, S.S.; Bhattacharya, M.; Bhattacharya, J. Antimicrobial Activity of CaO Nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Magalhães, L.; Henriques, M.; Oliveira, R. Antimicrobial Activity Assessment of Textiles: Standard Methods Comparison. Ann. Microbiol. 2011, 61, 493–498. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef]
- Muthuchamy, M.; Muthusamy, A.; Govindhan, M.; Santhanakrishnan, S.; Akbar Sait Hameedha, B.; Radhakrishnan Jeeva, P. Investigation on the Electrical Conductivity of ZnO Nanoparticles-Decorated Bacterial Nanowires. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 45011. [Google Scholar]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Shabanzadeh, P.; Sangpour, P.; Jahangirian, H.; Gharayebi, Y. Investigation of Antibacterial Properties Silver Nanoparticles Prepared via Green Method. Chem. Cent. J. 2012, 6, 73. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E.; Etemadifar, A. Fabricating Multifunctional Silver Nanoparticles-Coated Cotton Fabric. Arab. J. Chem. 2017, 10, S2355–S2362. [Google Scholar] [CrossRef]
- Jiang, X.C.; Chen, W.M.; Chen, C.Y.; Xiong, S.X.; Yu, A.B. Role of Temperature in the Growth of Silver Nanoparticles Through a Synergetic Reduction Approach. Nanoscale Res. Lett. 2011, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Abdallah, S.; Khalek, A.A. Characterization and Photocatalytic Properties of Cotton Fibers Modified with ZnO Nanoparticles Using Sol–gel Spin Coating Technique. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 277–283. [Google Scholar] [CrossRef]
