Intramolecular Metal Exchange Reaction Promoted by Thiol Ligands
Abstract
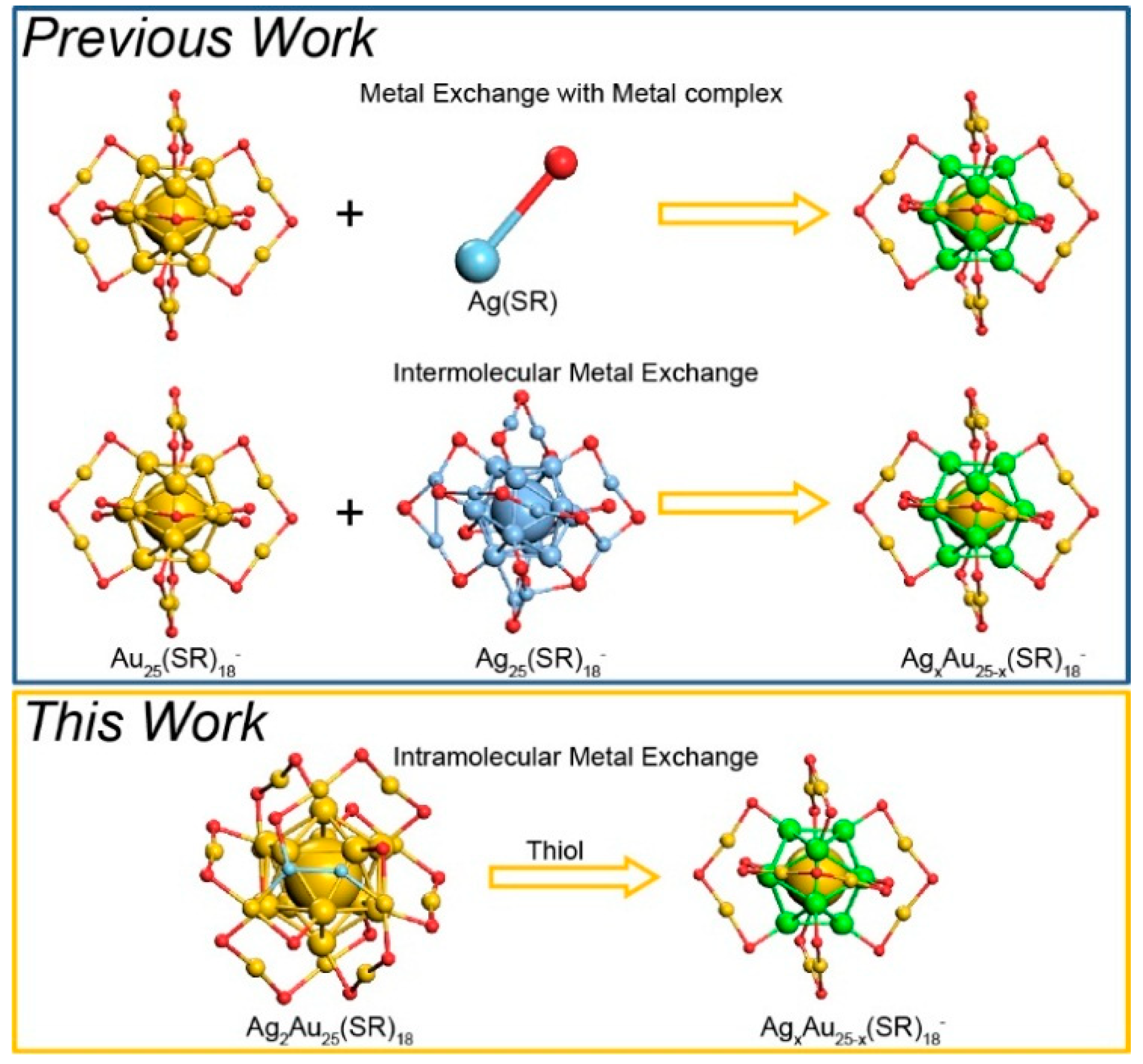
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Au25(PET)18− Nanocluster
2.3. Synthesis of the Ag2Au25(PET)18+ Nanocluster
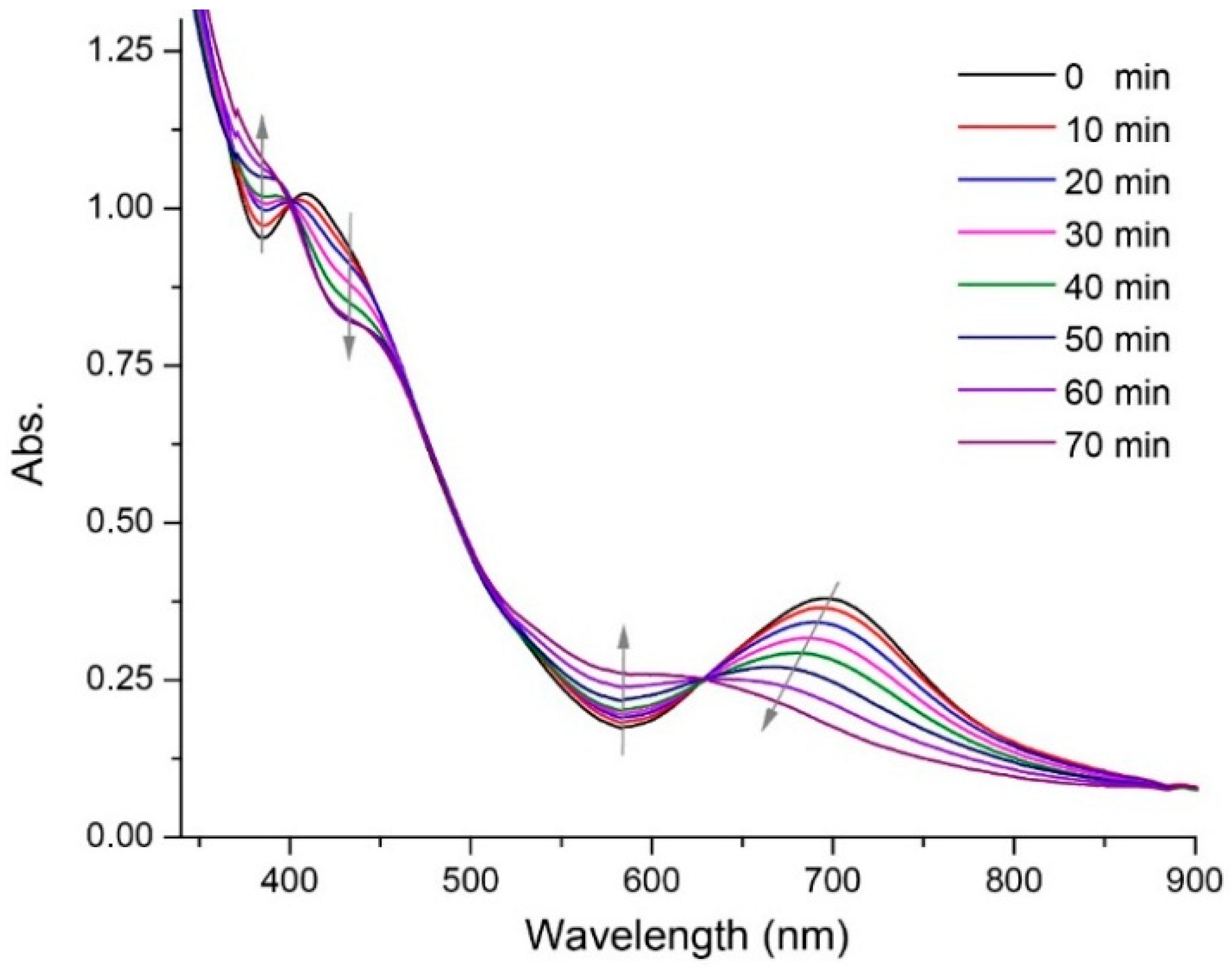
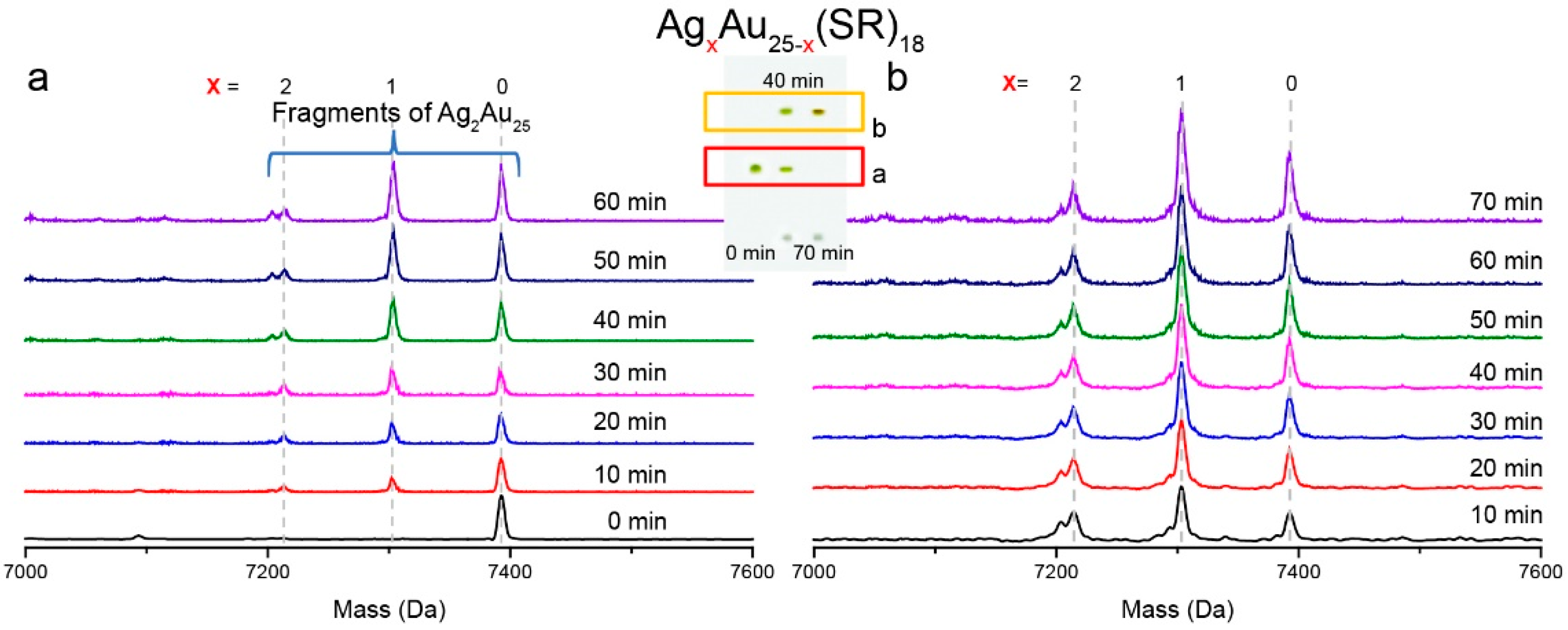
2.4. Intramolecular Metal Exchange of the Ag2Au25 Nanocluster
2.5. Characterization
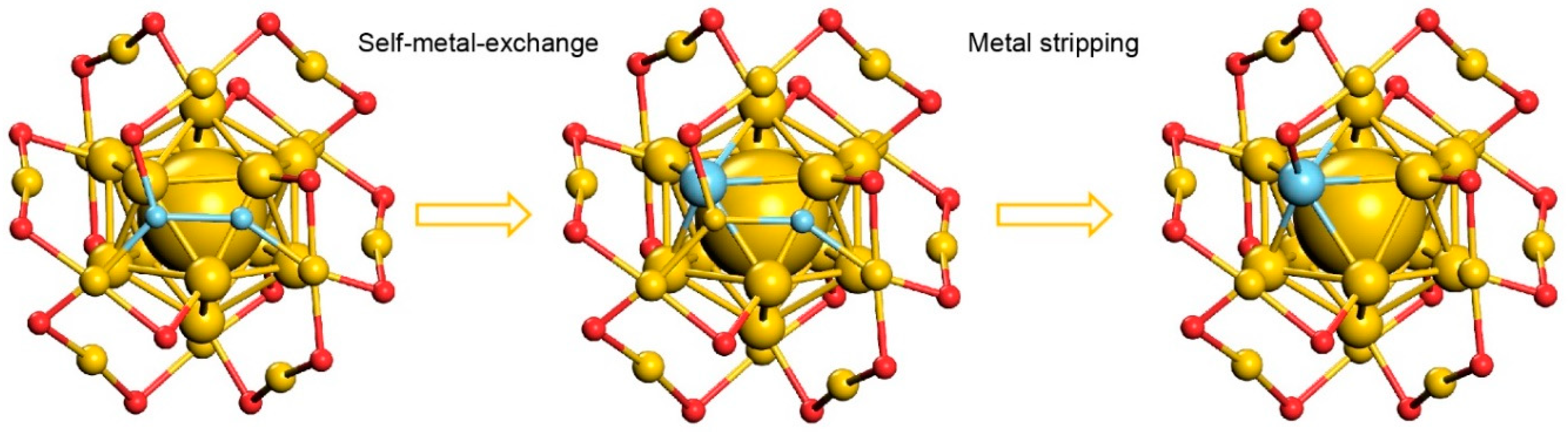
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.-C.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Jones, Z.R.; Goldsmith, B.R.; Buratto, W.R.; Wu, G.; Scott, S.L.; Hayton, T.W. A Cu25 Nanocluster with Partial Cu(0) Character. J. Am. Chem. Soc. 2015, 137, 13319–13324. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Q.; Kang, X.; Zhu, M. Customizing the Structure, Composition, and Properties of Alloy Nanoclusters by Metal Exchange. Acc. Chem. Res. 2018, 51, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, Y.; Jin, S.; Liu, X.; Zhang, J.; Pei, Y.; Meng, X.; Chen, M.; Li, P.; Zhu, M. Metal Exchange Method Using Au25 Nanoclusters as Templates for Alloy Nanoclusters with Atomic Precision. J. Am. Chem. Soc. 2015, 137, 4018–4021. [Google Scholar] [CrossRef]
- Wang, S.; Abroshan, H.; Liu, C.; Luo, T.-Y.; Zhu, M.; Kim, H.J.; Rosi, N.L.; Jin, R. Shuttling single metal atom into and out of a metal nanoparticle. Nat. Commun. 2017, 8, 848. [Google Scholar] [CrossRef]