- Pelicano, C.M.; Rapadas, N.J.; Magdaluyo, E., Jr. X-Ray Peak Profile Analysis of Zinc Oxide Nanoparticles Formed by Simple Precipitation Method. AIP Conf. Proc. 2017, 1901, 20016. [Google Scholar] [CrossRef]
- Saoud, K.; Alsoubaihi, R.; Bensalah, N.; Bora, T.; Bertino, M.; Dutta, J. Synthesis of Supported Silver Nano-Spheres on Zinc Oxide Nanorods for Visible Light Photocatalytic Applications. Mater. Res. Bull. 2015, 63, 134–140. [Google Scholar] [CrossRef]
- Shao, D.; Gao, Y.; Cao, K.; Wei, Q. Rapid Surface Functionalization of Cotton Fabrics by Modified Hydrothermal Synthesis of ZnO. J. Text. Inst. 2017, 108, 1391–1397. [Google Scholar] [CrossRef]
- Fahmy, A.; El-Zomrawy, A.; Saeed, A.M.; Sayed, A.Z.; El-Arab, M.A.E.; Shehata, H.A.; Friedrich, J. One-Step Synthesis of Silver Nanoparticles Embedded with Polyethylene Glycol as Thin Films. J. Adhes. Sci. Technol. 2017, 31, 1422–1440. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-Gel Synthesis of Thorn-like ZnO Nanoparticles Endorsing Mechanical Stirring Effect and Their Antimicrobial Activities: Potential Role as Nano-Antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef]
- Alireza, S.; Ismail, A.F.; Hadi, N.; Othaman, Z.; Mustafa, M.K. Spectral Features and Antibacterial Properties of Cu-Doped ZnO Nanoparticles Prepared by Sol-Gel Method. Chin. Phys. B 2016, 25, 77803. [Google Scholar]
- Kumar, S.; Sharma, V.; Bhattacharyya, K.; Krishnan, V. Synergetic Effect of MoS2-RGO Doping to Enhance the Photocatalytic Performance of ZnO Nanoparticles. New J. Chem. 2016, 40, 5185–5197. [Google Scholar] [CrossRef]