- Yang, S.; Wang, S.; Jin, S.; Chen, S.; Sheng, H.; Zhu, M. A metal exchange method for thiolate-protected tri-metal M1AgxAu24−x(SR)18 (M = Cd/Hg) nanoclusters. Nanoscale 2015, 7, 10005–10007. [Google Scholar] [CrossRef]
- Yang, S.; Chen, S.; Xiong, L.; Liu, C.; Yu, H.; Wang, S.; Rosi, N.L.; Pei, Y.; Zhu, M. Total Structure Determination of Au16(S-Adm)12 and Cd1Au14(StBu)12 and Implications for the Structure of Au15(SR)13. J. Am. Chem. Soc. 2018, 140, 10988–10994. [Google Scholar] [CrossRef]
- Jin, S.; Du, W.; Wang, S.; Kang, X.; Chen, M.; Hu, D.; Chen, S.; Zou, X.; Sun, G.; Zhu, M. Thiol-Induced Synthesis of PhosphineProtected Gold Nanoclusters with Atomic Precision and Controlling the Structure by Ligand/Metal Engineering. Inorg. Chem. 2017, 56, 11151–11159. [Google Scholar] [CrossRef]
- Li, Q.; Lambright, K.J.; Taylor, M.G.; Kirschbaum, K.; Luo, T.-Y.; Zhao, J.; Mpourmpakis, G.; Mokashi-Punekar, S.; Rosi, N.L.; Jin, R. Reconstructing the Surface of Gold Nanoclusters by Cadmium Doping. J. Am. Chem. Soc. 2017, 139, 17779–17782. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Kirschbaum, K.; Lambright, K.J.; Das, A.; Jin, R. Heavily doped Au25−xAgx(SC6H11)18 nanoclusters: Silver goes from the core to the surface. Chem. Commun. 2016, 52, 5194–5197. [Google Scholar] [CrossRef]
- Du, W.; Jin, S.; Xiong, L.; Chen, M.; Zhang, J.; Zou, X.; Pei, Y.; Wang, S.; Zhu, M. Ag50(Dppm)6(SR)30 and Its Homologue AuxAg50−x(Dppm)6(SR)30 Alloy Nanocluster: Seeded Growth, Structure Determination, and Differences in Properties. J. Am. Chem. Soc. 2017, 139, 1618–1624. [Google Scholar] [CrossRef]
- Kang, X.; Xiong, L.; Wang, S.; Yu, H.; Jin, S.; Song, Y.; Chen, T.; Zheng, L.; Pan, C.; Pei, Y.; et al. Shape-Controlled Synthesis of Trimetallic Nanoclusters: Structure Elucidation and Properties Investigation. Chem. Eur. J. 2016, 22, 17145–17150. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Ono, T.; Yoshioka, M.; Hu, G.; Hosoi, M.; Chen, Z.; Nair, L.V.; Niihori, Y.; Kurashige, W.; Jiang, D.-E.; et al. Thiolate-Protected Trimetallic Au∼20Ag∼4Pd and Au∼20Ag∼4Pt Alloy Clusters with Controlled Chemical Composition and Metal Positions. J. Phys. Chem. Lett. 2018, 9, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Krishnadas, K.R.; Baksi, A.; Ghosh, A.; Natarajan, G.; Pradeep, T. Structure-conserving spontaneous transformations between nanoparticles. Nat. Commun. 2016, 7, 13447. [Google Scholar] [CrossRef]
- Zhang, B.; Salassa, G.; Bürgi, T. Silver migration between Au38(SC2H4Ph)24 and doped AgxAu38−x(SC2H4Ph)24 nanoclusters. Chem. Commun. 2016, 52, 9205–9207. [Google Scholar] [CrossRef]