- Ferrreira, A.; Rocha, J.G.; Ansón-Casaos, A.; Martínez, M.T.; Vaz, F.; Lanceros-Mendez, S. Electromechanical Performance of Poly(Vinylidene Fluoride)/Carbon Nanotube Composites for Strain Sensor Applications. Sens. Actuators A Phys. 2012, 178, 10–16. [Google Scholar] [CrossRef]
- Paleo, A.J.; van Hattum, F.W.J.; Pereira, J.; Rocha, J.G.; Silva, J.; Sencadas, V.; Lanceros-Méndez, S. The Piezoresistive Effect in Polypropylene—Carbon Nanofibre Composites Obtained by Shear Extrusion. Smart Mater. Struct. 2010, 19, 65013. [Google Scholar] [CrossRef]
- Mano, J.F.; Sencadas, V.; Costa, A.M.; Lanceros-Méndez, S. Dynamic Mechanical Analysis and Creep Behaviour of β-PVDF Films. Mater. Sci. Eng. A 2004, 370, 336–340. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-Ray Peak Broadening Analysis in ZnO Nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Huang, X.; Netravali, A. Characterization of Flax Fiber Reinforced Soy Protein Resin Based Green Composites Modified with Nano-Clay Particles. Compos. Sci. Technol. 2007, 67, 2005–2014. [Google Scholar] [CrossRef]
- Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Countering Drug Resistance, Infectious Diseases, and Sepsis Using Metal and Metal Oxides Nanoparticles: Current Status. Colloids Surf. B Biointerfaces 2016, 146, 70–83. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial Mechanism of Silver Nanoparticles in Pseudomonas Aeruginosa: Proteomics Approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Basha, S.K.; Lakshmi, K.V.; Kumari, V.S. Ammonia Sensor and Antibacterial Activities of Green Zinc Oxide Nanoparticles. Sens. Bio-Sens. Res. 2016, 10, 34–40. [Google Scholar] [CrossRef]