- Zhang, B.; Safonova, O.V.; Pollitt, S.; Salassa, G.; Sels, A.; Kazan, R.; Wang, Y.; Rupprechter, G.; Barrabes, N.; Bürgi, T. On the mechanism of rapid metal exchange between thiolate-protected gold and gold/silver clusters: A time-resolved in situ XAFS study. Phys. Chem. Chem. Phys. 2018, 20, 5312–5318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Bürgi, T. Doping Silver Increases the Au38(SR)24 Cluster Surface Flexibility. J. Phys. Chem. C 2016, 120, 4660–4666. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.; Das, A.; Li, T.; Song, Y.; Cao, T.; Zhu, X.; Zhu, M.; Jin, R. A 200-fold Quantum Yield Boost in the Photoluminescence of Silver Doped AgxAu25−x Nanoclusters: The 13th Silver Atom Matters. Angew. Chem. Int. Ed. 2014, 53, 2376–2380. [Google Scholar] [CrossRef]
- Bootharaju, M.S.; Joshi, C.P.; Parida, M.R.; Mohammed, O.F.; Bakr, O.M. Templated Atom-Precise Galvanic Synthesis and Structure Elucidation of a [Ag24Au(SR)18]− Nanocluster. Angew. Chem. 2016, 128, 934–938. [Google Scholar] [CrossRef]
- Deng, H.; Wang, S.; Jin, S.; Yang, S.; Xu, Y.; Liu, L.; Xiang, J.; Hu, D.; Zhu, M. Active metal (cadmium) doping enhanced the stability of inert metal (gold) nanocluster under O2 atmosphere and the catalysis activity of benzyl alcohol oxidation. Gold Bull. 2015, 48, 161–167. [Google Scholar] [CrossRef]