- Shateri-Khalilabad, M.; Yazdanshenas, M.E. Bifunctionalization of Cotton Textiles by ZnO Nanostructures: Antimicrobial Activity and Ultraviolet Protection. Text. Res. J. 2013, 83, 993–1004. [Google Scholar] [CrossRef]
- Duta, L.; Popescu, A.C.; Zgura, I.; Preda, N.; Mihailescu, I.N. Wettability of Nanostructured Surfaces. In Wetting and Wettability; Popescu, A.C., Ed.; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Abdel-Halim, E.S.; Konczewicz, W.; Zimniewska, M.; Al-Deyab, S.S.; El-Newehy, M.H. Enhancing Hydrophilicity of Bioscoured Flax Fabric by Emulsification Post-Treatment. Carbohydr. Polym. 2010, 82, 195–201. [Google Scholar] [CrossRef]
- Rajavel, K.; Gomathi, R.; Pandian, R.; Rajendra Kumar, R.T. In Situ Attachment and Its Hydrophobicity of Size- and Shape-Controlled Silver Nanoparticles on Fabric Surface for Bioapplication. Inorg. Nano-Metal Chem. 2017, 47, 1196–1203. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, S.; Maity, J. In Situ Generation and Deposition of ZnO Nanoparticles on Cotton Surface to Impart Hydrophobicity: Investigation of Antibacterial Activity. Mater. Technol. 2018, 1–8. [Google Scholar] [CrossRef]
- Takuya, T.; Xungai, W. Nanoparticle Coatings for UV Protective Textiles. Res. J. Text. Appar. 2010, 14, 9–20. [Google Scholar] [CrossRef]
- Xuan, L.; Han, G.; Wang, D.; Cheng, W.; Gao, X.; Chen, F.; Li, Q. Effect of Surface-Modified TiO2 Nanoparticles on the Anti-Ultraviolet Aging Performance of Foamed Wheat Straw Fiber/Polypropylene Composites. Materials 2017, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Shaarawy, S.; Hebeish, A.A. Multifunctional Properties of Cotton Fabrics Coated with in Situ Synthesis of Zinc Oxide Nanoparticles Capped with Date Seed Extract. Carbohydr. Polym. 