- Parker, J.F.; Weaver, J.E.F.; McCallum, F.; Fields-Zinna, C.A.; Murray, R.W. Synthesis of Monodisperse [Oct4N]+ [Au25(SR)18]− Nanoparticles, with Some Mechanistic Observations. Langmuir 2010, 26, 13650–13654. [Google Scholar] [CrossRef]
- Yao, C.; Chen, J.; Li, M.; Liu, L.; Yang, J.; Wu, Z. Adding Two Active Silver Atoms on Au25 Nanoparticle. Nano Lett. 2015, 15, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
| Results | Au Atom | Ag Atom |
|---|---|---|
| ICP experimental ratio | 96.22% | 3.78% |
| XPS experimental ratio | 96.04% | 3.96% |
| Theoretical | 24/25 (96%) | 1/25 (4%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Chen, M.; Wang, S.; Zhu, M. Intramolecular Metal Exchange Reaction Promoted by Thiol Ligands. Nanomaterials 2018, 8, 1070. https://doi.org/10.3390/nano8121070
Li Y, Chen M, Wang S, Zhu M. Intramolecular Metal Exchange Reaction Promoted by Thiol Ligands. Nanomaterials. 2018; 8(12):1070. https://doi.org/10.3390/nano8121070
Chicago/Turabian StyleLi, Yangfeng, Man Chen, Shuxin Wang, and Manzhou Zhu. 2018. "Intramolecular Metal Exchange Reaction Promoted by Thiol Ligands" Nanomaterials 8, no. 12: 1070. https://doi.org/10.3390/nano8121070
APA StyleLi, Y., Chen, M., Wang, S., & Zhu, M. (2018). Intramolecular Metal Exchange Reaction Promoted by Thiol Ligands. Nanomaterials, 8(12), 1070. https://doi.org/10.3390/nano8121070