2018, 181, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Desuo, Z.; Guangyu, Z.; Ling, C.; Yanfen, L.; Yuyue, C.; Hong, L. Multifunctional Finishing of Cotton Fabric Based on in Situ Fabrication of Polymer-Hybrid Nanoparticles. J. Appl. Polym. Sci. 2013, 130, 3778–3784. [Google Scholar] [CrossRef]
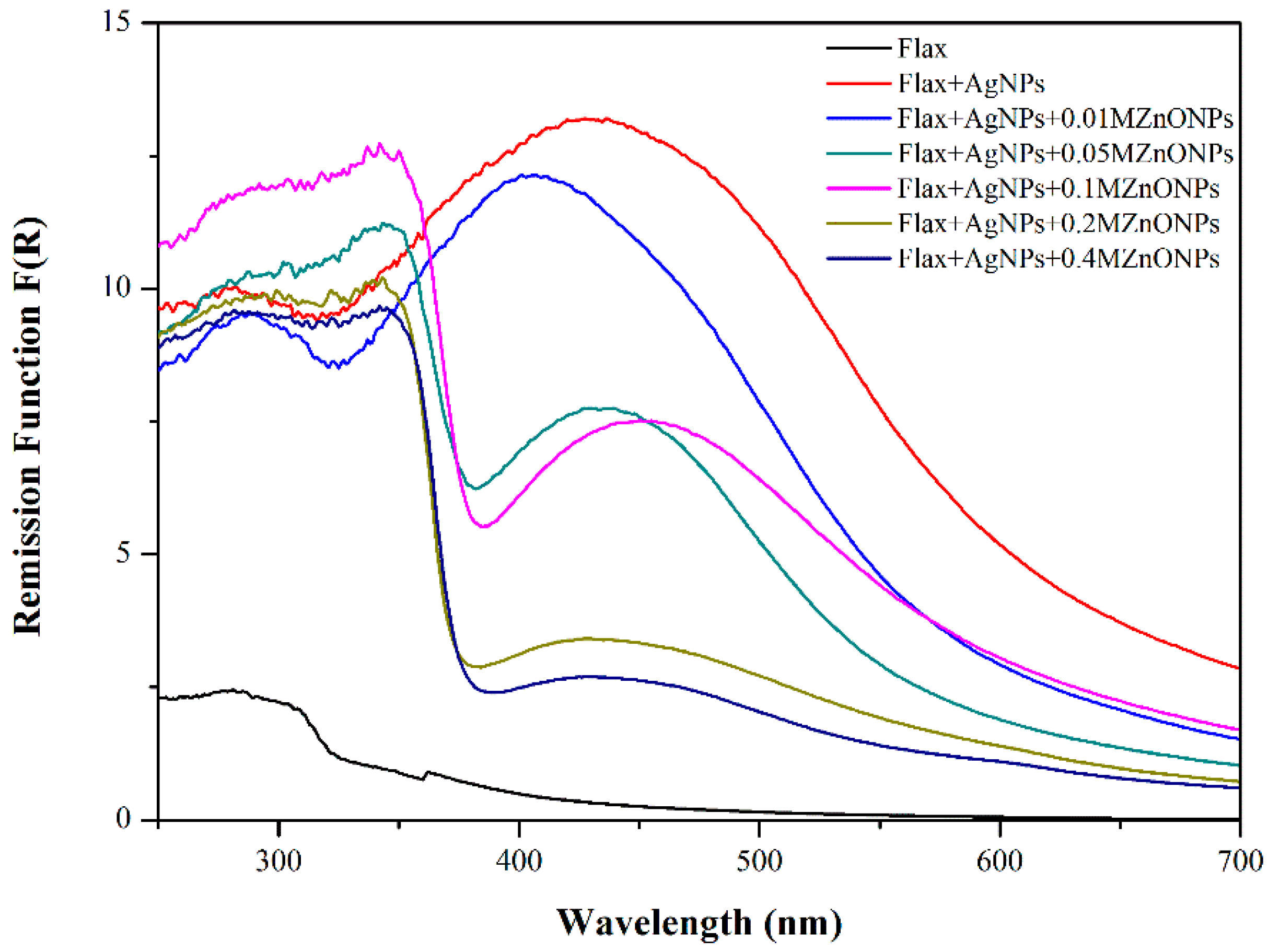
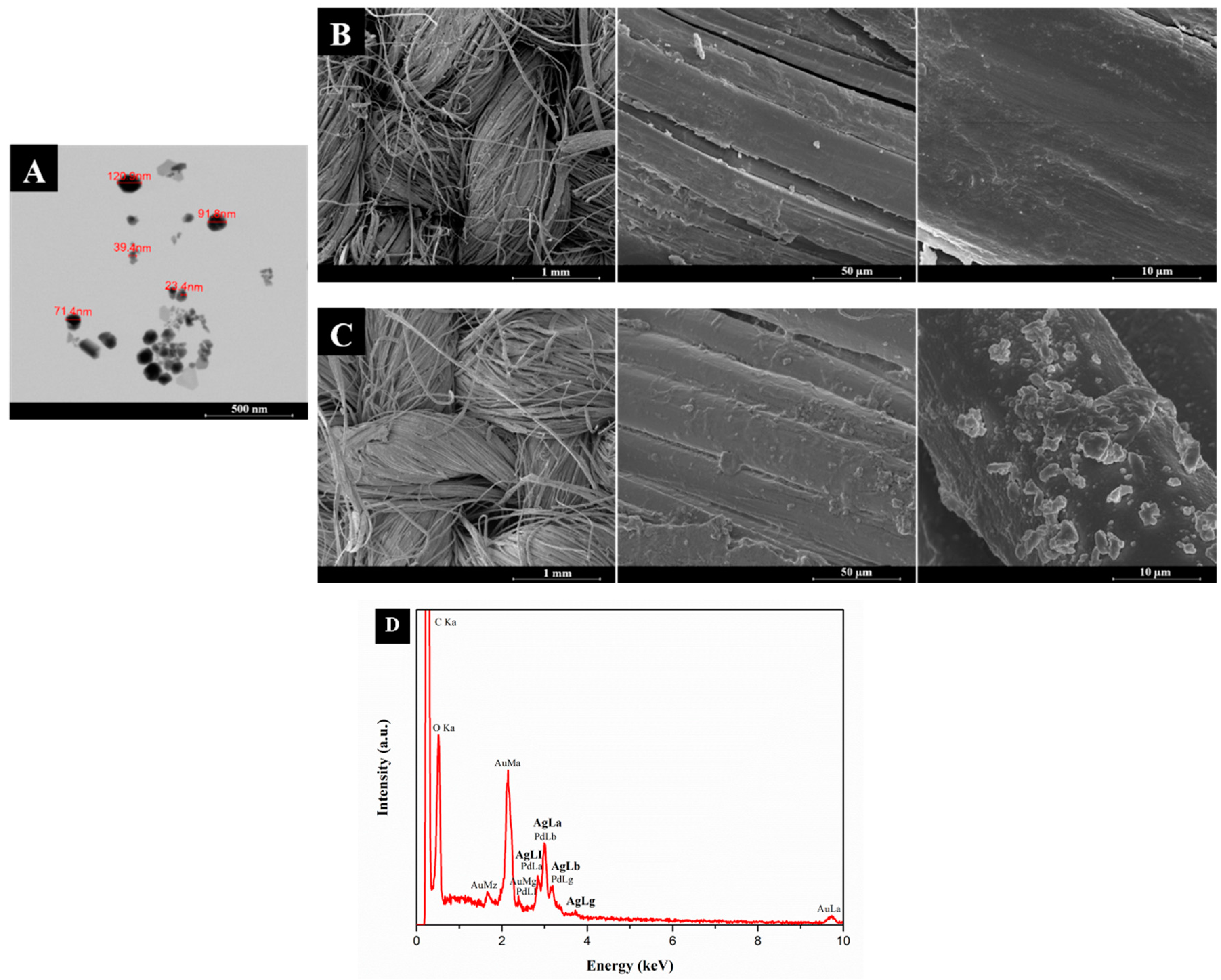

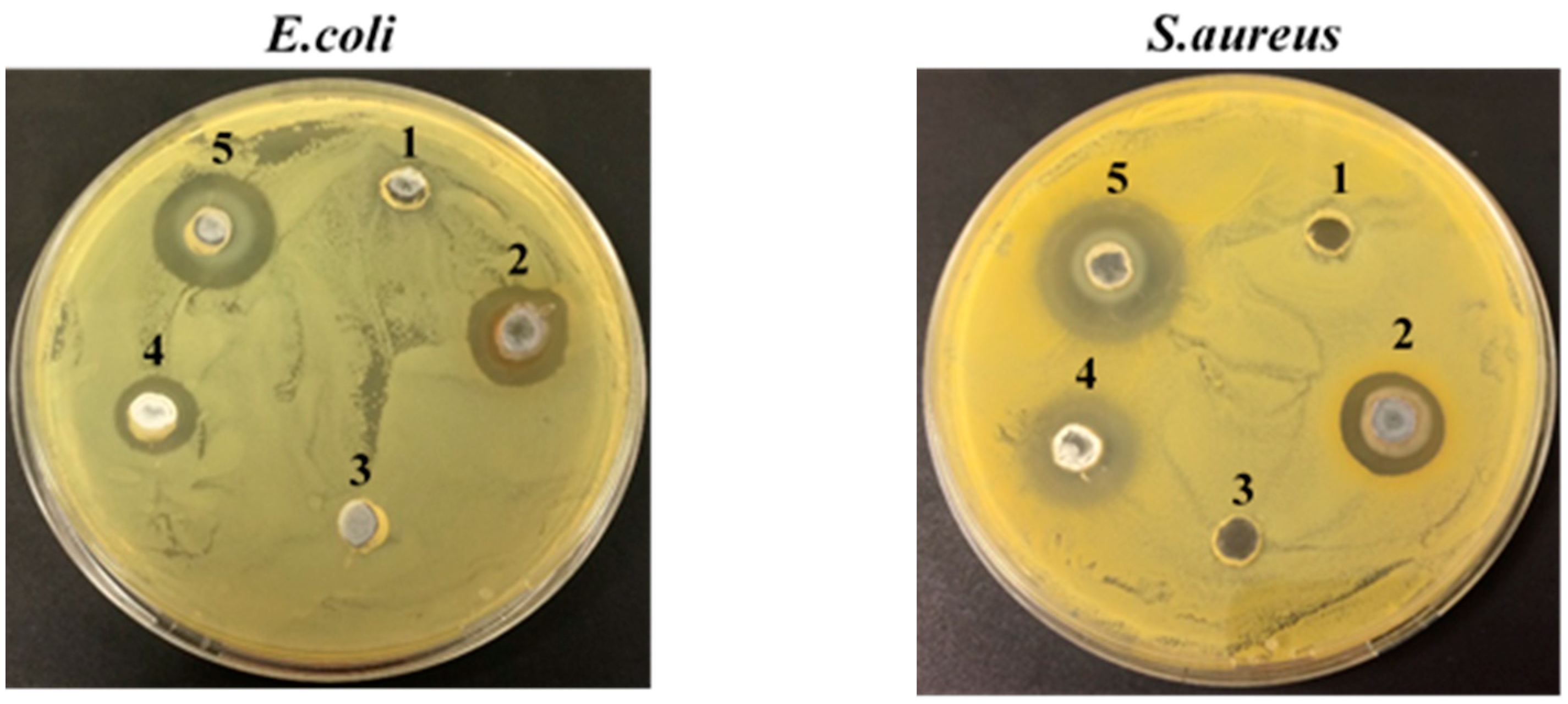

| Samples | Mean Diameter of the Halos (mm) | |
|---|---|---|
| E. coli | S. aureus | |
| Flax | 0 | 0 |
| Flax + AgNPs | 4 ± 0.08 | 6.33 ± 0.33 |
| Flax + AgNPs + 0.2 MZnONPs | 6 ± 0.22 | 9 ± 0.28 |
| Flax + AgNPs + 0.4 MZnONPs | 14.33 ± 0.09 | 13.67 ± 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, S.M.; Ferreira, D.P.; Ferreira, A.; Vaz, F.; Fangueiro, R. Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures. Nanomaterials 2018, 8, 1069. https://doi.org/10.3390/nano8121069
Costa SM, Ferreira DP, Ferreira A, Vaz F, Fangueiro R. Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures. Nanomaterials. 2018; 8(12):1069. https://doi.org/10.3390/nano8121069
Chicago/Turabian StyleCosta, Sofia M., Diana P. Ferreira, Armando Ferreira, Filipe Vaz, and Raul Fangueiro. 2018. "Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures" Nanomaterials 8, no. 12: 1069. https://doi.org/10.3390/nano8121069
APA StyleCosta, S. M., Ferreira, D. P., Ferreira, A., Vaz, F., & Fangueiro, R. (2018). Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures. Nanomaterials, 8(12), 1069. https://doi.org/10.3390/nano8121069